Blends of Cyanate Ester and Phthalonitrile–Polyhedral Oligomeric Silsesquioxane Copolymers: Cure Behavior and Properties
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Preparation of Cyanate and POSS-Phthalonitrile Blends
2.3. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Keller, T.M. Phthalonitrile-based high temperature resin. J. Polym. Sci. Part. A Polym. Chem. 1988, 26, 3199–3212. [Google Scholar] [CrossRef]
- Keller, T.M. Phthalonitrile conductive polymer. J. Polym. Sci. Part. A Polym. Chem. 1987, 25, 2569–2576. [Google Scholar] [CrossRef]
- Keller, T.M. A stable intrinsically conductive polymer. J. Polym. Sci. Part. C Polym. Lett. 1986, 24, 211–214. [Google Scholar] [CrossRef]
- Sastri, S.B.; Keller, T.M. Phthalonitrile polymers: Cure behavior and properties. J. Polym. Sci. Part. A Polym. Chem. 1999, 37, 2105–2111. [Google Scholar] [CrossRef]
- Laskoski, M.; Keller, T.M.; Qadri, S.B. Direct conversion of highly aromatic phthalonitrile thermosetting resins into carbon nanotube containing solids. Polymer 2007, 48, 7484–7489. [Google Scholar] [CrossRef]
- Sastri, S.B.; Armistead, J.P.; Keller, T.M. Phthalonitrile-glass fabric composites. In Proceedings of the 41th International SAMPE Symposium, Anaheim, CA, USA, 24–28 March 1996; pp. 24–28. [Google Scholar]
- Sastri, S.B.; Armistead, J.P.; Keller, T.M. Phthalonitrile-carbon fiber composites. Polym. Compos. 1996, 17, 816–822. [Google Scholar] [CrossRef]
