Characterization and Actuation of Ionic Polymer Metal Composites with Various Thicknesses and Lengths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the IPMCs
2.3. Scanning Electron Microscopy
2.4. Surface Resistance
2.5. Water Uptake Capability
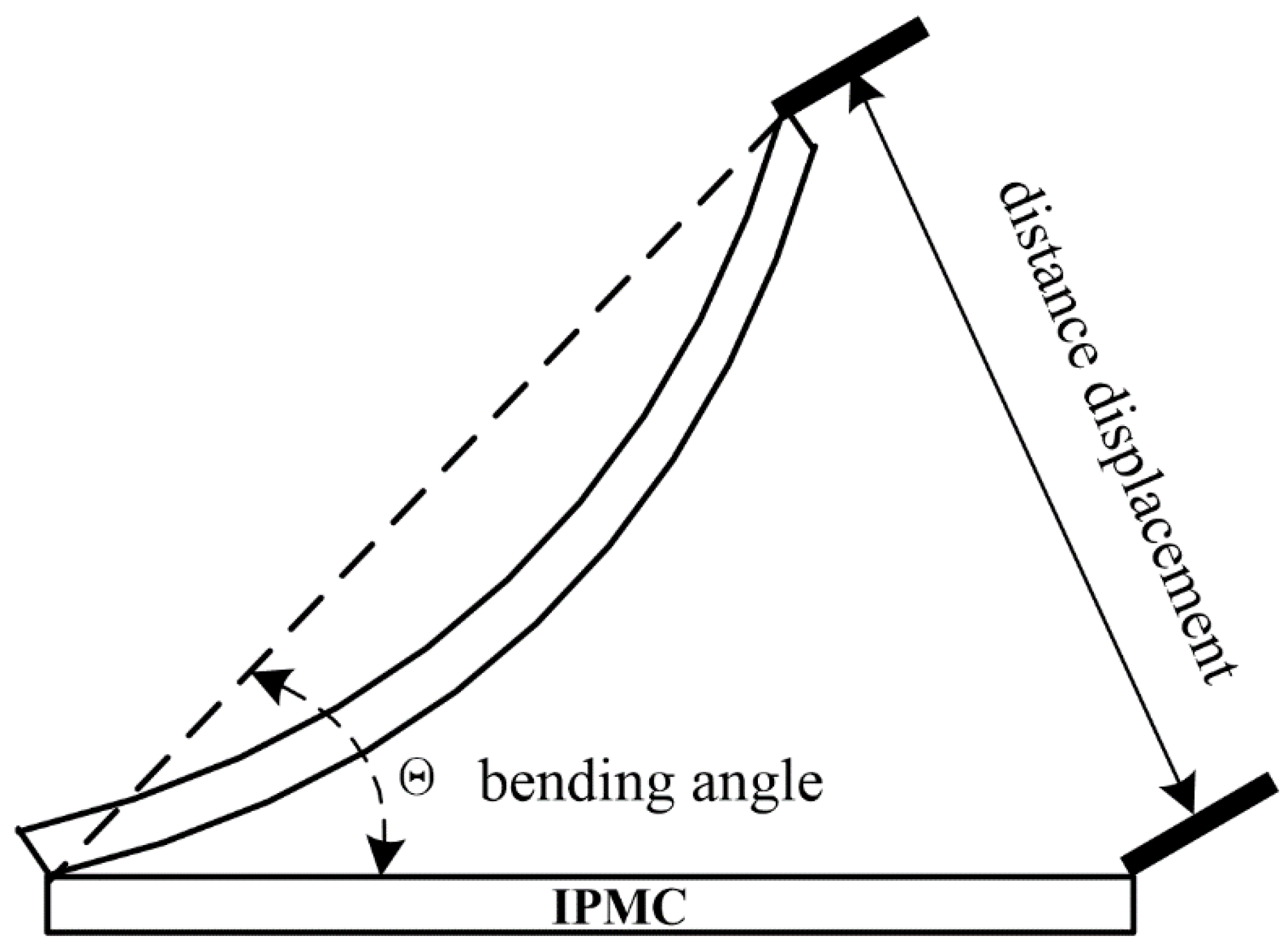
2.6. Actuation Performance
2.7. Repeated Actuations of the IPMCs
3. Results and Discussion
3.1. Morphology
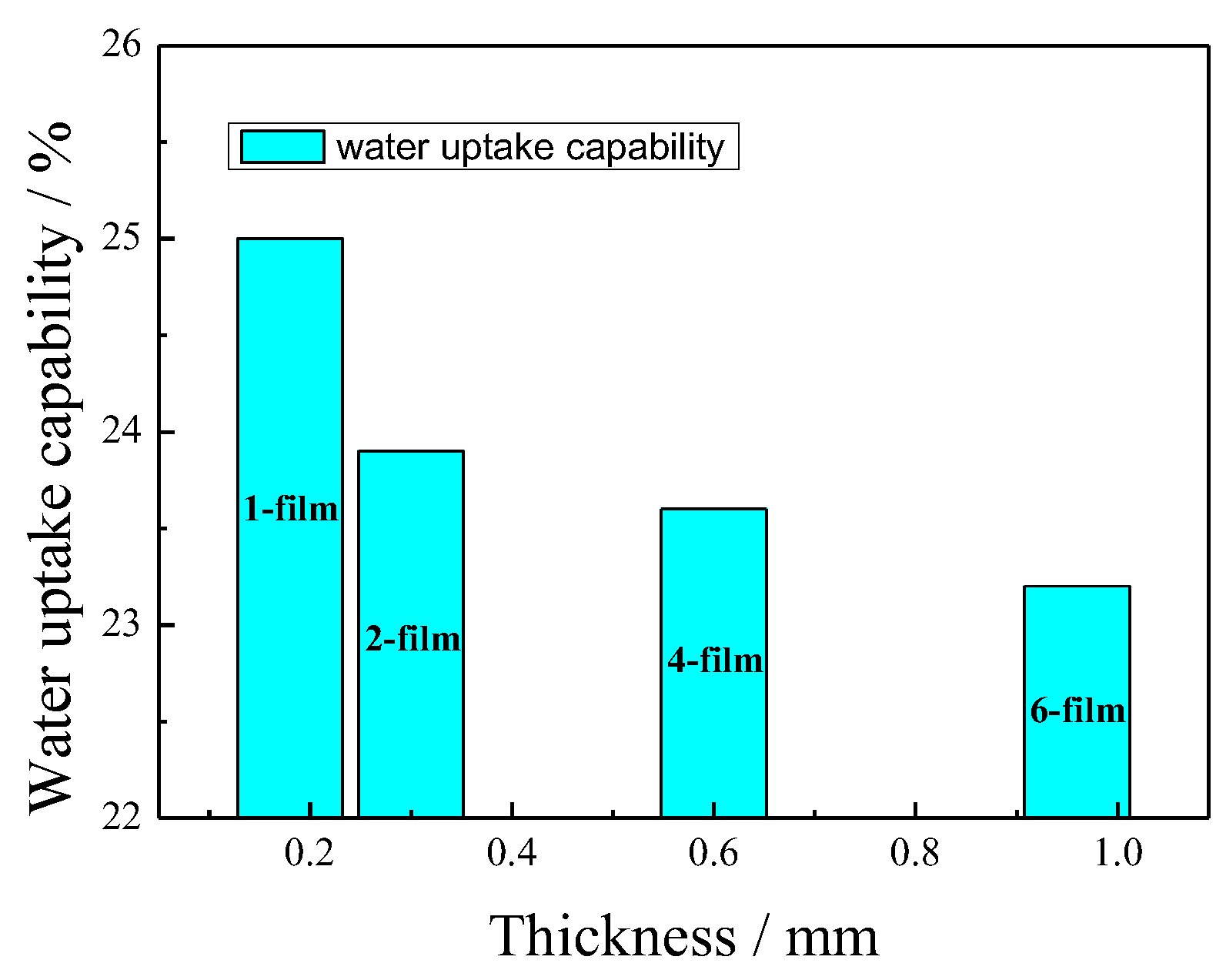
3.2. Water Uptake Capability
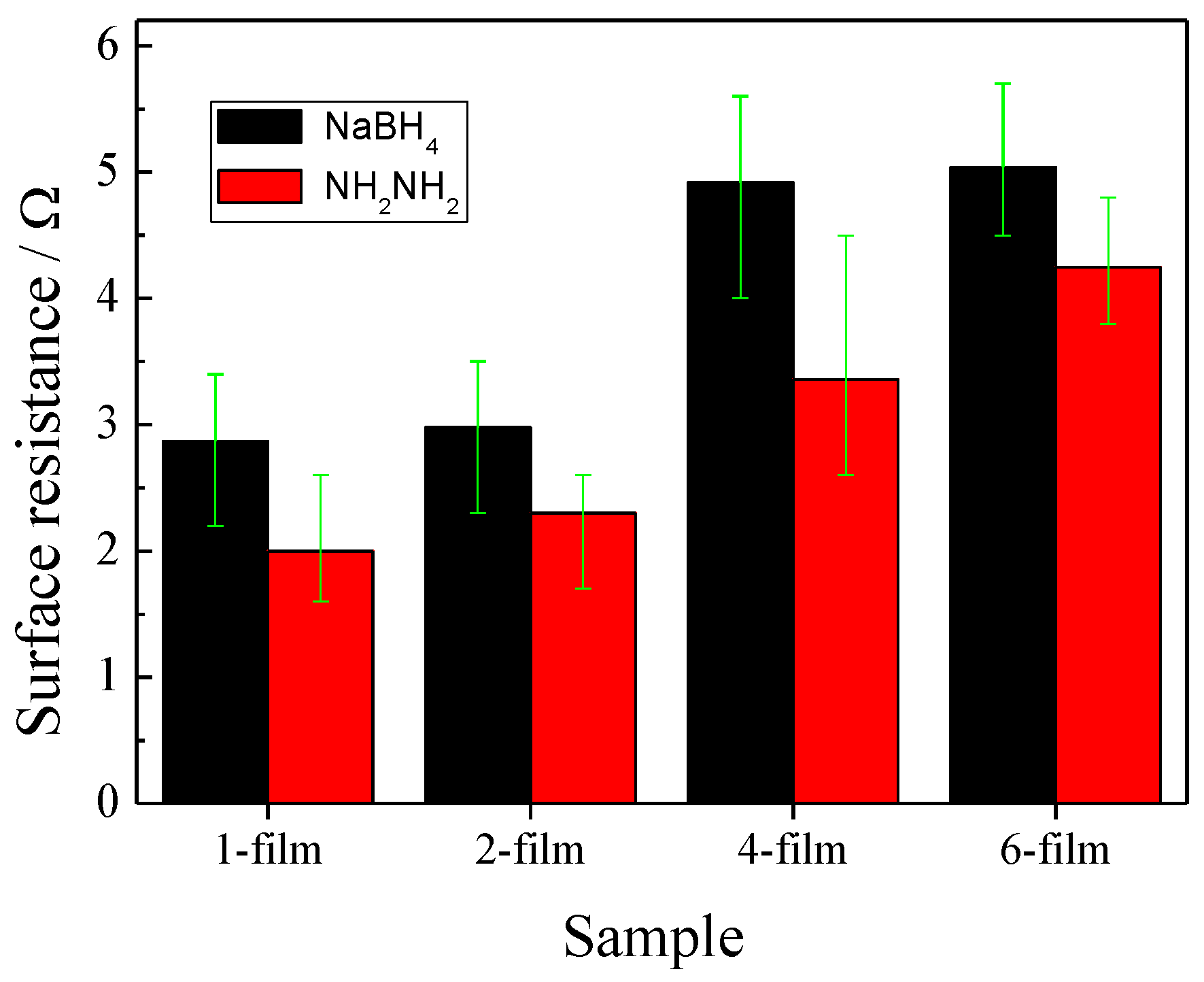
3.3. Surface Resistance
3.4. Effect of IPMC Thickness on Actuation
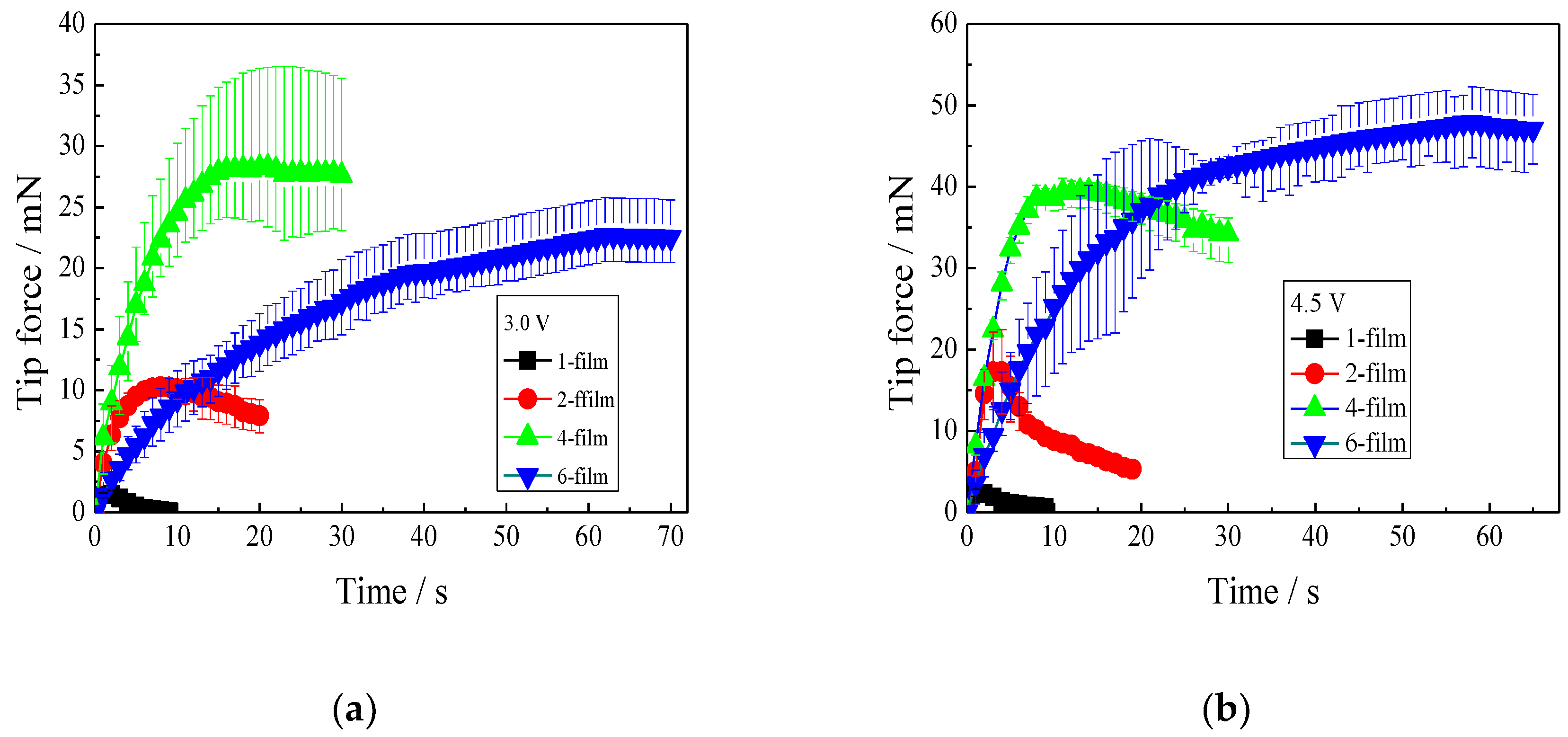
3.4.1. Effect of IPMC Thickness on Tip Force
3.4.2. Effect of IPMC Thickness on Displacement
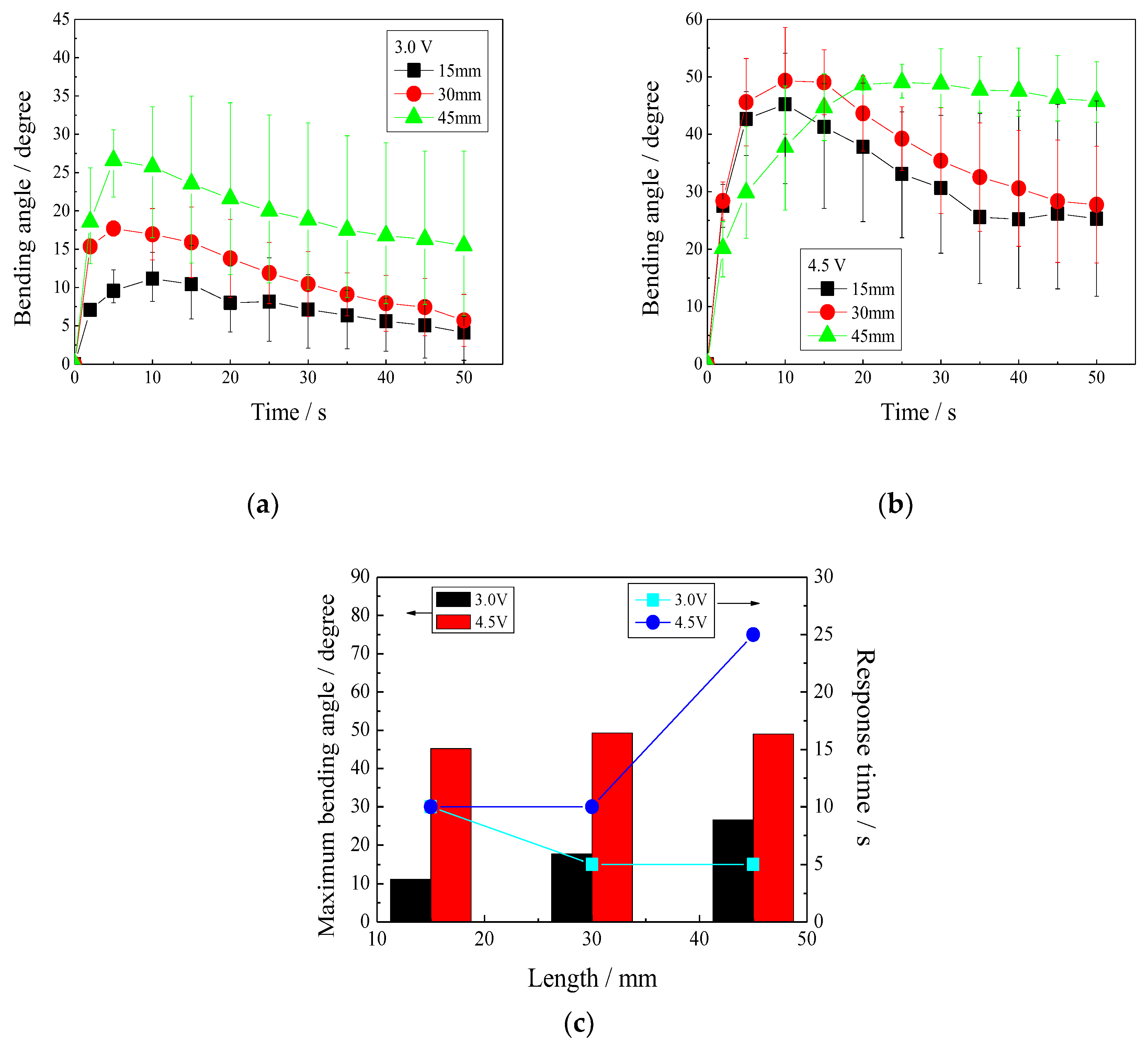
3.5. Effect of IPMC Length on Actuation
3.5.1. Effect of IPMC Length on Tip Force
3.5.2. Effect of IPMC Length on Bending Angle
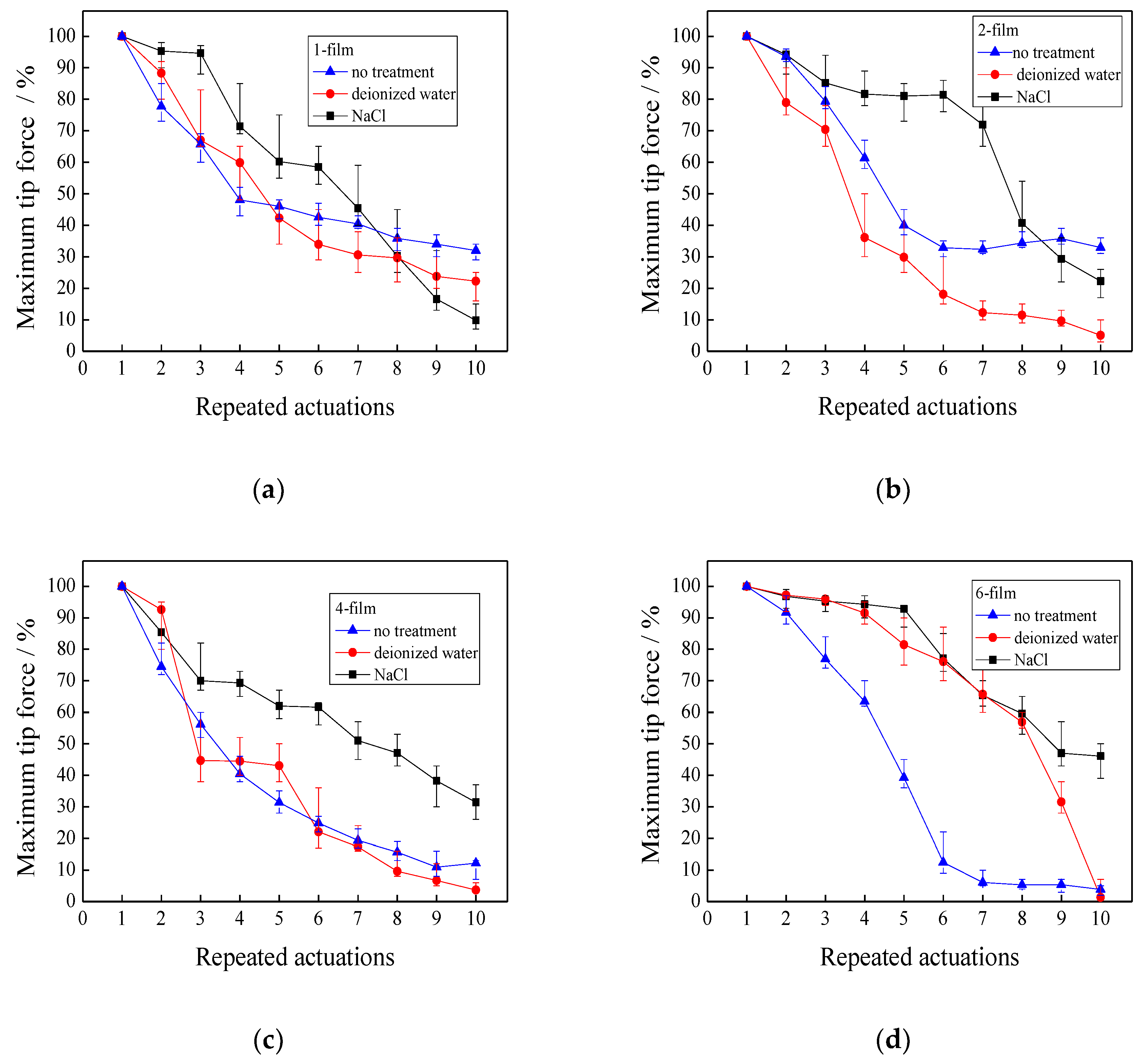
