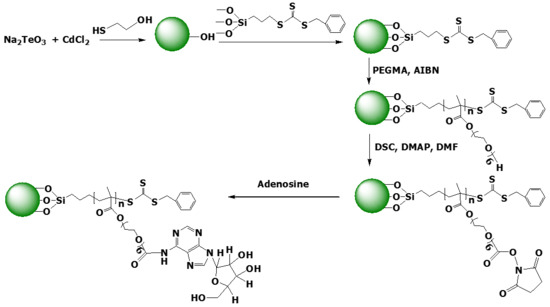
Chemical Synthesis and Characterization of Poly(poly(ethylene glycol) methacrylate)-Grafted CdTe Nanocrystals via RAFT Polymerization for Covalent Immobilization of Adenosine
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of S-Benzyl S′-Trimethoxysilylpropyltrithiocarbonate
2.3. Synthesis of Hydroxyl-Coated CdTe Nanocrystals
2.4. Immobilization of Chain Transfer Agent on CdTe QD Surface
2.5. Grafting of Poly(Ethylene glycol) Methacrylate Brushes on CdTe QDs by SI-RAFT
2.6. Conjugation of Adenosine onto PPEGMA-g-CdTe Nanocomposites
2.7. Measurements
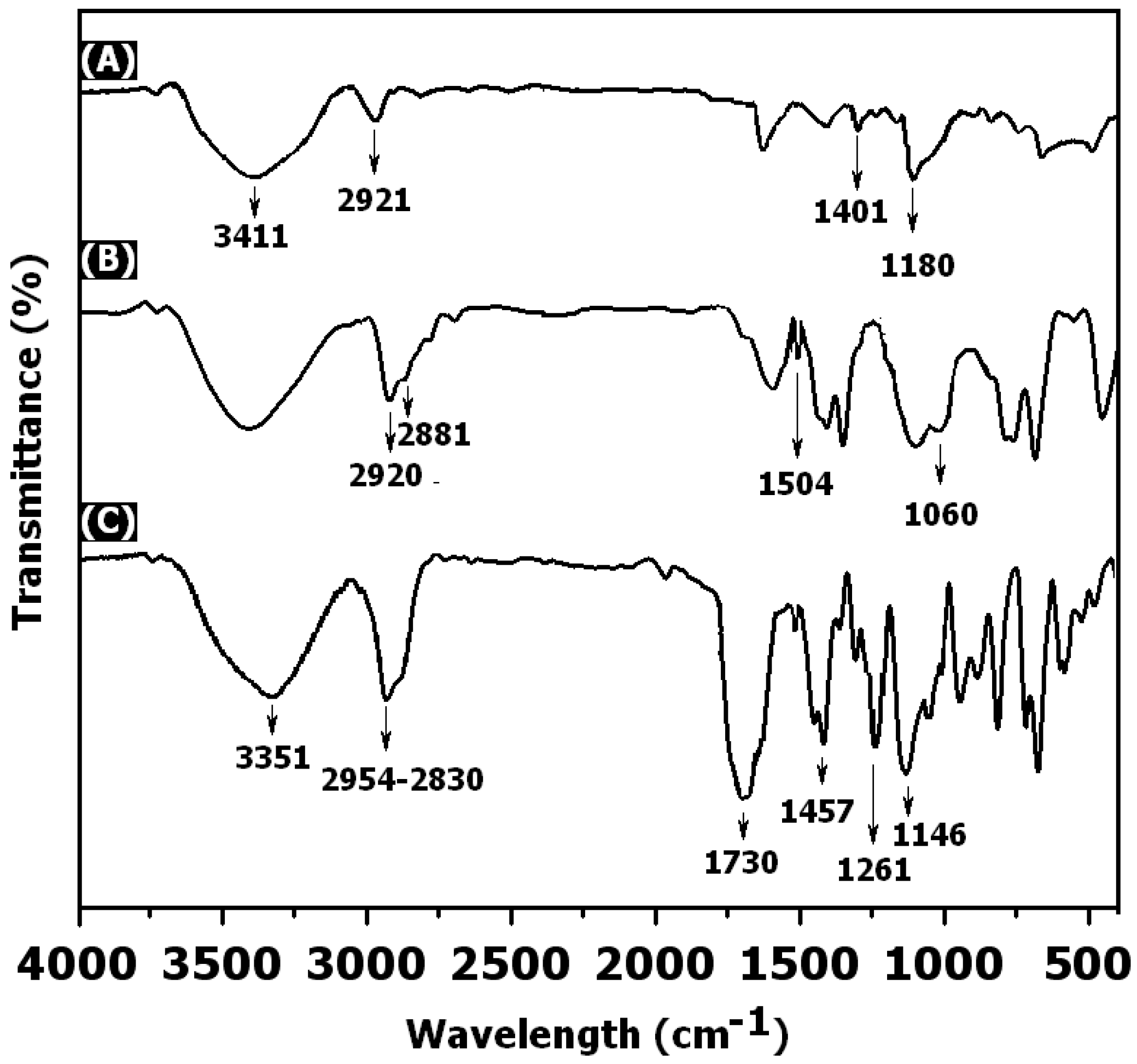
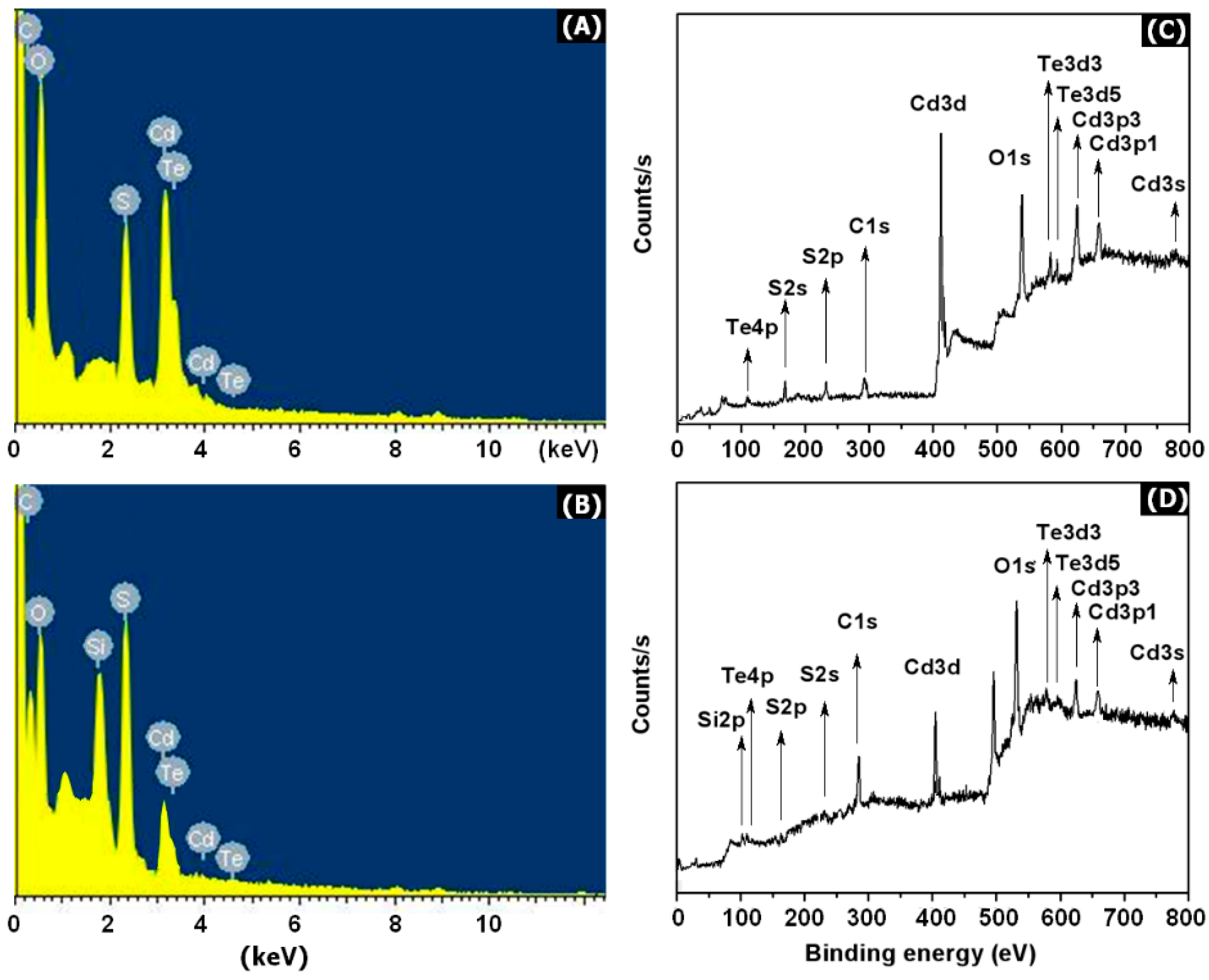
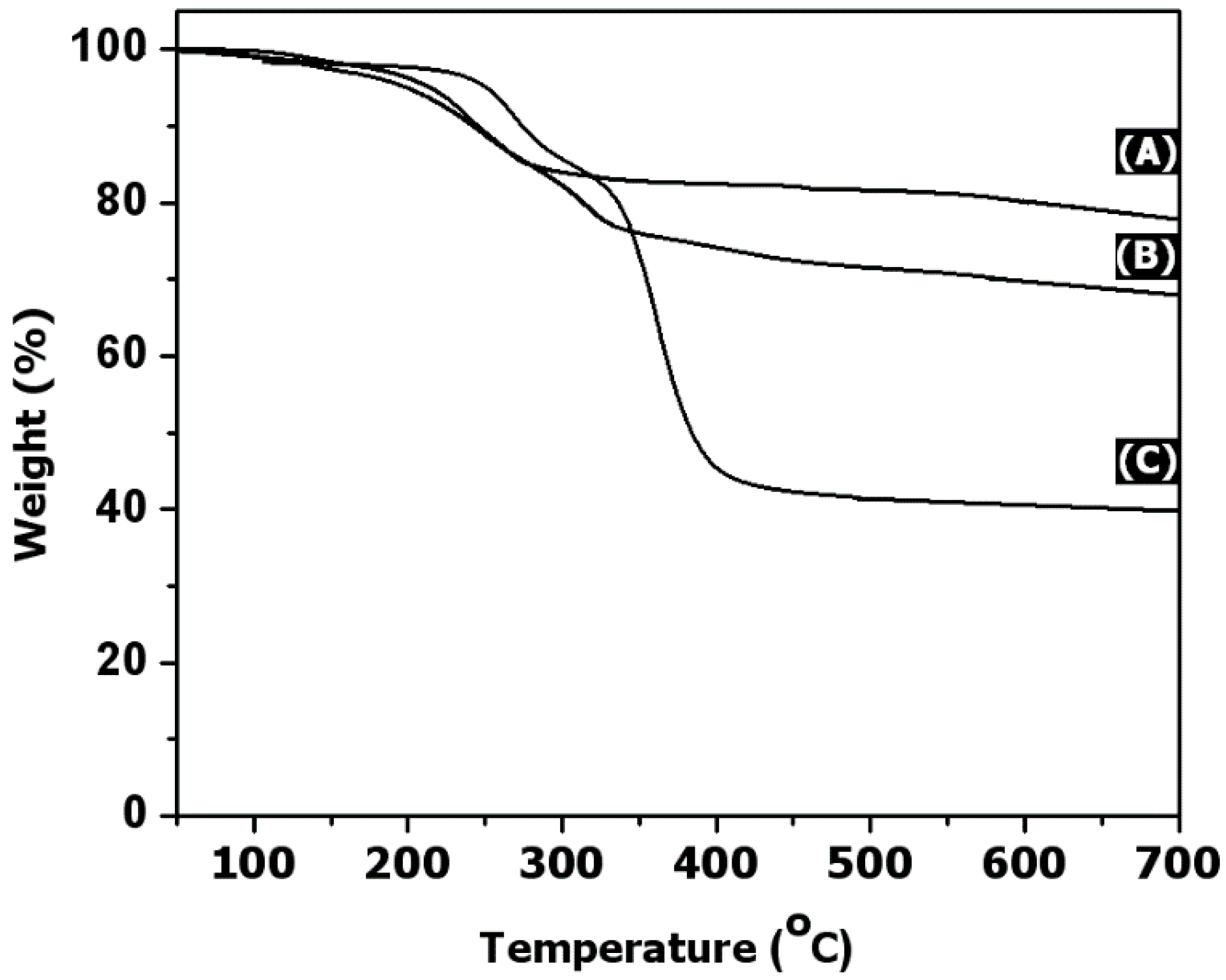
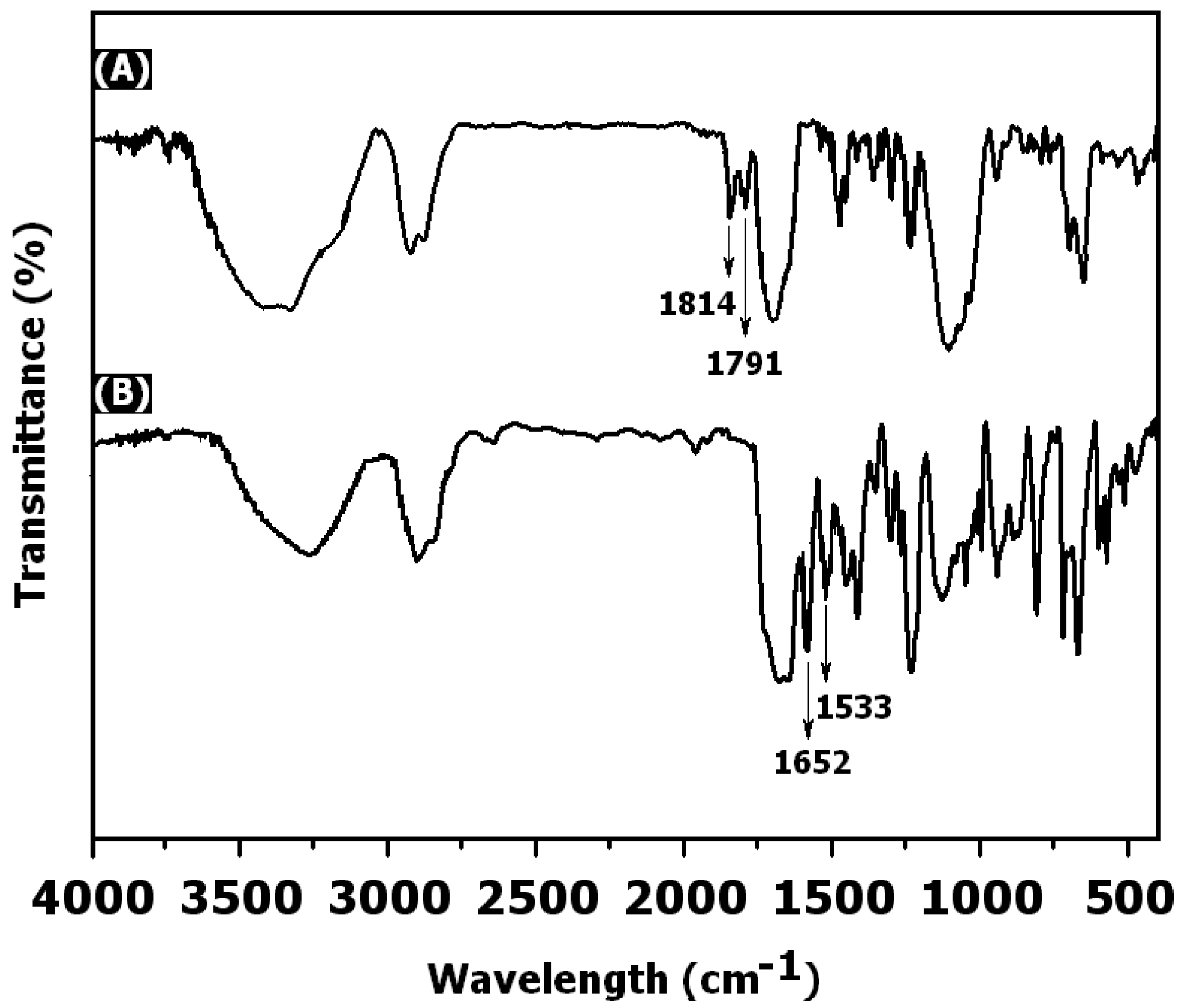
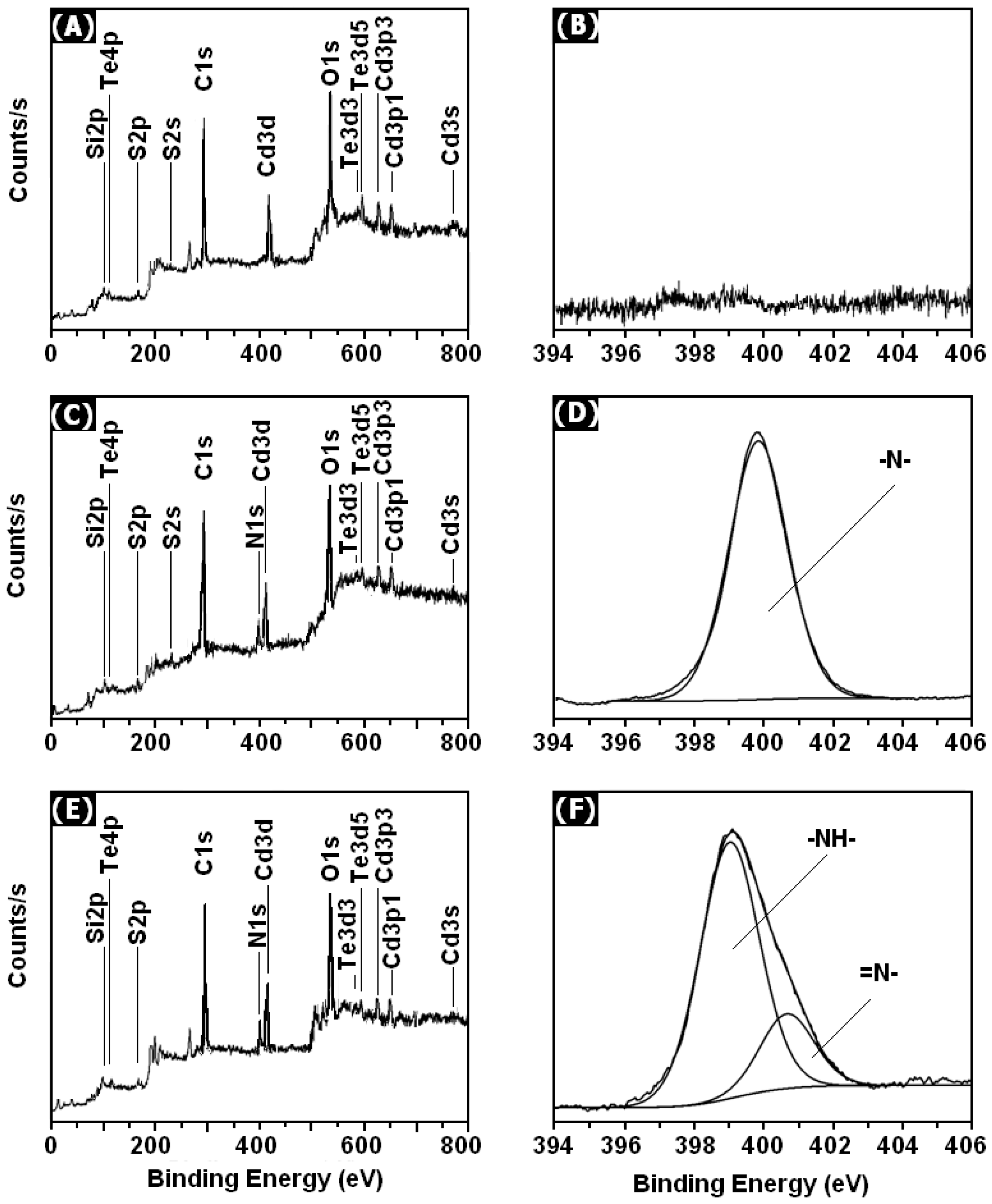
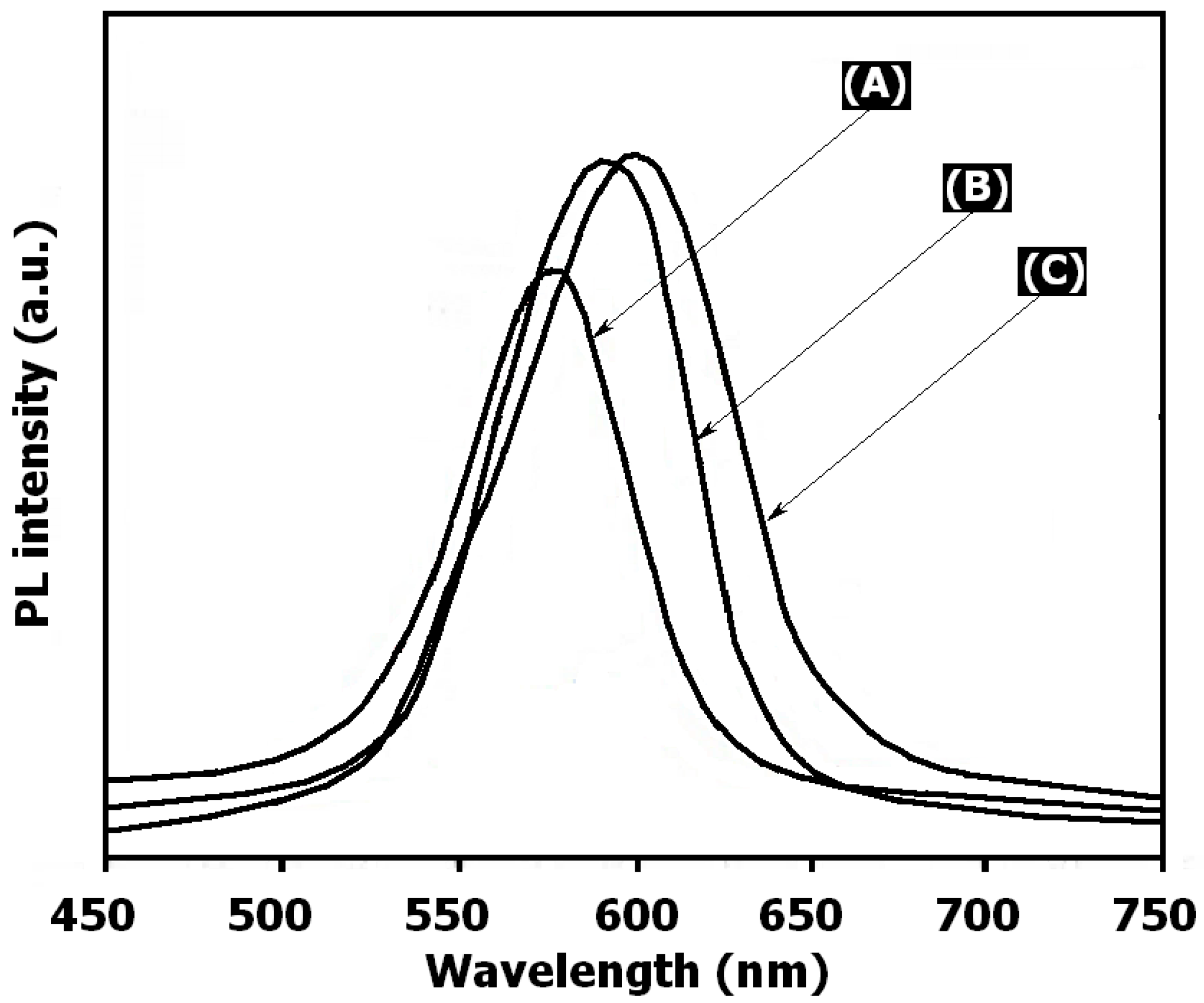
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brichkin, S.B.; Razumov, V.F. Colloidal Quantum Dots: Synthesis, Properties and Applications. Russ. Chem. Rev. 2016, 85, 1297–1312. [Google Scholar] [CrossRef]
- Rakovich, A.; Savateeva, D.; Rakovich, T.; Donegan, J.F.; Rakovich, Y.P.; Kelly, V.; Lesnyak, V.; Eychmüller, A. CdTe Quantum Dot/Dye Hybrid System as Photosensitizer for Photodynamic Therapy. Nanoscale Res. Lett. 2010, 5, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.B.; Ghaderi, S.; Keshtgar, M.; Seifalian, A.M. Semiconductor Quantum Dots as Fluorescent Probes for in Vitro and in Vivo Bio-Molecular and Cellular Imaging. Nanoscale Rev. 2010, 1, 5161. [Google Scholar]