- Dominguez, D.D.; Jones, H.N.; Trzaskoma-Paulette, P.P.; Keller, T.M. Mechanical properties of graphite fiber-reinforced phthalonitrile composites. In Proceedings of the 46th International SAMPE Symposium, Long Beach, CA, USA, 6–7 May 2001; pp. 94–107. [Google Scholar]
- Laskoski, M.; Dominguez, D.D.; Keller, T.M. Synthesis and properties of a bisphenolA based phthalonitrile resin. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4136–4143. [Google Scholar] [CrossRef]
- Dominguez, D.D.; Keller, T.M. Low-melting phthalonitrile oligomers: Preparation, polymerization and polymer properties. High. Perform. Polym. 2006, 18, 283–304. [Google Scholar] [CrossRef]
- Dominguez, D.D.; Jones, H.N.; Keller, T.M. The effect of curing Additive on the mechanical properties of phthalonitrile-carbon fiber composites. Polym. Compos. 2004, 25, 554–561. [Google Scholar] [CrossRef]
- Sastri, S.B.; Keller, T.M. Phthalonitrile Cure reaction with aromatic diamines. J. Polym. Sci. Part. A Polym. Chem. 1998, 36, 1885–1890. [Google Scholar] [CrossRef]
- Keller, T.M.; Dominguez, D.D. High temperature resorcinol-based phthalonitrile polymer. Polymer 2005, 46, 4614–4618. [Google Scholar] [CrossRef]
- Keller, T.M. Synthesis and polymerization of multiple aromatic ether phthalonitriles. Chem. Mater. 1994, 6, 302–305. [Google Scholar] [CrossRef]
- Laskoski, M.; Dominguez, D.D.; Keller, T.M. Synthesis and properties of aromatic ether phosphine oxide containing oligomericphthalonitrile resins with improved oxidative stability. Polymer 2007, 48, 6234–6240. [Google Scholar] [CrossRef]
