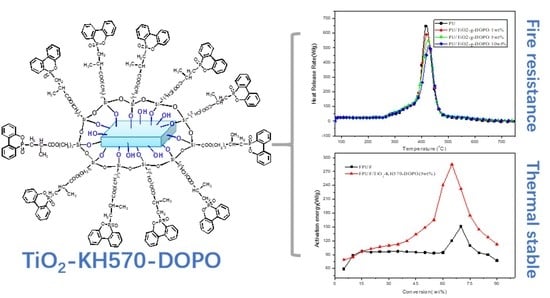
Investigation of Flame Retardant Flexible Polyurethane Foams Containing DOPO Immobilized Titanium Dioxide Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Measurements
2.3. Preparation of KH570-DOPO
2.4. Preparation of TiO2-KH570-DOPO
2.5. Preparation of FPUF/TiO2 Blends
3. Results and Discussions
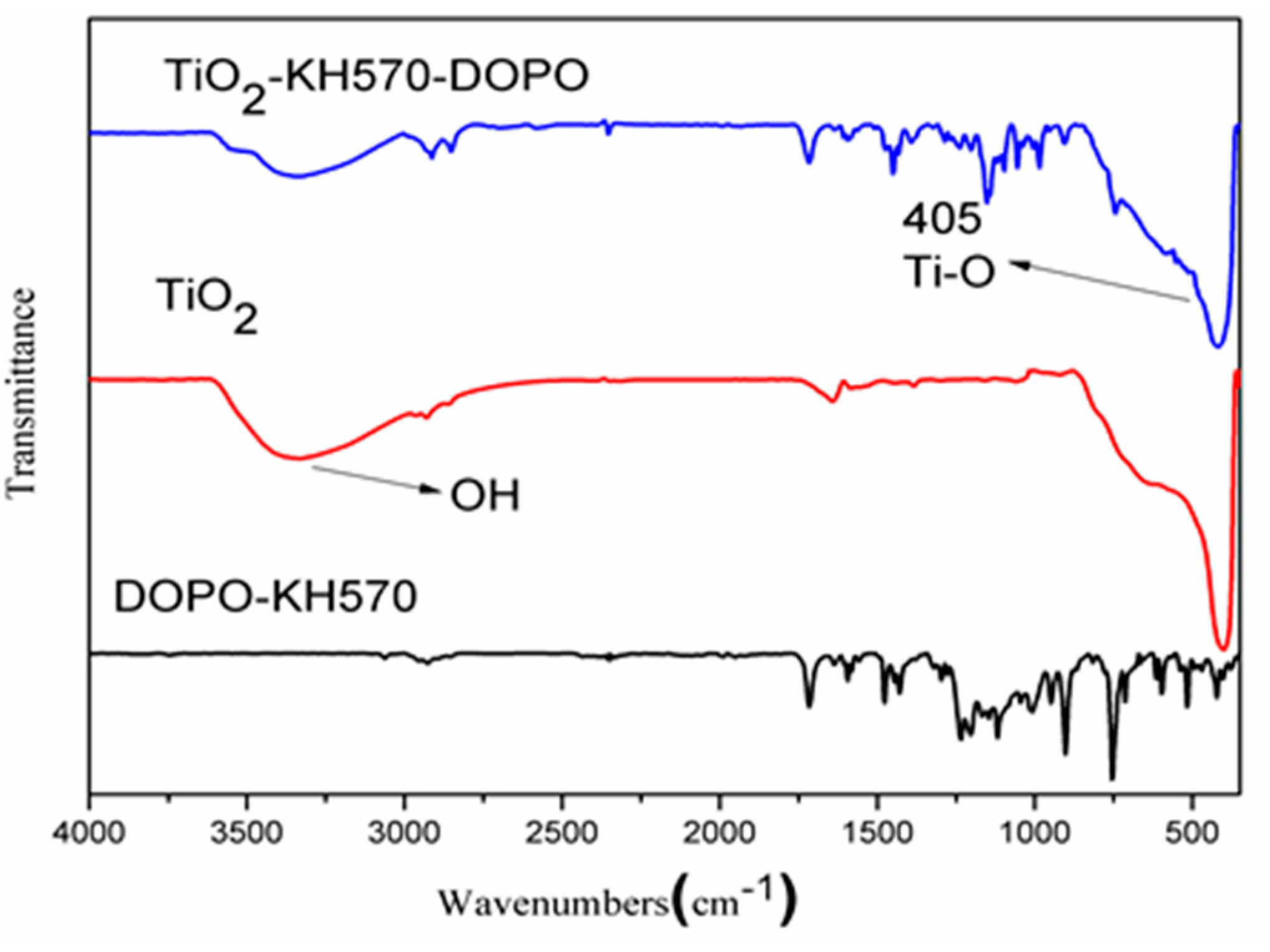
3.1. Characterization of TiO2-KH570-DOPO
3.1.1. FTIR Analysis
3.1.2. TGA Analysis
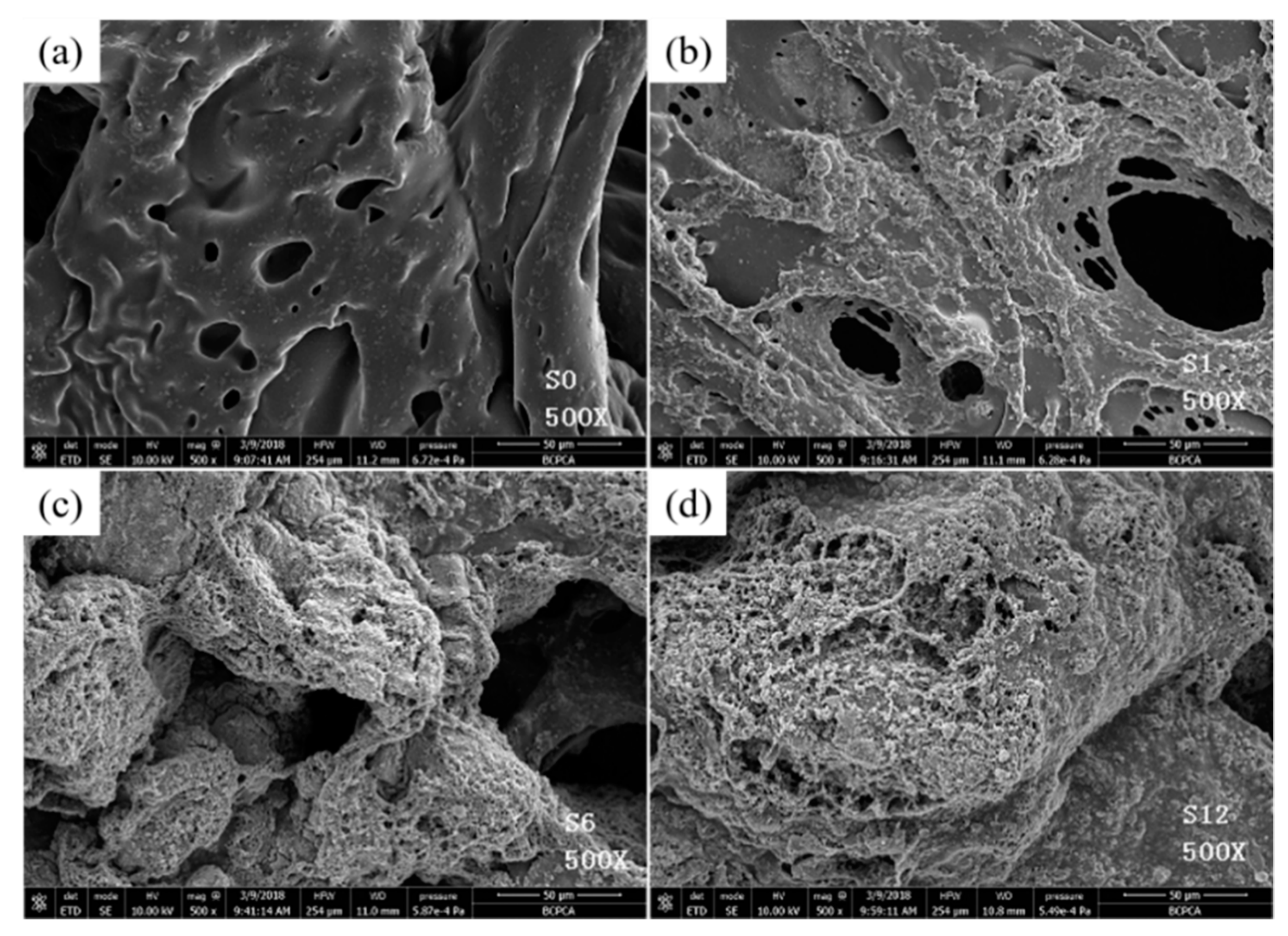
3.1.3. Surface Morphology
3.2. Characterization of FPUF
3.2.1. Flame Retardancy
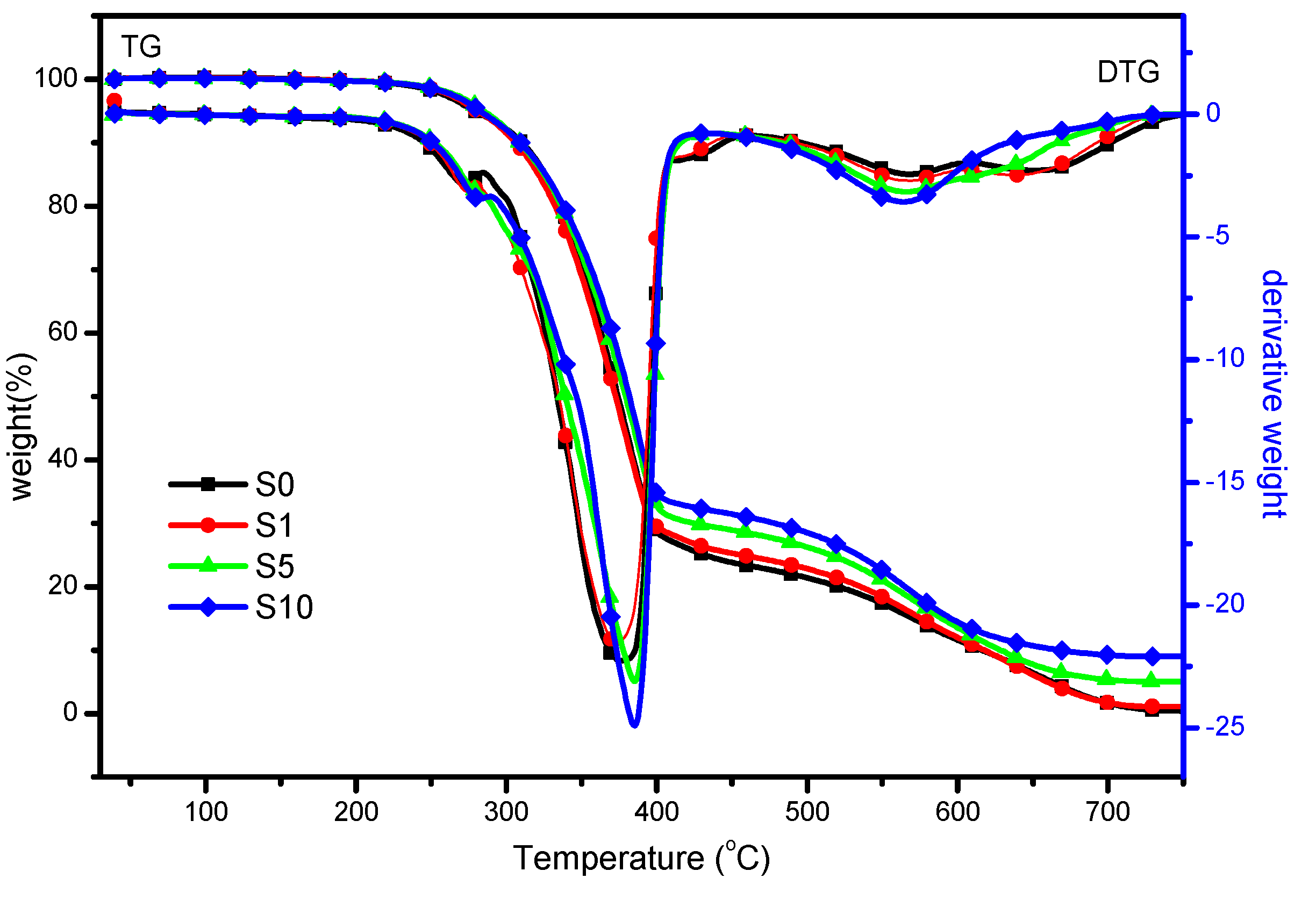
3.2.2. Thermal Stability
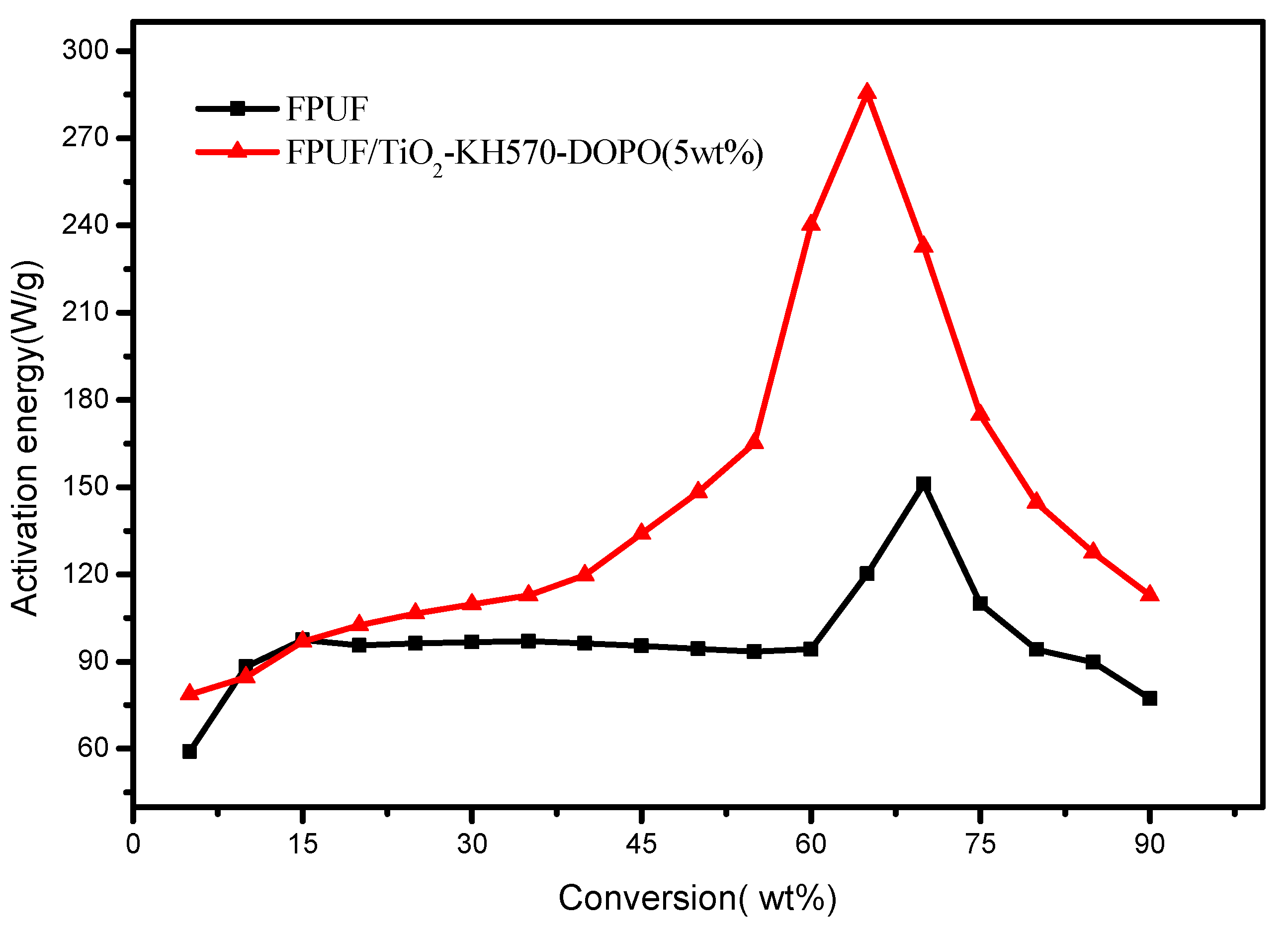
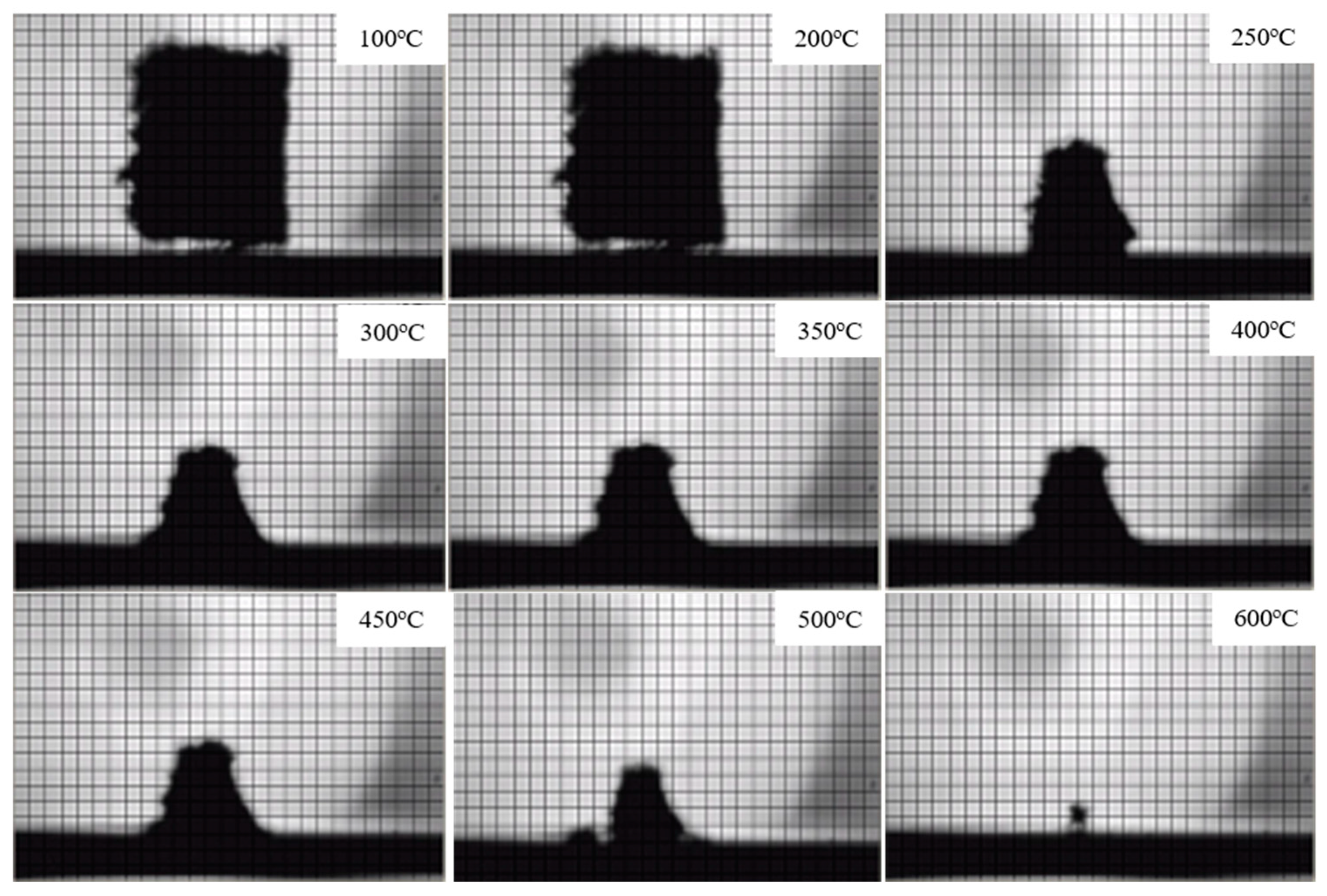
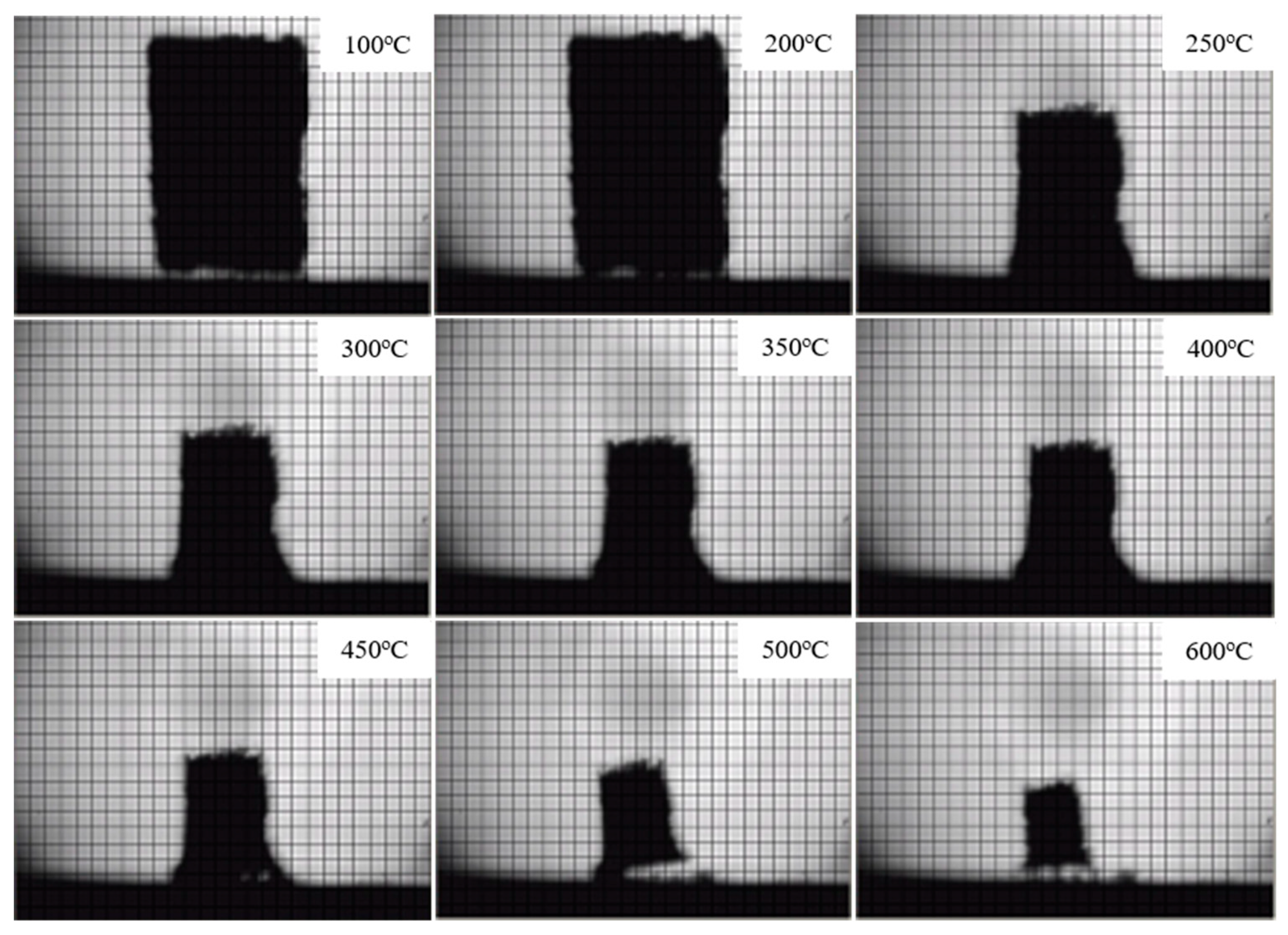
3.2.3. Condensed Phase Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Cho, J.H.; Vasagar, V.; Shanmuganathan, K.; Jones, A.R.; Nazarenko, S.; Ellison, C.J. Bioinspired catecholic flame retardant nanocoating for flexible polyurethane foams. Chem. Mater. 2015, 27, 6784–6790. [Google Scholar] [CrossRef]