- Bilan, R.; Nabiev, I.; Sukhanova, A. Quantum Dot-Based Nanotools for Bioimaging, Diagnostics, and Drug Delivery. ChemBioChem 2016, 17, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.X.; Zeng, E.Z. Application of Functional Quantum Dot Nanoparticles as Fluorescence Probes in Cell Labeling and Tumor Diagnostic Imaging. Nanoscale Res. Lett. 2015, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Goryacheva, O.A.; Mishra, P.K.; Goryacheva, I.Y. Luminescent Quantum Dots for MiRNA Detection. Talanta 2018, 179, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Biju, V. Relations of Exciton Dynamics in Quantum Dots to Photoluminescence, Lasing, and Energy Harvesting. J. Photochem. Photobiol. C. 2018, 137–151. [Google Scholar] [CrossRef]
- Zhou, W.; Coleman, J.J. Semiconductor Quantum Dots. Curr. Opin. Solid State Mater. Sci. 2016, 352–360. [Google Scholar] [CrossRef]
- Li Volsi, A.; Fiorica, C.; D’Amico, M.; Scialabba, C.; Palumbo, F.S.; Giammona, G.; Licciardi, M. Hybrid Gold/Silica/Quantum-Dots Supramolecular-Nanostructures Encapsulated in Polymeric Micelles as Potential Theranostic Tool for Targeted Cancer Therapy. Eur. Polym. J. 2018, 105, 38–47. [Google Scholar] [CrossRef]
- Aimé, A.; Beztsinna, N.; Patwa, A.; Pokolenko, A.; Bestel, I.; Barthélémy, P. Quantum Dot Lipid Oligonucleotide Bioconjugates: Toward a New Anti-MicroRNA Nanoplatform. Bioconjug. Chem. 2013, 24, 1345–1355. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.; Chen, J.; Wu, B.; Chen, H.; Jiang, X. Quantum Dots Bearing Lectin-Functionalized Nanoparticles as a Platform for in Vivo Brain Imaging. Bioconjug. Chem. 2008, 19, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, B.; Tang, W.; Chen, T.; Zhang, H.; Wang, Q. Glycopolypeptide-Encapsulated Mn-Doped ZnS Quantum Dots for Drug Delivery: Fabrication, Characterization, and in Vitro Assessment. Colloids Surf. B 2011, 88, 51–57. [Google Scholar] [CrossRef]