- Laskoski, M.; Schear, M.B.; Neal, A.; Dominguez, D.D.; Ricks-laskoski, H.L.; Hervey, J.; Keller, T.M. Improved synthesis and properties of aryl ether-based oligomericphthalonitrile resins and polymers. Polymer 2015, 67, 185–191. [Google Scholar] [CrossRef]
- Babkin, A.V.; Zodbinov, E.B.; Bulgakov, B.A.; Kepman, A.V.; Avdeev, V.V. Low-melting siloxane-bridged phthalonitriles for heat-resistant matrices. Eur. Polym. J. 2015, 66, 452–457. [Google Scholar] [CrossRef]
- Augustine, D.; Vijayalakshmi, K.P.; Sadhana, R.; Mathew, D.; Nair, C.P.R. Hydroxyl terminated peek-toughed epoxy–amino novolacphthalonitrile blends—Synthesis, cure studies and adhesive properties. Polymer 2014, 55, 6006–6016. [Google Scholar] [CrossRef]
- Augustine, D.; Mathew, D.; Nair, C.P.R. One component phthalonitrilenovolac: Synthesis and characterization. Eur. Polym. J. 2015, 71, 389–400. [Google Scholar] [CrossRef]
- Derradji, M.; Ramdani, N.; Zhang, T.; Wang, J.; Feng, T.T.; Wang, H.; Liu, W.B. Mechanical and thermal properties of phthalonitrile resin reinforced with silicon carbide particles. Mater. Des. 2015, 71, 48–55. [Google Scholar] [CrossRef]
- Zhao, F.H.; Liu, R.J.; Yu, X.Y.; Naito, K.; Tang, C.C.; Qu, X.W.; Zhang, Q.X. Systhesis of a novel naphthyl-based self-catalyzed phthalonitrile polymer. Chin. Chem. Lett. 2015, 26, 727–729. [Google Scholar] [CrossRef]