3.6. Repeated Actuations
3.6.1. Effects of Repeated Actuation Methods on the Maximum Tip Force
3.6.2. Effects of Repeated Actuation Methods on the Maximum Displacement
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shahinpoor, M. Ionic polymer-conductor composites as biomimetic sensors, robotic actuators and artificial muscles—A review. Electrochimica Act. 2003, 48, 2343–2353. [Google Scholar] [CrossRef]
- Yeom, S.W.; Oh, I.-K. A biomimetic jellyfish robot based on ionic polymer metal composite actuators. Smart Mater. Struct. 2009, 18, 085002. [Google Scholar] [CrossRef]
- Chung, C.K.; Hong, Y.Z.; Fung, P.K.; Ju, M.S.; Lin, C.C.K.; Wu, T.C. A novel fabrication of ionic polymer-metal composites (IPMC) actuator with silver nano-powders. Sens. Actuators B Chem. 2006, 117, 367–375. [Google Scholar] [CrossRef]
- Sen, I.; Seki, Y.; Sarikanat, M.; Cetin, L.; Gurses, B.O.; Ozdemir, O.; Yilmaz, O.C.; Sever, K.; Akar, E.; Mermer, O. Electroactive behavior of graphene nanoplatelets loaded cellulose composite actuators. Compos. Part B Eng. 2015, 69, 369–377. [Google Scholar] [CrossRef]
- Jung, J.H.; Vadahanambi, S.; Oh, I.K. Electro-active nano-composite actuator based on fullerene-reinforced Nafion. Compos. Sci. Technol. 2010, 7, 584–592. [Google Scholar] [CrossRef]
- Tamagawa, H.; Lin, W.; Kikuchi, K.; Sasaki, M. Bending control of Nafion-based electroactive polymer actuator coated with multi-walled carbon nanotubes. Sens. Actuators B Chem. 2001, 156, 375–382. [Google Scholar] [CrossRef]
- Lee, D.Y.; Park, I.S.; Lee, M.H.; Kim, K.J.; Heo, S. Ionic polymer–metal composite bending actuator loaded with multi-walled carbon nanotubes. Sens. Actuators A Phys. 2007, 133, 117–127. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, S.J.; Jang, J.H.; Wang, Z.; Kim, P.G.; Yoon, D.J.; Kim, J.; Hansen, G.; DeVries, K.L. Actuation of electrochemical, electro-magnetic, and electro-active actuators for carbon nanofiber and Ni nanowire reinforced polymer composites. Compos. Part B Eng. 2008, 39, 1161–1169. [Google Scholar] [CrossRef]
- Fang, B.K.; Ju, M.S.; Lin, C.C.K. A new approach to develop ionic polymer-metal composites (IPMC) actuator: Fabrication and control for active catheter systems. Sens. Actuators A Phys. 2007, 137, 321–329. [Google Scholar] [CrossRef]
- Jeon, J.H.; Kang, S.P.; Lee, S.; Oh, I.K. Novel biomimetic actuator based on SPEEK and PVDF. Sens. Actuators B Chem. 2009, 143, 357–364. [Google Scholar] [CrossRef]
- Dai, C.A.; Chang, C.J.; Kao, A.C.; Tsai, W.B.; Chen, W.S.; Liu, W.M.; Shih, W.P.; Ma, C.C. Polymer actuator based on PVA/PAMPS ionic membrane: Optimization of ionic transport properties. Sens. Actuators A Phys. 2009, 155, 152–162. [Google Scholar] [CrossRef]
