Recyclable and Mendable Cellulose-Reinforced Composites Crosslinked with Diels–Alder Adducts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the TH-Treated CFs
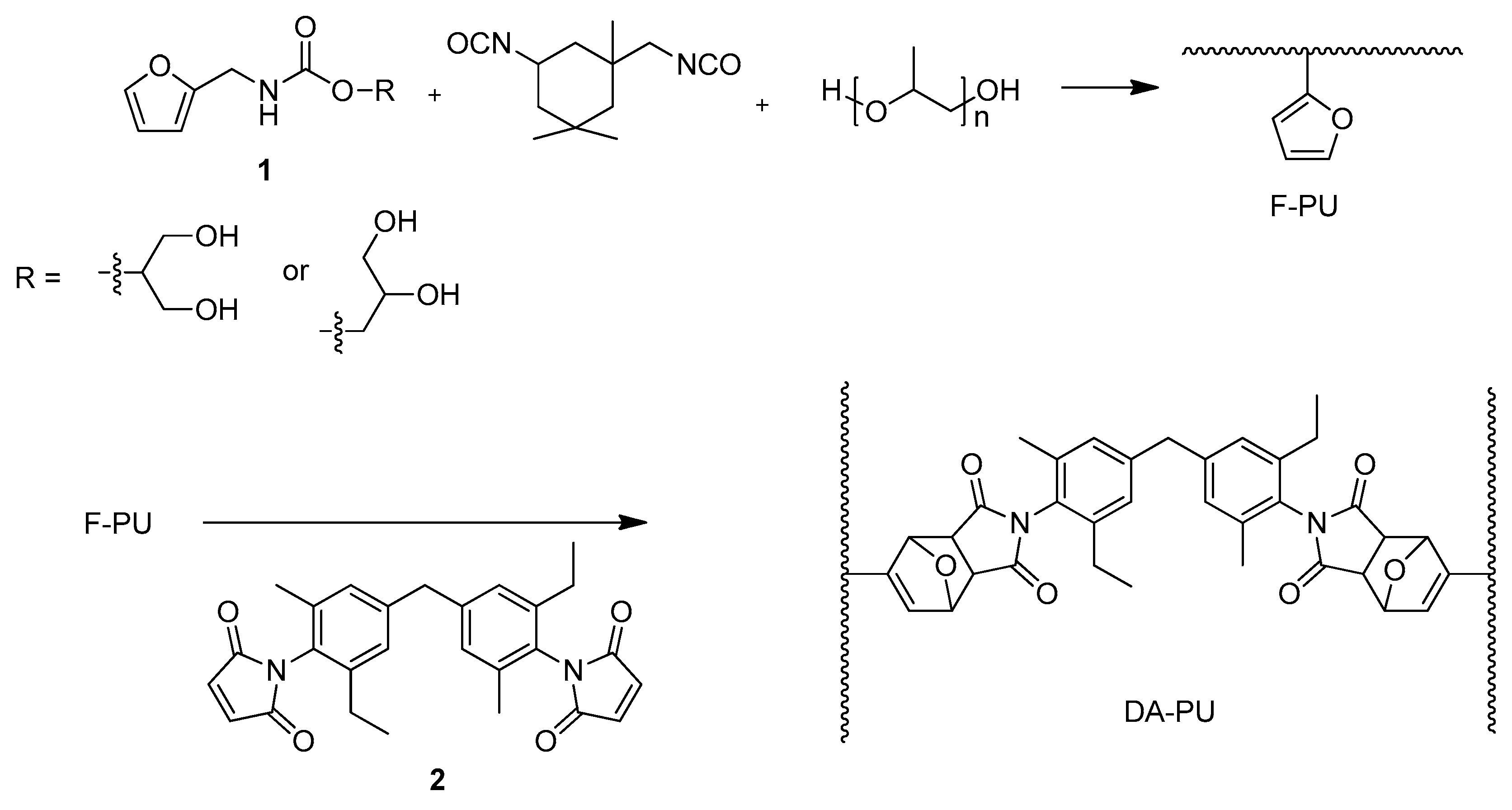
2.3. Preparation of the F-PU Solution
2.4. Synthesis of the DA-PU
2.5. Synthesis of the CF-Reinforced Composites
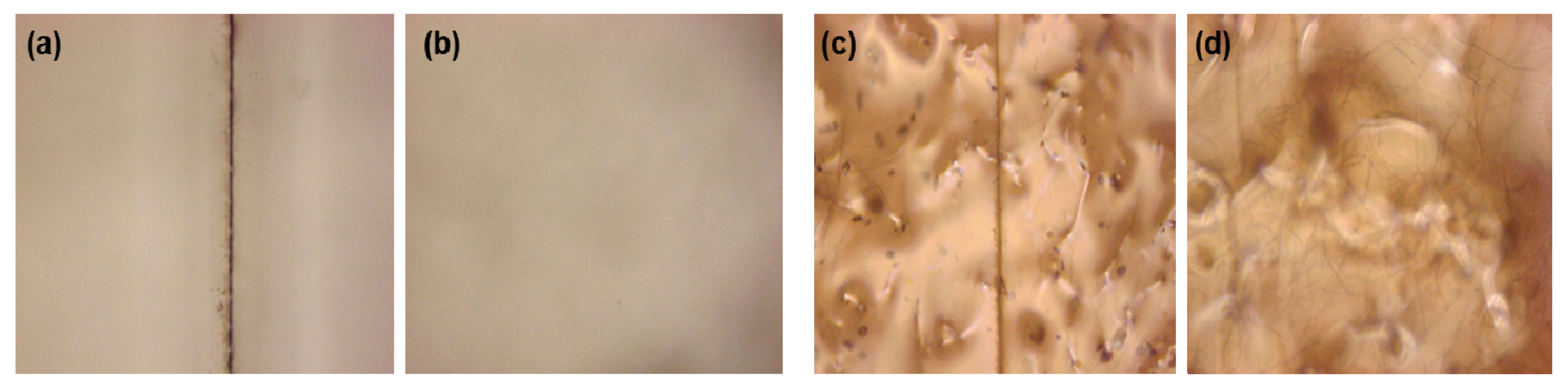
2.6. Healing Tests
2.7. Instrumentation
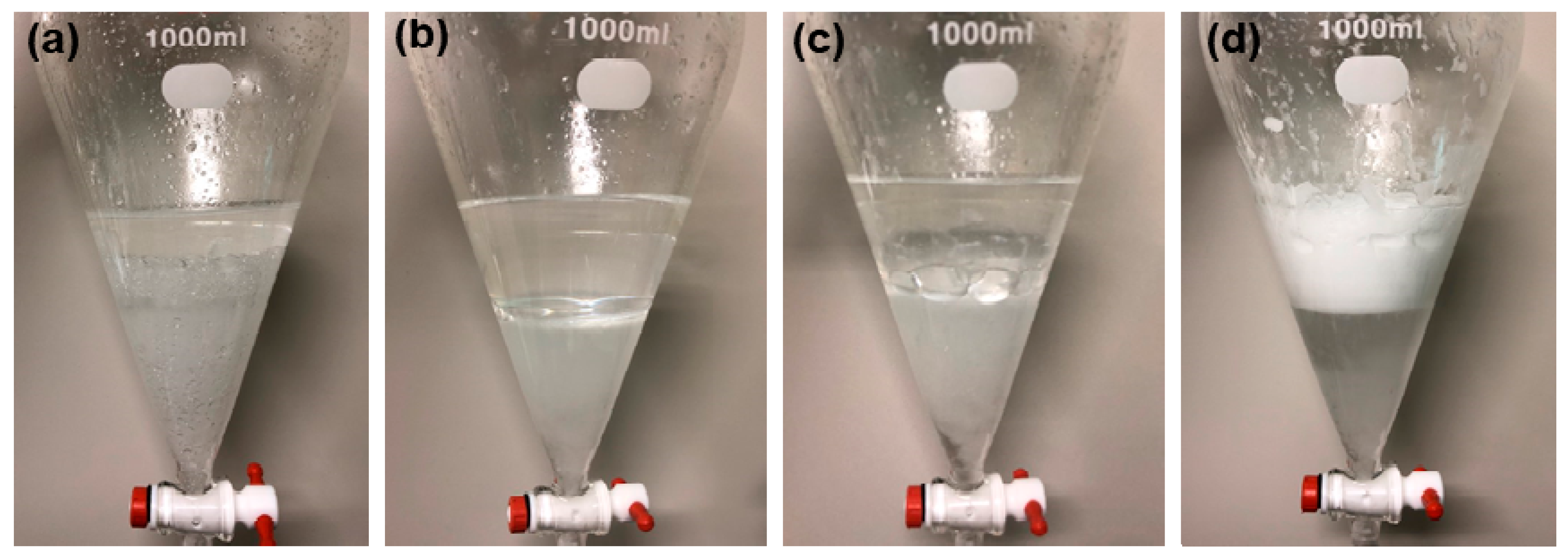
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, K.-Y.; Aitomäki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the use of nanocellulose as reinforcement in polymer matrix composites. Compos. Sci. Technol. 2014, 105, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Cazón, P.; Vázquez, M.; Velazquez, G. Novel composite films based on cellulose reinforced with chitosan and polyvinyl alcohol: Effect on mechanical properties and water vapour permeability. Polym. Test. 2018, 69, 536–544. [Google Scholar] [CrossRef]
- Sato, A.; Kabusaki, D.; Okumura, H.; Nakatani, T.; Nakatsubo, F.; Yano, H. Surface modification of cellulose nanofibers with alkenyl succinic anhydride for high-density polyethylene reinforcement. Compos. Part A 2016, 83, 72–79. [Google Scholar] [CrossRef]
- Liimatainen, H.; Visanko, M.; Sirviö, J.A.; Hormi, O.E.O.; Niinimaki, J. Enhancement of the Nanofibrillation of Wood Cellulose through Sequential Periodate–Chlorite Oxidation. Biomacromolecules 2012, 13, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Sirvio, J.; Hyvakko, U.; Liimatainen, H.; Niinimaki, J.; Hormi, O. Periodate oxidation of cellulose at elevated temperatures using metal salts as cellulose activators. Carbohydr. Polym. 2011, 83, 1293–1297. [Google Scholar] [CrossRef]
- Pandey, J.K.; Chu, W.S.; Kim, C.S.; Lee, C.S.; Ahn, S.H. Bio-nano reinforcement of environmentally degradable polymer matrix by cellulose whiskers from grass. Compos. Part B 2009, 40, 676–680. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Belgacem, M.N.; Gandini, A. Controlled heterogeneous modification of cellulose fibers with fatty acids: Effect of reaction conditions on the extent of esterification and fiber properties. J. Appl. Polym. Sci. 2006, 100, 1093–1102. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Pascoal Neto, C.; Rocha, R.M.A. An Efficient Method for Determination of the Degree of Substitution of Cellulose Esters of Long Chain Aliphatic Acids. Cellulose 2005, 12, 449–458. [Google Scholar] [CrossRef]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Nishiyama, Y.; Putaux, J.-L.; Vignon, M.; Isogai, A. Homogeneous Suspensions of Individualized Microfibrils from TEMPO-Catalyzed Oxidation of Native Cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Hosseinmardi, A.; Annamalai, P.K.; Wang, L.; Martin, D.; Amiralian, N. Reinforcement of natural rubber latex using lignocellulosic nanofibers isolated from spinifex grass. Nanoscale 2017, 9, 9510–9519. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose. Green Chem. 2015, 17, 3401–3406. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, J.; Song, X.; Zarrin, H.; Tian, X.; Qiao, J.; Rasen, L.; Li, K.; Chen, Z. A flexible solid-state electrolyte for wide-scale integration of rechargeable zinc–air batteries. Energy Environ. Sci. 2016, 9, 663–670. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, X.; Urban, M.W. Chemical and physical aspects of self-healing materials. Prog. Polym. Sci. 2015, 49–50, 34–59. [Google Scholar] [CrossRef]
- An, S.Y.; Arunbabu, D.; Noh, S.M.; Song, Y.K.; Oh, J.K. Recent strategies to develop self-healable crosslinked polymeric networks. Chem. Commun. 2015, 51, 13058–13070. [Google Scholar] [CrossRef] [PubMed]
- Rekondo, A.; Martin, R.; Ruiz de Luzuriaga, A.; Cabañero, G.; Grande, H.J.; Odriozola, I. Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horiz. 2014, 1, 237–240. [Google Scholar] [CrossRef]
- Canadell, J.; Goossens, H.; Klumperman, B. Self-Healing Materials Based on Disulfide Links. Macromolecules 2011, 44, 2536–2541. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, Y.; Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 3218. [Google Scholar] [CrossRef] [Green Version]
- Park, J.I.; Choe, A.; Kim, M.P.; Ko, H.; Lee, T.H.; Noh, S.M.; Kim, J.C.; Cheong, I.W. Water-adaptive and repeatable self-healing polymers bearing bulky urea bonds. Polym. Chem. 2018, 9, 11–19. [Google Scholar] [CrossRef]