- Li, X.; Yu, B.; Zhang, D.; Lei, J.; Nan, Z. Cure behavior and thermomechanical properties of phthalonitrile–polyhedral oligomeric silsesquioxane copolymers. Polymers 2017, 9, 334. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Sun, Y.; Zhang, D.; Nan, Z.; Lei, J. The effect of thermal ttreatment on the decomposition of phthalonitrile polymer and phthalonitrile-polyhedral oligomeric silsesquioxane (POSS) copolymer. Polym. Degrad. Stab. 2018, 156, 279–291. [Google Scholar] [CrossRef]
- Huang, J.; Wang, W.; Gu, J.; Li, W.; Zhang, Q.; Ding, Y.; Xi, K.; Zhen, Y.; Jia, X. New bead type and high symmetrical diallyl-POSS based emissive conjugated polyfluorene. Polymer 2014, 55, 6696–6707. [Google Scholar] [CrossRef]
- Florea, N.M.; Lungu, A.; Badica, P.; Craciun, L.; Enculescu, M.; Ghita, D.G.; Ionescu, C.; Zgirian, R.G.; Iuvo, H. Novel nanocomposites based on epoxy resin/epoxy-functionalized polydimethylsiloxane reinforced with POSS. Composites Part. B 2015, 75, 226–234. [Google Scholar] [CrossRef]
- Przadka, D.; Jeczalik, J.; Andrzejewska, E.; Marciniec, B.; Dutkiewicz, M.; Szlapka, M. Novel hybrid polyurethane/POSS materials via bulk polymerization. React. Funct. Polym. 2013, 73, 114–121. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Latteri, A.; Recca, A. A Kinetic study of the thermal and the thermal oxidative degradations of new bridge POSS/PS nanocomposites. Polym. Degrad. Stab. 2013, 98, 2564–2570. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, L.; Zhang, Y.; Zhang, Y.; Yin, N. Preparation and properties of POSS grafted polypropylene by reactive blending. Eur. Polym. J. 2008, 44, 3057–3066. [Google Scholar] [CrossRef]