- Bonilla, J.C.; Bozkurt, F.; Ansari, S.; Sozer, N.; Kokini, J.L. Applications of Quantum Dots in Food Science and Biology. Trends Food Sci. Technol. 2016, 53, 75–89. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, Properties and Biomedical Applications of Carbon-Based Quantum Dots: An Updated Review. Biomed. Pharmacother. 2017, 209–222. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. Review of in Vitro Toxicological Research of Quantum Dot and Potentially Involved Mechanisms. Sci. Total Environ. 2018, 940–962. [Google Scholar] [CrossRef] [PubMed]
- Bali Prasad, B.; Kumar, A.; Singh, R. Synthesis of Novel Monomeric Graphene Quantum Dots and Corresponding Nanocomposite with Molecularly Imprinted Polymer for Electrochemical Detection of an Anticancerous Ifosfamide Drug. Biosens. Bioelectron. 2017, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baslak, C.; Demirel Kars, M.; Karaman, M.; Kus, M.; Cengeloglu, Y.; Ersoz, M. Biocompatible Multi-Walled Carbon Nanotube-CdTe Quantum Dot-Polymer Hybrids for Medical Applications. J. Lumin. 2015, 160, 9–15. [Google Scholar] [CrossRef]
- Amjadi, M.; Jalili, R. A Molecularly Imprinted Dual-Emission Carbon Dot-Quantum Dot Mesoporous Hybrid for Ratiometric Determination of Anti-Inflammatory Drug Celecoxib. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 345–351. [Google Scholar] [CrossRef]
- Das, K.; Sanwlani, S.; Rawat, K.; Haughn, C.R.; Doty, M.F.; Bohidar, H.B. Spectroscopic Profile of Surfactant Functionalized CdSe Quantum Dots and Their Interaction with Globular Plasma Protein BSA. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 495–506. [Google Scholar] [CrossRef]
- Speranskaya, E.S.; Beloglazova, N.V.; Lenain, P.; De Saeger, S.; Wang, Z.; Zhang, S.; Hens, Z.; Knopp, D.; Niessner, R.; Potapkin, D.V.; et al. Polymer-Coated Fluorescent CdSe-Based Quantum Dots for Application in Immunoassay. Biosens. Bioelectron. 2014, 53, 225–231. [Google Scholar] [CrossRef]