- Burattini, S.; Colquhoun, H.M.; Fox, J.D.; Friedmann, D.; Greenland, B.W.; Harris, P.J.F.; Hayes, W.; Mackay, M.E.; Rowan, S.J. A self-repairing, supramolecular polymer system: Healability as a consequence of donor–acceptor π–π stacking interactions. Chem. Commun. 2009, 6717–6719. [Google Scholar] [CrossRef]
- Zhang, D.-D.; Ruan, Y.-B.; Zhang, B.-Q.; Qiao, X.; Deng, G.; Chen, Y.; Liu, C.-Y. A self-healing PDMS elastomer based on acylhydrazone groups and the role of hydrogen bonds. Polymer 2017, 120, 189–196. [Google Scholar] [CrossRef]
- Montarnal, D.; Cordier, P.; Soulié-Ziakovic, C.; Tournilhac, F.; Leibler, L. Synthesis of self-healing supramolecular rubbers from fatty acid derivatives, diethylene triamine, and urea. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7925–7936. [Google Scholar] [CrossRef]
- Folmer, B.J.B.; Sijbesma, R.P.; Versteegen, R.M.; van der Rijt, J.A.J.; Meijer, E.W. Supramolecular Polymer Materials: Chain Extension of Telechelic Polymers Using a Reactive Hydrogen-Bonding Synthon. Adv. Mater. 2000, 12, 874–878. [Google Scholar] [CrossRef]
- Garcia, S.J. Effect of polymer architecture on the intrinsic self-healing character of polymers. Eur. Polym. J. 2014, 53, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Zechel, S.; Geitner, R.; Abend, M.; Siegmann, M.; Enke, M.; Kuhl, N.; Klein, M.; Vitz, J.; Gräfe, S.; Dietzek, B.; et al. Intrinsic self-healing polymers with a high E-modulus based on dynamic reversible urea bonds. NPG Asia Mater. 2017, 9, e420. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-M.; Jeon, H.; Shin, S.-H.; Park, S.-A.; Jegal, J.; Hwang, S.Y.; Oh, D.X.; Park, J. Superior Toughness and Fast Self-Healing at Room Temperature Engineered by Transparent Elastomers. Adv. Mater. 2018, 30, 1705145. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels–Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A Thermally Re-mendable Cross-Linked Polymeric Material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Lee, D.H.; Heo, G.; Pyo, K.-H.; Kim, Y.; Kim, J.-W. Mechanically Robust and Healable Transparent Electrode Fabricated via Vapor-Assisted Solution Process. ACS Appl. Mater. Interfaces 2016, 8, 8129–8136. [Google Scholar] [CrossRef]