- Su, C.H.; Chiu, Y.P.; Teng, C.C.; Chiang, C.L. Preparation, characterization and Thermal properties of organic and inorganic composites involving epoxy and polyhedral oligomericSilsequioxane(POSS). J. Polym. Res. 2010, 17, 673–681. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A. Synthesis and thermal properties of new dumbbell-shaped isobutyl-substituted POSSs linked by aliphatic bridges. J. Therm. Anal. Calorim. 2014, 116, 5–13. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Antonelli, M.L.; Bottino, F.A.; Bottino, P. Phenyl heptacyclopentyl–polyhedral oligomericSilsequioxane (ph, hcp-POSS)/polystyrene (PS) nanocomposites: The influence of substituents in the phenyl group on the thermal stability. eXPRESSPolym. Lett. 2012, 6, 997–1006. [Google Scholar] [CrossRef]
- Levicki, J.P.; Pielichowski, K.; De La Croix, P.T.; Janowski, B.; Todd, D.; Liggat, J.J. Thermal degradation studies of polyurethane/POSS nanohybrid elastomers. Polym. Degrad. Stab. 2010, 95, 1099–1105. [Google Scholar] [Green Version]
- Raj, M.M.; Raj, L.M.; Dave, P.N. Glass fiber reinforced composites of phenolic-urea-epoxy resin blends. J. Saudi Chem. Soc. 2012, 16, 241–246. [Google Scholar] [CrossRef]
- Nishimuna, A.; Izumi, Y.; Imaizumi, M.; Nishijima, S.; Hemmi, T.; Shikama, T. Neutron and gamma ray irradiation effects on interlaminar shear strength of insulation materials with cyanate ester-epoxy blended resin. Fusion Eng. Des. 2011, 86, 1558–1561. [Google Scholar] [CrossRef]