- Allan, D.; Daly, J.; Liggat, J.J. Thermal volatilisation analysis of TDI-based flexible polyurethane foam. Polym. Degrad. Stab. 2013, 98, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Morgan, A.B.; Grunlan, J.C. Exceptionally flame retardant sulfur-based multilayer nanocoating for polyurethane prepared from aqueous polyelectrolyte solutions. ACS Macro Lett. 2013, 2, 361–365. [Google Scholar] [CrossRef]
- Troitzsch, J. Plastics Flammability Handbook; Hanser Publications: Munich, Germany, 2004. [Google Scholar]
- Dounis, D.V.; Wilkes, G.L. Structure-property relationships of flexible polyurethane foams. Polymer 1997, 38, 2819–2828. [Google Scholar] [CrossRef]
- Jiao, C.; Wang, H.; Zhang, Z.; Chen, X. Preparation and properties of an efficient smoke suppressant and flame-retardant agent for thermoplastic polyurethane. Polym. Adv. Technol. 2017, 28, 1690–1698. [Google Scholar] [CrossRef]
- Savas, L.A.; Deniz, T.K.; Tayfun, U.; Dogan, M. Effect of microcapsulated red phosphorus on flame retardant, thermal and mechanical properties of thermoplastic polyurethane composites filled with huntite & hydromagnesite mineral. Polym. Degrad. Stab. 2017, 135, 121–129. [Google Scholar]
- Chen, X.; Wang, W.; Jiao, C.A. Recycled environmental friendly flame retardant by modifying para-aramid fiber with phosphorus acid for thermoplastic polyurethane elastomer. J. Hazard. Mater. 2017, 331, 257–264. [Google Scholar] [CrossRef]