- Chen, M.L.; He, Y.J.; Chen, X.W.; Wang, J.H. Quantum Dots Conjugated with Fe3O4-Filled Carbon Nanotubes for Cancer-Targeted Imaging and Magnetically Guided Drug Delivery. Langmuir 2012, 28, 16469–16476. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Educ. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.A.; Fitzgerald, A.V.L.; Jenkins, M.J. Control of the Secondary Crystallisation Process in Poly(Hydroxybutyrate-Co-Hydroxyvalerate) through the Incorporation of Poly(Ethylene Glycol). Polym. Degrad. Stab. 2018, 148, 67–74. [Google Scholar] [CrossRef]
- Acosta-Vélez, G.F.; Zhu, T.Z.; Linsley, C.S.; Wu, B.M. Photocurable Poly(Ethylene Glycol) as a Bioink for the Inkjet 3D Pharming of Hydrophobic Drugs. Int. J. Pharm. 2018, 546, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Rivera, C.P.; Wu, C.J.; Schmidt, G. Transparent, Elastomeric and Tough Hydrogels from Poly(Ethylene Glycol) and Silicate Nanoparticles. Acta Biomater. 2011, 7, 4139–4148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rana, S.; Srivastava, R.S.; Misra, R.D.K. On the Chemical Synthesis and Drug Delivery Response of Folate Receptor-Activated, Polyethylene Glycol-Functionalized Magnetite Nanoparticles. Acta Biomater. 2008, 4, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Susumu, K.; Mei, B.C.; Mattoussi, H. Multifunctional Ligands Based on Dihydrolipoic Acid and Polyethylene Glycol to Promote Biocompatibility of Quantum Dots. Nat. Protoc. 2009, 4, 424–436. [Google Scholar] [CrossRef]
- Li, H.; Niu, Y. Synthesis and Characterization of Amphiphilic Block Polymer Poly(Ethylene Glycol)-Poly(Propylene Carbonate)-Poly(Ethylene Glycol) for Drug Delivery. Mater. Sci. Eng. C 2018, 89, 160–165. [Google Scholar] [CrossRef]