- Harvey, B.G.; Guenthner, A.J.; Yandek, G.R.; Gambrea, L.R.; Meylemans, H.A.; Bladwin, L.C.; Reams, J.T. Synthesis and characterization of a renewable cyanate ester/polycarbonate network derived from eugenol. Polymer 2014, 55, 5073–5079. [Google Scholar] [CrossRef]
- Dominguez, D.D.; Keller, T.M. Phthalonitrile-epoxy blends: Cure behavior and copolymer properties. J. Appl. Polym. Sci. 2008, 110, 2504–2514. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Li, X.; Fan, H.; Ran, Q.; Fu, Q.; Gu, Y. A novel ultra low-k nanocomposites of benzoxazinyl modified polyhedral oligomeric silsesquioxane and cyanate ester. Eur. Polym. J. 2018, 103, 124–132. [Google Scholar] [CrossRef]
- Lin, Y.; Song, M.; Stone, C.A.; Shaw, S.J. A comprehensive study on the curing kinetics and network formation of cyanate ester resin/clay nanocomposites. Thermochim. Acta 2013, 552, 77–86. [Google Scholar] [CrossRef]
- Wang, C.S.; Lin, C.H. Novel phosphorus-containing epoxy resins. Part II: Curing kinetics. Polymer 2000, 41, 8579–8586. [Google Scholar] [CrossRef]
- Metalwala, Z.; Khoshroo, K.; Rasoulianboroujeni, M.; Tahriri, M.; Johnsonb, A.; Baeten, J.; Fahimipour, F.; Ibrahim, M.; Tayebi, L. Rheological properties of contemporary nanohybrid dental resin composites: The influence of preheating. Polym. Test. 2018, 72, 157–163. [Google Scholar] [CrossRef]