- Heo, G.; Pyo, K.-H.; Lee, D.H.; Kim, Y.; Kim, J.-W. Critical Role of Diels–Adler Adducts to Realise Stretchable Transparent Electrodes Based on Silver Nanowires and Silicone Elastomer. Sci. Rep. 2016, 6, 25358. [Google Scholar] [CrossRef] [Green Version]
- Du, P.; Wu, M.; Liu, X.; Zheng, Z.; Wang, X.; Sun, P.; Joncheray, T.; Zhang, Y. Synthesis of linear polyurethane bearing pendant furan and cross-linked healable polyurethane containing Diels–Alder bonds. New J. Chem. 2014, 38, 770–776. [Google Scholar] [CrossRef]
- Pyo, K.-H.; Lee, D.H.; Kim, Y.; Kim, J.-W. Extremely rapid and simple healing of a transparent conductor based on Ag nanowires and polyurethane with a Diels–Alder network. J. Mater. Chem. C 2016, 4, 972–977. [Google Scholar] [CrossRef]
- Vadukumpully, S.; Paul, J.; Valiyaveettil, S. Cationic surfactant mediated exfoliation of graphite into graphene flakes. Carbon 2009, 47, 3288–3294. [Google Scholar] [CrossRef]
- Kim, J.-W.; Lee, D.H.; Jeon, H.-J.; Jang, S.I.; Cho, H.M.; Kim, Y. Recyclable thermosetting thermal pad using silicone-based polyurethane crosslinked by Diels-Alder adduct. Appl. Surf. Sci. 2018, 429, 128–133. [Google Scholar] [CrossRef]
- Blond, D.; Barron, V.; Ruether, M.; Ryan, K.P.; Nicolosi, V.; Blau, W.J.; Coleman, J.N. Enhancement of Modulus, Strength, and Toughness in Poly(methyl methacrylate)-Based Composites by the Incorporation of Poly(methyl methacrylate)-Functionalized Nanotubes. Adv. Funct. Mater. 2006, 16, 1608–1614. [Google Scholar] [CrossRef]
- Yang, B.-X.; Shi, J.-H.; Pramoda, K.P.; Goh, S.H. Enhancement of the mechanical properties of polypropylene using polypropylene-grafted multiwalled carbon nanotubes. Compos. Sci. Technol. 2008, 68, 2490–2497. [Google Scholar] [CrossRef]
- Yang, B.-X.; Shi, J.-H.; Pramoda, K.P.; Goh, S.H. Enhancement of stiffness, strength, ductility and toughness of poly(ethylene oxide) using phenoxy-grafted multiwalled carbon nanotubes. Nanotechnology 2007, 18, 125606. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, J.; Liu, X.; Wang, Y.; Lin, J.; Peng, N.; Li, J.; Zhao, F. Performance enhancement of polyvinyl chloride ultrafiltration membrane modified with graphene oxide. J. Colloid Interface Sci. 2016, 480, 1–8. [Google Scholar] [CrossRef]