- Elsaid, Z.; Taylor, K.M.G.; Puri, S.; Eberlein, C.A.; Al-Jamal, K.; Bai, J.; Klippstein, R.; Wang, J.T.-W.; Forbes, B.; Chana, J.; et al. Mixed Micelles of Lipoic Acid-Chitosan-Poly(Ethylene Glycol) and Distearoylphosphatidylethanolamine-Poly(Ethylene Glycol) for Tumor Delivery. Eur. J. Pharm. Sci. 2017, 101, 228–242. [Google Scholar] [CrossRef]
- Jung, S.; Tang, Y.; Shim, G.; Lee, C.-S.; Choi, C.-H.; Yi, H. Controlled Network Structures of Chitosan-Poly(Ethylene Glycol) Hydrogel Microspheres and Their Impact on Protein Conjugation. Biochem. Eng. J. 2018, 135, 123–132. [Google Scholar] [CrossRef]
- Racine, L.; Costa, G.; Bayma-Pecit, E.; Texier, I.; Auzély-Velty, R. Design of Interpenetrating Chitosan and Poly(Ethylene Glycol) Sponges for Potential Drug Delivery Applications. Carbohydr. Polym. 2017, 170, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xue, J.; Xu, S.; You, Y.; Yan, X.Q.; Zhao, X.; Cao, J. Polyelectrolyte Complex Nanoparticles Based on Chitosan and Methoxy Poly(Ethylene Glycol) Methacrylate-Co-Poly(Methylacrylic Acid) for Oral Delivery of Ibuprofen. Colloids Surf. B 2018, 165, 235–242. [Google Scholar] [CrossRef] [PubMed]
- González-Villegas, J.; Kan, Y.; Bakhmutov, V.I.; García-Vargas, A.; Martínez, M.; Clearfield, A.; Colón, J.L. Poly(Ethylene Glycol)-Modified Zirconium Phosphate Nanoplatelets for Improved Doxorubicin Delivery. Inorg. Chim. Acta 2017, 468, 270–279. [Google Scholar] [CrossRef]
- Garg, N.K.; Dwivedi, P.; Campbell, C.; Tyagi, R.K. Site Specific/Targeted Delivery of Gemcitabine through Anisamide Anchored Chitosan/Poly Ethylene Glycol Nanoparticles: An Improved Understanding of Lung Cancer Therapeutic Intervention. Eur. J. Pharm. Sci. 2012, 47, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Alavi, A.S.; Meshkini, A. Fabrication of Poly(Ethylene Glycol)-Coated Mesoporous Nanocomposite ZnO@Fe2O3 for Methotrexate Delivery: An