- Dodero, A.; Williams, R.; Gagliardi, S.; Vicini, S.; Alloisio, M.; Castellano, M. A micro-rheological and rheological study of biopolymers solutions: Hyaluronic acid. Carbohydr. Polym. 2019, 203, 349–355. [Google Scholar] [CrossRef]
- Vashchuk, A.; de Anda, A.R.; Starostenko, O.; Grigoryeva, O.; Sotta, P.; Rogalsky, S.; Smertenko, P.; Fainleib, A.; Grande, D. Structure–property relationships in nanocomposites based on cyanate ester resins and 1-heptyl pyridinium tetrafluoroborate ionic liquid. Polymer 2018, 148, 12–26. [Google Scholar] [CrossRef]
- Bartolomeo, P.; Chailan, J.F.; Vernet, J.L. Curing of cyanate ester resin: A novel approach based on FTIR spectroscopy and comparison with other techniques. Eur. Polym. J. 2001, 37, 659–670. [Google Scholar] [CrossRef]
- Burchill, P.J. On the formation and properties of a high-temperature resin from a bisphthalonitrile. J. Polym. Sci Part A Polym. Chem. 1994, 32, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bershtein, V.; Fainleib, A.; Egorova, L.; Grigoryeva, O.; Kirilenko, D.; Konnikov, S.; Ryzhov, V.; Starostenko, O.; Yakushev, P.; Yagovkina, M.; et al. The impact of ultra-low amounts of introduced reactive POSS nanoparticles on structure, dynamics and properties of densely cross-linked cyanate ester resins. Eur. Polym. J. 2015, 67, 128–142. [Google Scholar] [CrossRef]
- Tao, Q.; Pinter, G.; Krivec, T. Influence of cooling rate and annealing on the DSC Tg of an epoxy resin. Microelectro. Reliab. 2017, 78, 396–400. [Google Scholar] [CrossRef]