- Phillips, A.K.; Moore, R.B. Ionic actuators based on novel sulfonated ethylene vinyl alcohol copolymer membranes. Polymer 2005, 46, 7788–7802. [Google Scholar] [CrossRef]
- Wang, X.L.; Oh, I.K.; Xu, L. Electro-active artificial muscle based on irradiation-crosslinked sulfonated poly(styrene-ran-ethylene). Sens. Actuators B Chem. 2010, 145, 635–642. [Google Scholar] [CrossRef]
- Lu, J.; Kim, S.G.; Lee, S.; Oh, I.K. A Biomimetic Actuator Based on an Ionic Networking Membrane of Poly(styrene-alt-maleimide)-Incorporated Poly(vinylidene fluoride). Adv. Funct. Mater. 2008, 18, 1290–1298. [Google Scholar] [CrossRef]
- Lee, J.W.; Hong, S.M.; Kim, J.; Chong, M.K. Novel sulfonated styrenic pentablock copolymer/silicate nanocomposite membranes with controlled ion channels and their IPMC transducers. Sens. Actuators B Chem. 2012, 162, 369–376. [Google Scholar] [CrossRef]
- Kunitomo, K.; Shigeki, T. Nafion®-based polymer actuators with ionic liquids as solvent incorporated at room temperature. J. Appl. Phys. 2008, 106, 053519. [Google Scholar]
- Lee, H.K.; Choi, N.J.; Jung, S.; Park, K.H.; Kim, J. Ionic polymer-metal composites (IPMCs) containing Cu/Ni electrodes and ionic liquids for durability. Proc. SPIE-Int. Soc. Opt. Eng. 2009, 7362, 73620I. [Google Scholar]
- Shahinpoor, M.; Kim, K.J. The effect of surface-electrode resistance on the performance of ionic polymer–metal composite (IPMC) artificial muscles. Smart Mater. Struct. 2000, 9, 543–551. [Google Scholar] [CrossRef]
- Saher, S.; Moon, S.; Kim, S.J.; Kim, H.J.; Kim, Y.H. O2 plasma treatment for ionic polymer metal nano composites (IPMC) actuator. Sens. Actuators B Chem. 2010, 1, 170–179. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, I.T.; Kim, Y.H. Performance enchancement of IPMC actuator by plasma surface treatment. Smart Mater. Struct. 