Integrated Nanoplatform for Dual-Mode Cancer Therapy. Eur. J. Pharm. Sci. 2018, 115, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zeng, G.; Xu, D.; Liu, M.; Wang, K.; Li, Z.; Fu, L.; Zhang, Q.; Zhang, X.; Wei, Y. Biomimetic PEGylation of Carbon Nanotubes through Surface-Initiated RAFT Polymerization. Mater. Sci. Eng. C 2017, 80, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Decker, C.G.; Maynard, H.D. Degradable PEGylated Protein Conjugates Utilizing RAFT Polymerization. Eur. Polym. J. 2015, 65, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.-F.; Jiang, X.-Q.; Zhou, L.-J. Surface Modification of Poly Ethylene Glycol to Resist Nonspecific Adsorption of Proteins. Chin. J. Anal. Chem. 2013, 41, 445–453. [Google Scholar] [CrossRef]
- Pal, A.; Pal, S. Synthesis of Triblock Copolymeric Micelle Based on Poly (Ethylene Glycol) and Poly (Vinyl Acetate) through Reversible Addition–fragmentation Chain Transfer Polymerization. J. Colloid Interface Sci. 2018, 524, 122–128. [Google Scholar] [CrossRef]
- Chong, J.Y.T.; Keddie, D.J.; Postma, A.; Mulet, X.; Boyd, B.J.; Drummond, C.J. RAFT Preparation and the Aqueous Self-Assembly of Amphiphilic Poly(Octadecyl Acrylate)-Block-Poly(Polyethylene Glycol Methyl Ether Acrylate) Copolymers. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 60–69. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Gunatillake, P.A.; Meagher, L. Biomedical Applications of Polymers Derived by Reversible Addition—Fragmentation Chain-Transfer (RAFT). Adv. Drug Deliv. Rev. 2015, 91, 141–152. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.; Kennedy, R.; Duffy, P.; Vasquez, J.M.; Wall, J.G.; Tai, H.; Wang, W. Poly(Ethylene Glycol)-Based Hyperbranched Polymer from RAFT and Its Application as a Silver-Sulfadiazine-Loaded Antibacterial Hydrogel in Wound Care. ACS Appl. Mater. Interfaces 2016, 8, 26648–26656. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.G.; Rafiqul Islam, M.; Vo, T.-S.; Kim, S.-K.; Lim, K.T. Poly(allyl methacrylate) Functionalized Hydroxyapatite Nanocrystals via Combination of Surface-Initiated RAFT Polymerization and Thiol-Ene Protocol: A Potential Anticancer Drug Nanocarrier. J. Colloid Interface Sci. 2013, 394, 132–140. [Google Scholar] [CrossRef]
- Bach, L.G.; Islam, M.R.; Jeong, Y.T.; Gal, Y.S.; Lim, K.T. Synthesis and characterization of chemically anchored adenosine with PHEMA grafted gold nanoparticles. Appl. Surf. Sci. 2012, 258, 2816–2822. [Google Scholar] [CrossRef]
- Islam, M.R.; Bach, L.G.; Lim, K.T. Poly(2-hydroxyethyl methacrylate) grafted halloysite nanotubes as a molecular host matrix for luminescent ions prepared by surface-initiated RAFT polymerization and coordination chemistry. Appl. Surf. Sci. 2013, 276, 298–305. [Google Scholar] [CrossRef]
- Islam, M.R.; Bach, L.G.; Park, J.M.; Hong, S.-S.; Lim, K.T. Synthesis and Characterization of Poly(HEMA-co-MMA)-g-POSS Nanocomposites by Combination of Reversible Addition Fragmentation Chain Transfer Polymerization and Click Chemistry. J. Appl. Polym. Sci. 2013, 127, 1569–1577. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Vo, S.T.; Islam, M.R.; Lim, K.T.; Vo, D.V.N.; Bach, L.G. Functionalization of halloysite nanotube surfaces via controlled living radical polymerization: Covalent immobilization of penicillin for a bioactive interface. J. Chem. Technol. Biotechnol. 2018. [Google Scholar] [CrossRef]
- Kim, Y.; Klutz, A.M.; Hechler, B.; Gao, Z.-G.; Gachet, C.; Jacobson, K.A. Application of the functionalized congener approach to dendrimer-based signaling agents acting through A2A adenosine receptors. Purinergic Signal. 2009, 5, 39–50. [Google Scholar] [CrossRef]