- Kim, Y.S.; Davis, R.; Cain, A.A.; Grunlan, J.C. Development of layer-by-layer assembled carbon nanofiber-filled coatings to reduce polyurethane foam flammability. Polymer 2011, 52, 2847–2855. [Google Scholar] [CrossRef]
- Smith, R.J.; Holder, K.M.; Ruiz, S.; Hahn, W.; Song, Y.X.; Lvov, Y.M.; Grunlan, J.C. Environmentally Benign Halloysite Nanotube Multilayer Assembly Significantly Reduces Polyurethane Flammability. Adv. Funct. Mater. 2017, 28, 1703289–1703297. [Google Scholar] [CrossRef]
- Richardson, J.J.; Bjornmalm, M.; Caruso, F. Multilayer assembly Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.D.; Sun, J.; Zhang, J.S.; Gu, X.Y.; Bourbigot, S.; Li, H.F.; Tang, W.F.; Zhang, S. Preparation of a Novel Intumescent Flame Retardant Based on Supramolecular Interactions and Its Application in Polyamide 11. ACS Appl. Mater. Interface 2017, 9, 24964–24975. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Hu, Z.W.; Zhang, S.; Gu, X.Y.; Wang, H.J.; Jiang, P.; Zhao, Q. Effects of titanium dioxide on the flammability and char formation of water-based coatings containing intumescent flame retardants. Prog. Org. Coat. 2015, 78, 318–324. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Ruch, D. Layer-by-layer deposition of a TiO2-filled intumescent coating and its effect on the flame retardancy of polyamide and polyester fabrics. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 1–10. [Google Scholar] [CrossRef]
- Jiang, P.; Gu, X.Y.; Zhang, S.; Sun, J.; Xu, R.W.; Bourbigot, S.; Duquesne, S.; Casetta, M. Flammability and thermal degradation of poly (lactic acid)/polycarbonate alloys containing a phosphazene derivative and trisilanollsobutyl POSS. Polymer 2015, 79, 221–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Wang, B.B.; Liew, K.M.; Song, L.; Wang, C.M.; Hu, Y. Highly Effective P-P synergy of a Novel DOPO-based Flame Retardant for Epoxy Resin. Ind. Eng. Chem. Res. 2017, 56, 1245–1255. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Qian, L.J.; Huang, Z.G.; Tang, S.; Qiu, Y. Synergistic charring effect of triazinetrione-alkyl-phosphinate and phosphaphenanthrene derivatives in epoxy thermosets. RSC Adv. 2017, 7, 46505–46513. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.J.; Yan, H.; Liu, B.; Wei, L.Q.; Xu, B.S. The influence of KH-550 on properties of ammonium polyphosphate and polypropylene flame retardant composites. Polym. Degrad. Stab. 2011, 96, 1382–1388. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, D.D.; Li, W.M.; Cai, X.F. Flame retardant efficiency of KH-550 modified urea-formaldehyde resin cooperating with ammonium polyphosphate on polypropylene. Polym. Degrad. Stab. 2018, 151, 160–171. [Google Scholar] [CrossRef]
- Dong, Q.X.; Ding, Y.F.; Wen, B.; Wang, F.; Dong, H.C.; Zhang, S.M.; Wang, T.X.; Yang, M.S. Improvement of thermal stability of polypropylene using DOPO-immobilized silica nanoparticles. Colloids Polym. Sci. 2012, 290, 1371–1380. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.X.; Liu, M.M.; Ding, Y.F.; Wang, F.; Gao, C.; Liu, P.; Wen, B.; Zhang, S.M.; Yang, M.S. Synergistic effect of DOPO immobilized silica nanoparticles in the intumescent flame retarded polypropylene composites. Polym. Adv. Technol. 2013, 24, 732–739. [Google Scholar] [CrossRef]
- Stojanović, D.B.; Brajović, L.; Orlović, A.; Dramlić, D.; Radmilović, V.; Uskoković, P.S.; Aleksića, R. Transparent PMMA/silica nanocomposites containing silica nanoparticles coating under supercritical conditions. Prog. Org. Coat. 2013, 76, 626–631. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.Q.; Chen, T.; Hu, H.; Zhang, L.; Xue, H.; Hu, H.Q. Supercritical Carbon-Dioxide-Assisted Deposition of Pt Nanoparticles on Graphene Sheets and Their Application as an Electrocatalyst for Direct Methanol Fuel Cells. J. Phys. Chem. C 2012, 116, 21374–21381. [Google Scholar] [CrossRef]
- Kuang, T.R.; Chen, F.; Chang, L.Q.; Zhao, Y.N.; Fu, D.J.; Gong, X.; Peng, X.F. Facile Preparation of Open-cellular Porous Poly (L-lactic acid) Scaffold by Supercritical Carbon Dioxide Foaming for Potential Tissue Engineering Applications. Chem. Eng. J. 2017, 307, 1017–1025. [Google Scholar] [CrossRef]
- Chen, S.H.; Wang, X.D.; Ma, X.Q.; Wang, K.S. Morphology and properties of polypropylene/nano-CaCO3, composites prepared by supercritical carbon dioxide-assisted extrusion. J. Mater. Sci. 2016, 51, 708–718. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Yu, J.R.; Wang, X.X.; Wang, Y.; Zhu, J.; Hu, Z.M. Preparation of KH570-SiO2 and their modification on the MF/PVA composite membrane. Fibers Polym. 2015, 16, 1772–1780. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Yang, H.Y.; Xing, W.Y.; Lu, H.D. Synthesis and characterization of a DOPO-substituted organophosphorus oligomer and its application in flame retardant epoxy resins. Prog. Org. Coat. 2011, 71, 72–82. [Google Scholar] [CrossRef]