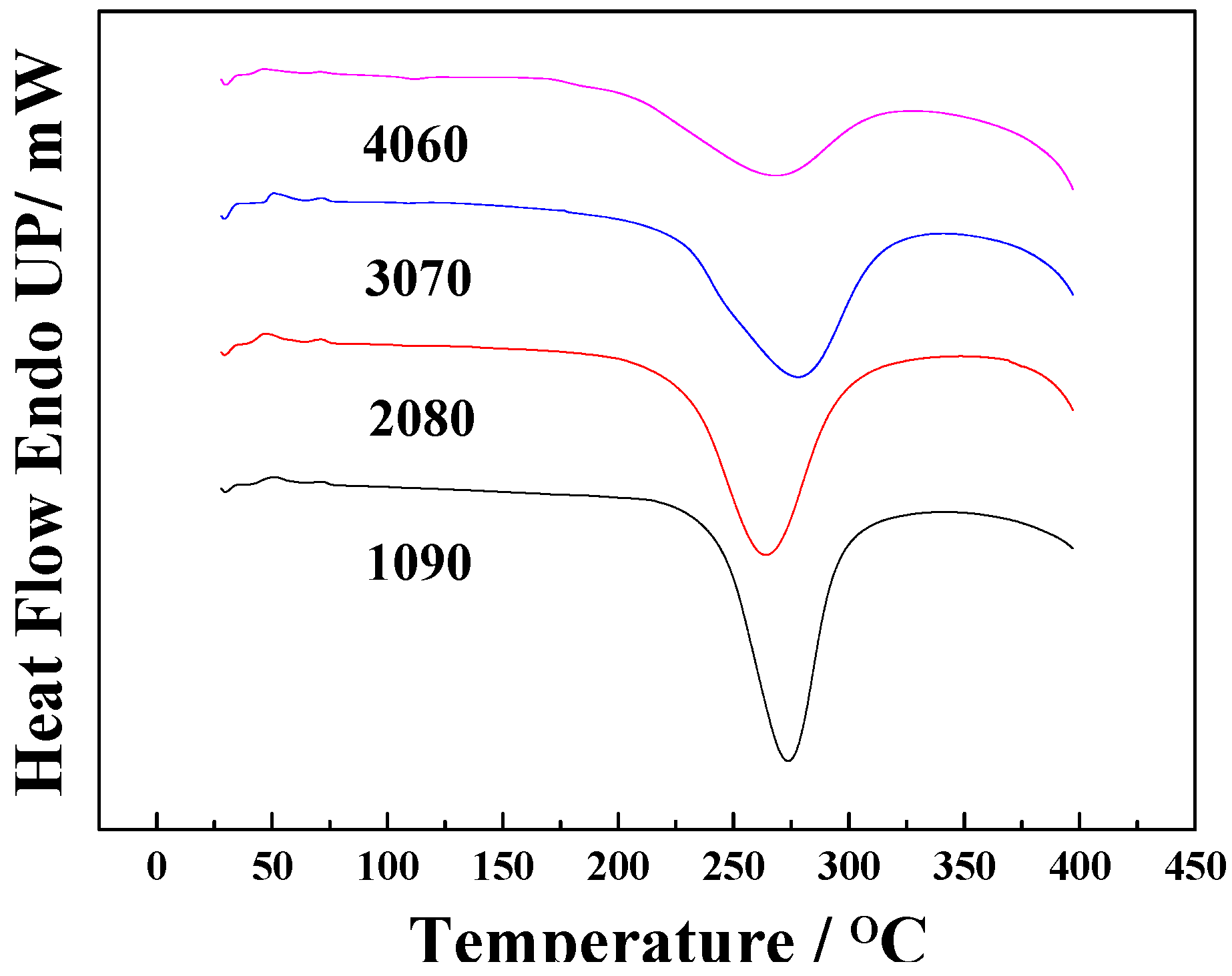
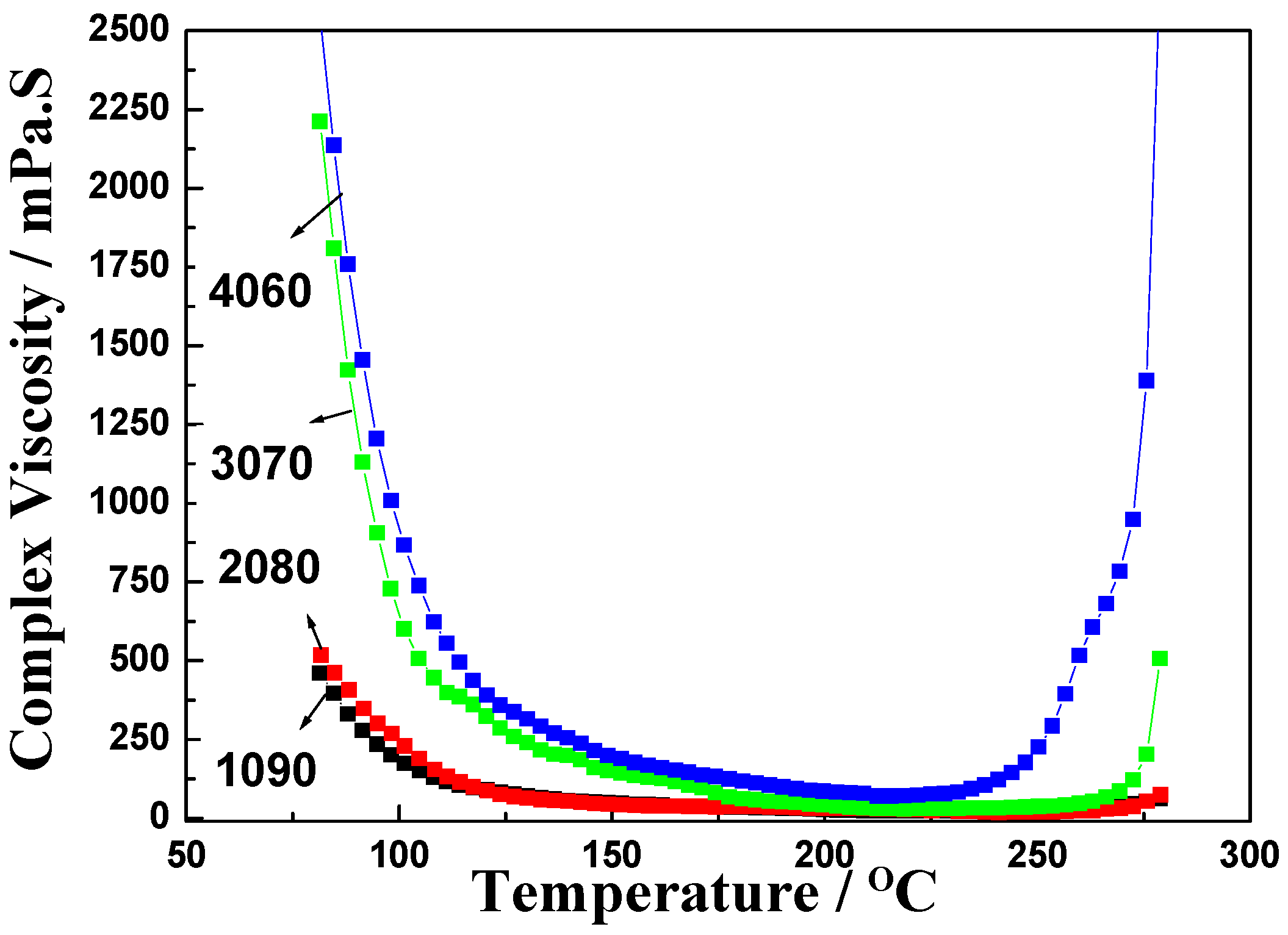
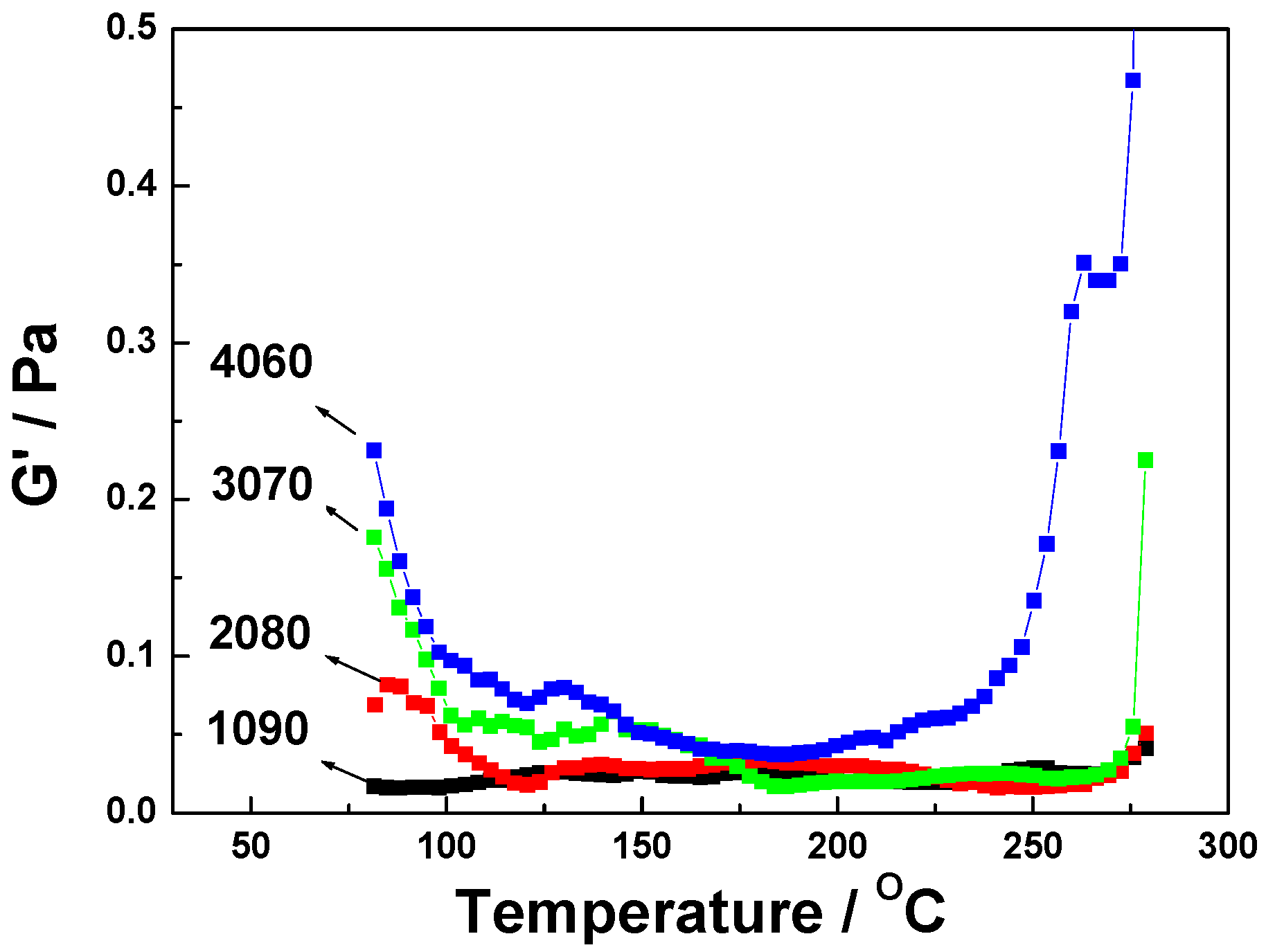
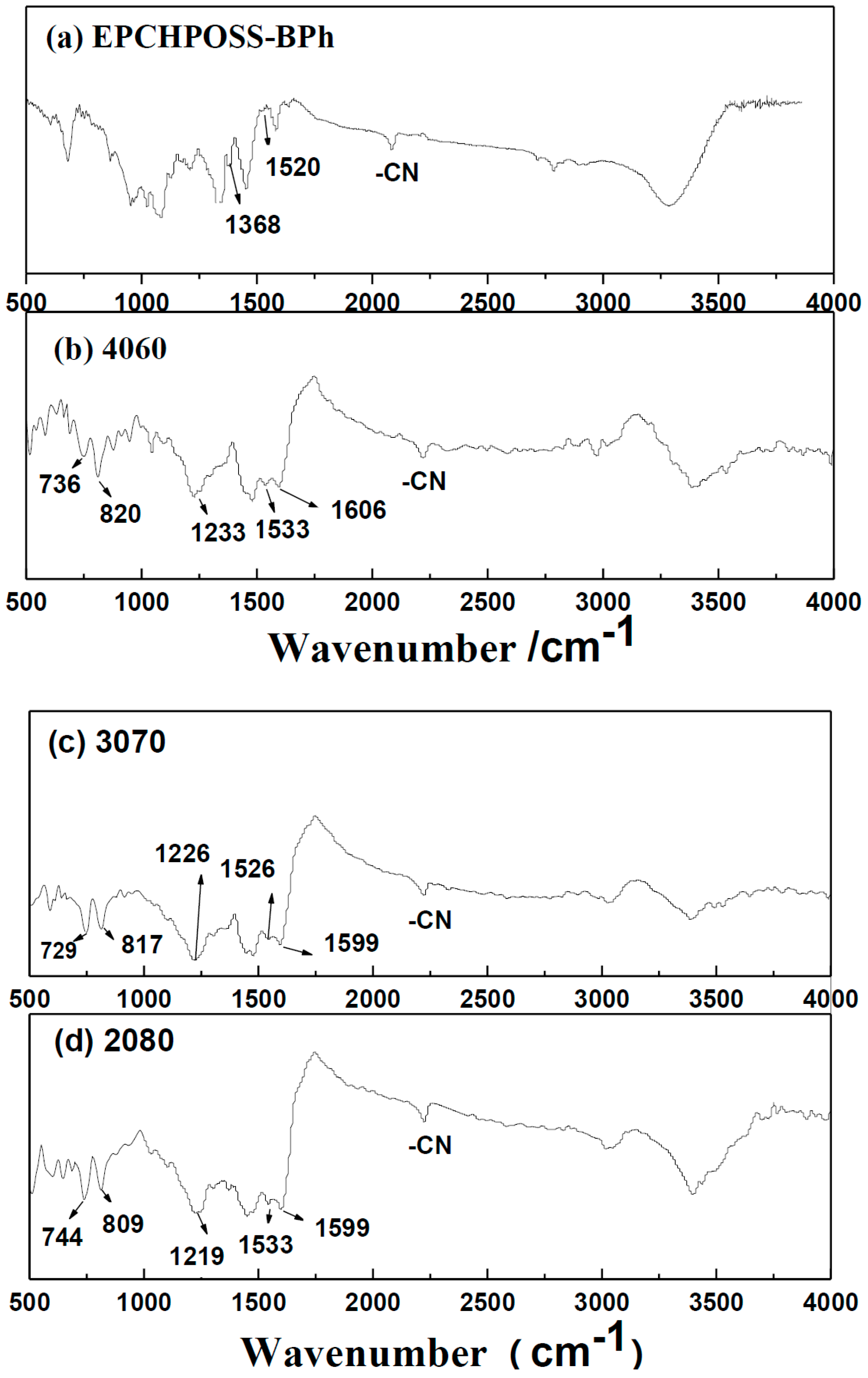
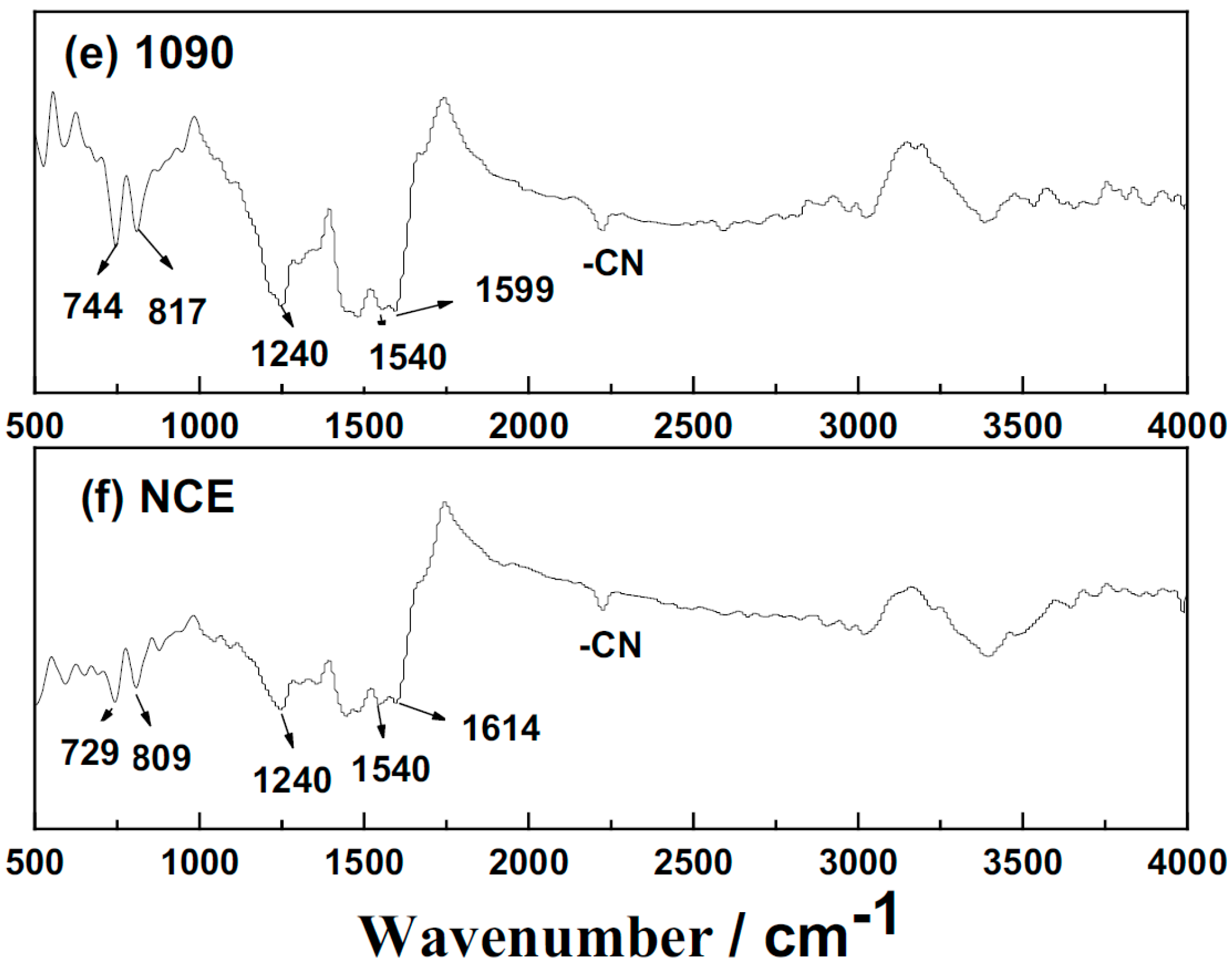


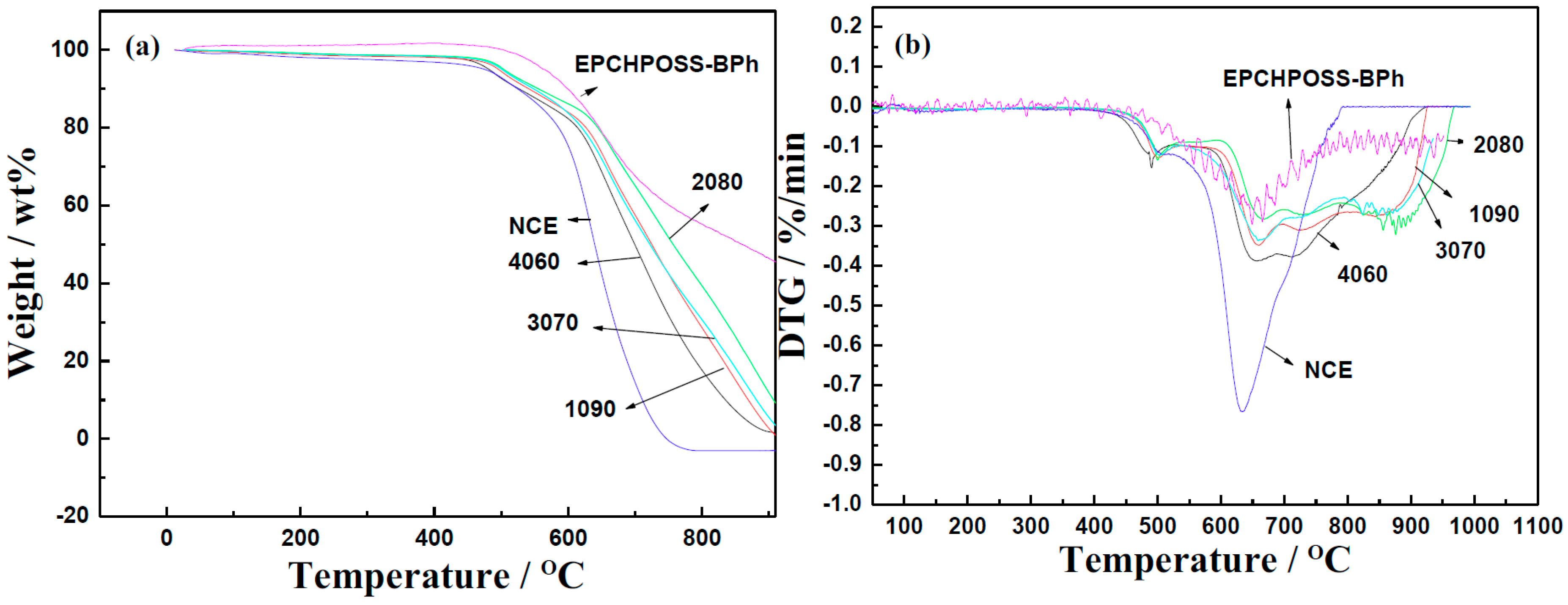

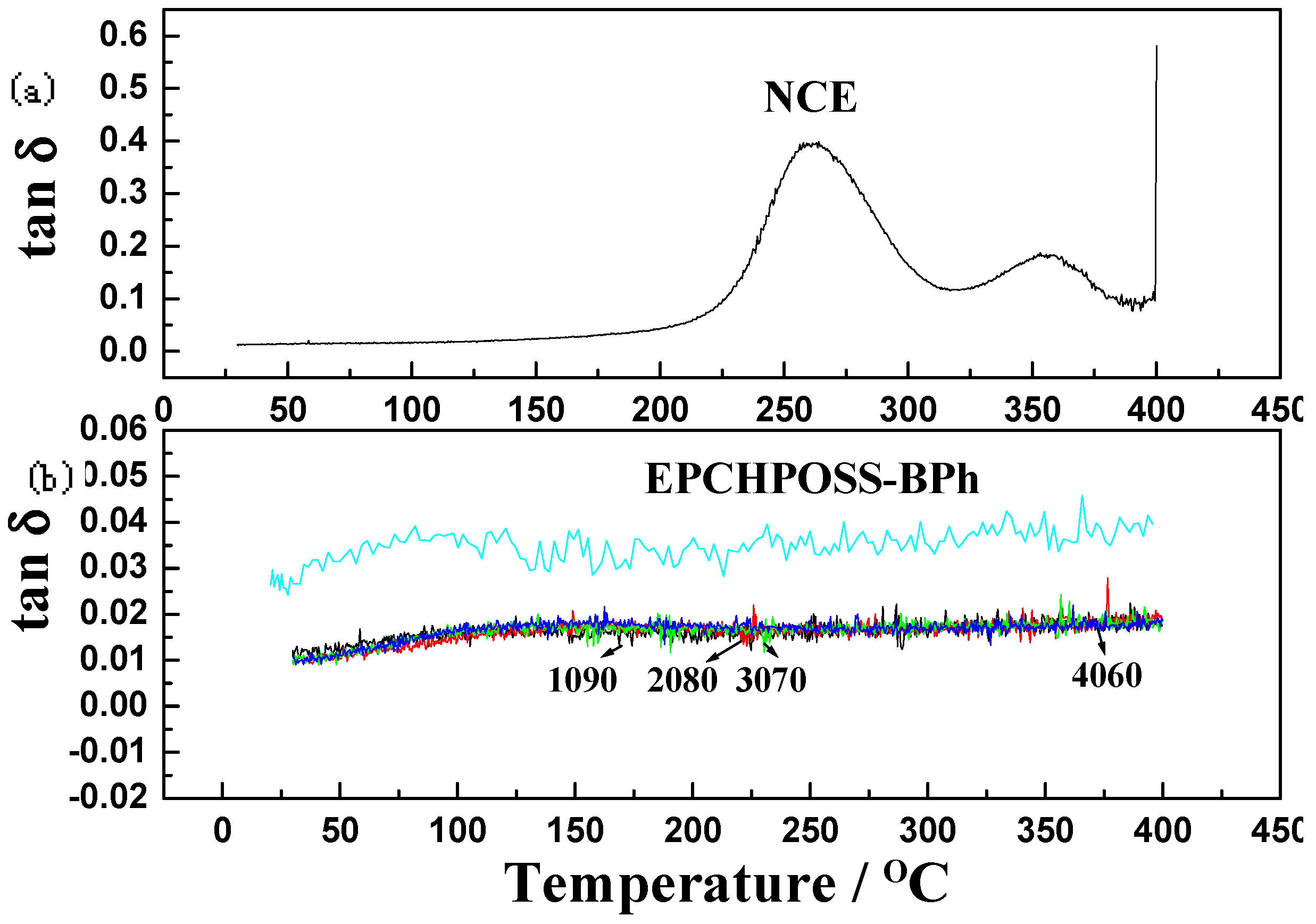

| Properties | EPCHPOSS–BPh | 1090 | 2080 | 3070 | 4060 | NCE |
|---|---|---|---|---|---|---|
| T5% (°C) | 564 | 496 | 503 | 500 | 481 | 475 |
| Char yield (%) | 48 | 2.7 | 12 | 5.5 | 1.8 | 0 |
| Tlost fast (°C) | 664 | 660 | 669 | 662 | 648 | 630 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhou, F.; Zheng, T.; Wang, Z.; Zhou, H.; Chen, H.; Xiao, L.; Zhang, D.; Wang, G. Blends of Cyanate Ester and Phthalonitrile–Polyhedral Oligomeric Silsesquioxane Copolymers: Cure Behavior and Properties. Polymers 2019, 11, 54. https://doi.org/10.3390/polym11010054
Li X, Zhou F, Zheng T, Wang Z, Zhou H, Chen H, Xiao L, Zhang D, Wang G. Blends of Cyanate Ester and Phthalonitrile–Polyhedral Oligomeric Silsesquioxane Copolymers: Cure Behavior and Properties. Polymers. 2019; 11(1):54. https://doi.org/10.3390/polym11010054
Chicago/Turabian StyleLi, Xiaodan, Fei Zhou, Ting Zheng, Ziqiao Wang, Heng Zhou, Haoran Chen, Lin Xiao, Dongxing Zhang, and Guanhui Wang. 2019. "Blends of Cyanate Ester and Phthalonitrile–Polyhedral Oligomeric Silsesquioxane Copolymers: Cure Behavior and Properties" Polymers 11, no. 1: 54. https://doi.org/10.3390/polym11010054