2007, 16, 6–11. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, I.T.; Lee, H.K.; Kim, Y.H. Performance improvement of an ionic polymer–metal composite actuator by parylene thin film coating. Smart Mater. Struct. 2006, 15, 1540–1546. [Google Scholar] [CrossRef]
- Yip, J.; Li, S.; Hang, C.W.; Marcus, Y.C.W.; Wai, K.C. Experimentally validated improvement of IPMC performance through alternation of pretreatment and electroless plating processes. Smart Mater. Struct. 2011, 20, 015009. [Google Scholar] [CrossRef]
- Ma, C.H.; Yu, T.L.; Lin, H.L.; Huang, Y.T.; Chen, Y.L.; Jeng, U.S.; Lai, Y.H.; Sun, Y.S. Morphology and properties of Nafion membranes prepared by solution casting. Polymer 2009, 50, 1764–1777. [Google Scholar] [CrossRef]
- Lee, S.J.; Han, M.J.; Kim, S.J.; Jho, J.Y.; Lee, H.Y.; Kim, Y.H. A new fabrication method for IPMC actuators and application to artificial fingers. Smart Mater. Struct. 2006, 15, 1217–1224. [Google Scholar] [CrossRef]
- Lian, H.; Qian, W.; Estevez, L.; Liu, H.; Liu, Y.; Jiang, T.; Wang, K.; Guo, W.; Giannelis, E.P. Enhanced actuation in functionalized carbon nanotube-Nafion composites. Sens. Actuators B Chem. 2011, 156, 187–193. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yip, J. Characterization and Actuation of Ionic Polymer Metal Composites with Various Thicknesses and Lengths. Polymers 2019, 11, 91. https://doi.org/10.3390/polym11010091
Li S, Yip J. Characterization and Actuation of Ionic Polymer Metal Composites with Various Thicknesses and Lengths. Polymers. 2019; 11(1):91. https://doi.org/10.3390/polym11010091
Chicago/Turabian StyleLi, Shufeng, and Joanne Yip. 2019. "Characterization and Actuation of Ionic Polymer Metal Composites with Various Thicknesses and Lengths" Polymers 11, no. 1: 91. https://doi.org/10.3390/polym11010091