- Jin, X.D.; Chen, C.; Sun, J.; Zhang, X.; Gu, X.Y.; Zhang, S. The synergism between melamine and expandable graphite on improving the flame retardancy of polyamide 11. High Perform. Polym. 2017, 29, 77–86. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick direct method for determination of activation energy from thermogravimetric data. J. Polym. Sci. B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Flynn, J.H. The isoconversional method for determination of energy of activation at constant heating rates—Corrections for the Doyle approximation. J. Therm. Anal. 1983, 27, 95–102. [Google Scholar] [CrossRef]
- Jin, X.D.; Gu, X.Y.; Chen, C.; Tang, W.F.; Li, H.F.; Liu, X.D.; Bourbigot, S.; Zhang, Z.W.; Sun, J.; Zhang, S. The fire performance of polylactic acid containing a novel intumescent flame retardant and intercalated layered double hydroxides. J. Mater. Sci. 2017, 52, 12235–12250. [Google Scholar] [CrossRef]



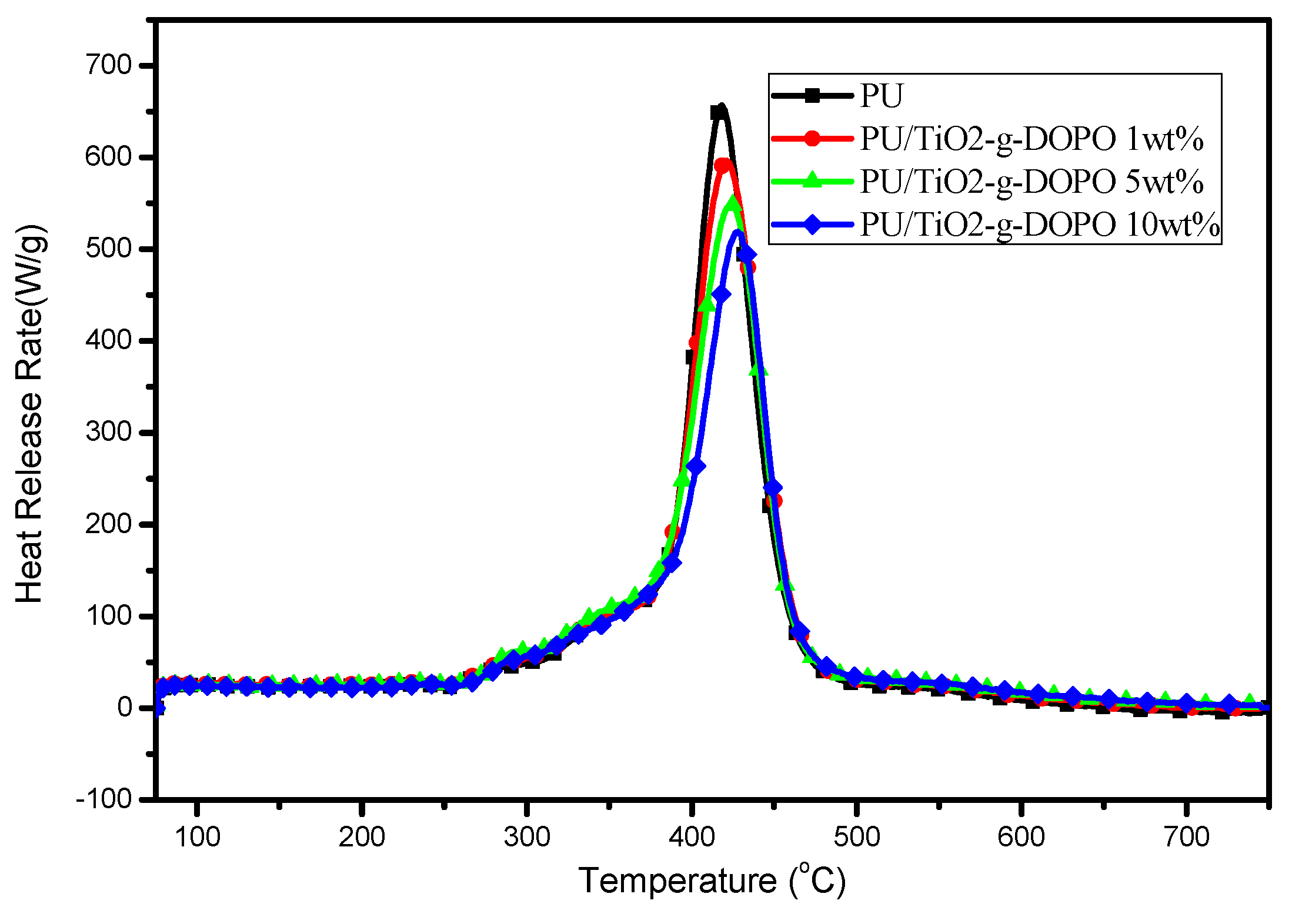

| Samples | pHRR/(W/g) | THR/(kJ/g) | TP/°C |
|---|---|---|---|
| S0 | 657.0 | 28.9 | 418.4 |
| S1 | 596.5 (↓9.3%) | 28.5 (↓1.4%) | 421.1 (+2.7) |
| S5 | 549.7 (↓16.3%) | 27.7 (↓4.2%) | 423.9 (+5.5) |