- Zou, H.; Xu, W.; Zhang, Q.; Fu, Q. Effect of alkylammonium salt on the dispersion and properties of Poly(p-phenylene sulfide)/clay nanocomposites via melt intercalation. J. Appl. Polym. Sci. 2006, 99, 1724–1731. [Google Scholar] [CrossRef]


| CF Content | Tensile Strength (MPa) | Elongation at Break | Young’s Modulus (MPa) | Toughness (MJ/m3) |
|---|---|---|---|---|
| 0 wt % | 2.83 ± 0.06 | 53.55 ± 3.35 | 10.10 ± 0.32 | 1.09 ± 0.02 |
| 1 wt % | 3.70 ± 0.24 | 73.33 ± 8.63 | 10.80 ± 0.87 | 1.96 ± 0.04 |
| 3 wt % | 3.95 ± 0.31 | 88.33 ± 6.20 | 23.80 ± 2.67 | 2.62 ± 0.02 |
| 5 wt % | 4.83 ± 0.82 | 151.57 ± 28.01 | 17.74 ± 2.29 | 5.67 ± 0.06 |
| 7 wt % | 1.38 ± 0.25 | 36.66 ± 10.42 | 7.21 ± 1.60 | 0.35 ± 0.29 |
| CF Content | Tensile Strength (MPa) | Elongation at Break | Young’s Modulus (MPa) | Toughness (MJ/m3) |
|---|---|---|---|---|
| 0 wt % | 2.44 ± 0.09 | 81.67 ± 9.36 | 4.61 ± 0.25 | 1.48 ± 0.03 |
| 1 wt % | 2.61 ± 0.20 | 76.66 ±17.14 | 9.83 ± 1.50 | 1.63 ± 0.06 |
| 3 wt % | 2.96 ± 0.37 | 113.33 ± 12.73 | 9.14 ± 1.74 | 2.70 ± 0.04 |
| 5 wt % | 3.01 ± 0.81 | 245.00 ± 44.33 | 6.57 ± 1.36 | 4.80 ± 0.08 |
| 7 wt % | 1.73 ± 0.44 | 78.33 ± 42.30 | 4.00 ± 1.15 | 0.98 ± 0.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Shin, C.; Song, Y.-S.; Lee, H.-J.; Shin, C.; Kim, Y. Recyclable and Mendable Cellulose-Reinforced Composites Crosslinked with Diels–Alder Adducts. Polymers 2019, 11, 117. https://doi.org/10.3390/polym11010117
Park K, Shin C, Song Y-S, Lee H-J, Shin C, Kim Y. Recyclable and Mendable Cellulose-Reinforced Composites Crosslinked with Diels–Alder Adducts. Polymers. 2019; 11(1):117. https://doi.org/10.3390/polym11010117
Chicago/Turabian StylePark, KeumHwan, Cheolmin Shin, Ye-Seul Song, Hee-Jin Lee, Chiho Shin, and Youngmin Kim. 2019. "Recyclable and Mendable Cellulose-Reinforced Composites Crosslinked with Diels–Alder Adducts" Polymers 11, no. 1: 117. https://doi.org/10.3390/polym11010117