- Bach, L.G.; Islam, M.R.; Lee, D.C.; Lim, K.T. Poly(glycidyl methacrylate) grafted CdSe quantum dots by surface-initiated atom transfer radical polymerization: Novel synthesis, characterization, properties, and cytotoxicity studies. Appl. Surf. Sci. 2013, 283, 546–553. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.D.; Vu-Quang, H.; Vo, T.S.; Nguyen, D.C.; Vo, D.-V.N.; Nguyen, D.H.; Lim, K.T.; Tran, D.L.; Bach, L.G. Chemical Synthesis and Characterization of Poly(poly(ethylene glycol) methacrylate)-Grafted CdTe Nanocrystals via RAFT Polymerization for Covalent Immobilization of Adenosine. Polymers 2019, 11, 77. https://doi.org/10.3390/polym11010077
Nguyen TD, Vu-Quang H, Vo TS, Nguyen DC, Vo D-VN, Nguyen DH, Lim KT, Tran DL, Bach LG. Chemical Synthesis and Characterization of Poly(poly(ethylene glycol) methacrylate)-Grafted CdTe Nanocrystals via RAFT Polymerization for Covalent Immobilization of Adenosine. Polymers. 2019; 11(1):77. https://doi.org/10.3390/polym11010077
Chicago/Turabian StyleNguyen, Trinh Duy, Hieu Vu-Quang, Thanh Sang Vo, Duy Chinh Nguyen, Dai-Viet N. Vo, Dai Hai Nguyen, Kwon Taek Lim, Dai Lam Tran, and Long Giang Bach. 2019. "Chemical Synthesis and Characterization of Poly(poly(ethylene glycol) methacrylate)-Grafted CdTe Nanocrystals via RAFT Polymerization for Covalent Immobilization of Adenosine" Polymers 11, no. 1: 77. https://doi.org/10.3390/polym11010077
APA StyleNguyen, T. D., Vu-Quang, H., Vo, T. S., Nguyen, D. C., Vo, D.-V. N., Nguyen, D. H., Lim, K. T., Tran, D. L., & Bach, L. G. (2019). Chemical Synthesis and Characterization of Poly(poly(ethylene glycol) methacrylate)-Grafted CdTe Nanocrystals via RAFT Polymerization for Covalent Immobilization of Adenosine. Polymers, 11(1), 77. https://doi.org/10.3390/polym11010077