| S10 | 519.2 (↓21.0%) | 26.8 (↓7.3%) | 427.8 (+9.4) |
| Samples | T−5% a (°C) | T−50% b (°C) | Weight Loss (wt %) | Residue(wt %) | |
|---|---|---|---|---|---|
| Step 2 | Step 3 | ||||
| S0 | 278 | 374 | 73.9 | 25.7 | 0.4 |
| S1 | 284 | 380 | 72.8 | 26.1 | 2.6 |
| S5 | 285 | 379 | 69.8 | 25.2 | 5.0 |
| S10 | 282 | 381 | 67.3 | 23.7 | 9.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Chen, K.; Jin, X.; Sun, S.; Tian, Y.; Wang, F.; Liu, P.; Yang, M. Investigation of Flame Retardant Flexible Polyurethane Foams Containing DOPO Immobilized Titanium Dioxide Nanoparticles. Polymers 2019, 11, 75. https://doi.org/10.3390/polym11010075
Dong Q, Chen K, Jin X, Sun S, Tian Y, Wang F, Liu P, Yang M. Investigation of Flame Retardant Flexible Polyurethane Foams Containing DOPO Immobilized Titanium Dioxide Nanoparticles. Polymers. 2019; 11(1):75. https://doi.org/10.3390/polym11010075
Chicago/Turabian StyleDong, Quanxiao, Keyu Chen, Xiaodong Jin, Shibing Sun, Yingliang Tian, Feng Wang, Peng Liu, and Mingshu Yang. 2019. "Investigation of Flame Retardant Flexible Polyurethane Foams Containing DOPO Immobilized Titanium Dioxide Nanoparticles" Polymers 11, no. 1: 75. https://doi.org/10.3390/polym11010075