Proposed Mechanism for the High-Yield Polymerization of Oxyethyl Propiolates with Rh Complex Catalyst Using the Density Functional Theory Method
Abstract
:1. Introduction
2. Experimental
2.1. Measurements
2.2. Materials
Synthesis of 2-Methoxyethyl propiolate, OP(1)
2.3. Polymerization
Synthesis of Poly(2-Methoxyethyl propiolate), POP(1)
2.4. Computation
3. Results and Discussion
3.1. Polymerization Behavior of OPs in MeOH
3.2. Polymerization Behavior of OP(2) in Various Solvents
3.3. Polymerization Behavior of OP(1) and BP(1) Dependent on Reaction Time When Using MeOH
3.4. Relevance of Monomer Structure and Polymerization Behavior Based on DFT Calculation
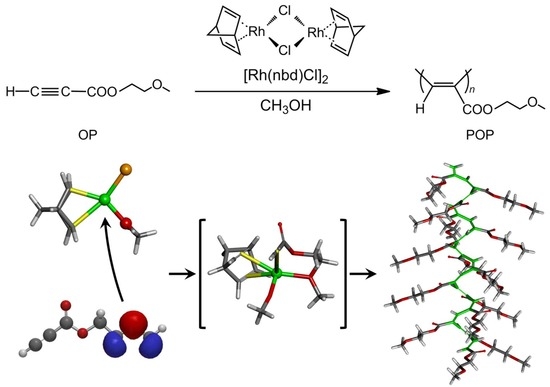
3.5. Proposal Polymerization Mechanism of OP(1) Using the Rh Complex Catalyst in MeOH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tabata, M.; Mawatari, Y. Emerging π-conjugated stretched and contracted helices and their mutual conversions of substituted polyacetylenes prepared using an organo-rhodium catalyst. Polym. Rev. 2016, 57, 65–88. [Google Scholar] [CrossRef]
- Tabata, M.; Mawatari, Y.; Yoshida, Y.; Sasaki, T. Mutual conversion between stretched and contracted helices and its external stimuli induced drastic colors and geometrical structures changes of substituted polyacetylenes prepared with an organo-rhodium catalyst. Adv. Sci. Tech. 2016, 97, 18–23. [Google Scholar] [CrossRef]
- Yoshida, Y.; Mawatari, Y.; Tabata, M. synthesis and helix pitch control of π-conjugated helical polymers with accordion-like oscillation. J. Synth. Org. Chem. Jpn. 2014, 72, 292–302. [Google Scholar] [CrossRef]
- Masuda, T. substituted polyacetylenes. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 165–180. [Google Scholar] [CrossRef]
- Shiotsuki, M.; Sanda, F.; Masuda, T. polymerization of substituted acetylenes and features of the formed polymers. Polym. Chem. 2010, 2, 1044–1058. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: Synthesis, structures, and functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef]
- Shimomura, K.; Ikai, T.; Kanoh, S.; Yashima, E.; Maeda, K. Switchable enantioseparation based on macromolecular memory of a helical polyacetylene in the solid state. Nat. Chem. 2014, 6, 429–434. [Google Scholar] [CrossRef]
- Aoki, T.; Kaneko, T.; Teraguchi, M. Synthesis of functional π-conjugated polymers from aromatic acetylenes. Polymer 2006, 47, 4867–4892. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lam, J.; Tang, B. Acetylenic polymers: Syntheses, structures, and functions. Chem. Rev. 2009, 109, 5799–5867. [Google Scholar] [CrossRef]
- Kern, R.J. Preparation and properties of isomeric polyphenylacetylenes. J. Polym. Sci. Part 1 Polym. Chem. 1969, 7, 621–631. [Google Scholar] [CrossRef]
- Luttinger, L.B. Hydridic reducing agent—Group VIII metal compound. A new catalyst system for the polymerization of acetylenes and related compounds. I. J. Org. Chem. 1962, 27, 1591–1596. [Google Scholar] [CrossRef]
- Masuda, T.; Higashimura, T. Polyacetylenes with substituents: Their synthesis and properties. Adv. Polym. Sci. 1986, 81, 121–165. [Google Scholar]
- Furlani, A.; Napoletano, C.; Russo, M.; Feast, W.J. Stereoregular polyphenylacetylene. Polym. Bull. 1986, 16, 311–317. [Google Scholar] [CrossRef]
- Tabata, M.; Yang, W.; Yokota, K. Polymerization of m-chlorophenylacetylene initiated by [Rh(norbornadiene)Cl]2-triethylamine catalyst containing long-lived propagation species. Polym. J. 1990, 22, 1105–1107. [Google Scholar] [CrossRef]
- Yang, W.; Tabata, M.; Kobayashi, S.; Yokota, K.; Shimizu, A. Synthesis of ultra-high-molecular-weight aromatic polyacetylenes with [Rh(norbornadiene)Cl]2-triethylamine and solvent-induced crystallization of the obtained amorphous polyacetylenes. Polym. J. 1991, 23, 1135–1138. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Eckerle, P.; Miyatake, T.; Kainosho, M.; Ono, A.; Ikariya, T.; Noyori, R. Well-controlled polymerization of phenylacetylenes with organorhodium(I) complexes: Mechanism and structure of the polyenes. J. Am. Chem. Soc. 1999, 121, 12035–12044. [Google Scholar] [CrossRef]
- Aoki, T.; Kaneko, T.; Maruyama, N.; Sumi, A.; Takahashi, M.; Sato, T.; Teraguchi, M. Helix-sense-selective polymerization of phenylacetylene having two hydroxy groups using a chiral catalytic system. J. Am. Chem. Soc. 2003, 125, 6346–6347. [Google Scholar] [CrossRef]
- Kumazawa, S.; Castanon, J.; Onishi, N.; Kuwata, K.; Shiotsuki, M.; Sanda, F. Characterization of the polymerization catalyst [(2,5-norbornadiene)Rh{C(Ph)═CPh2}(PPh3)] and identification of the end structures of poly(phenylacetylenes) obtained by polymerization using this catalyst. Organometallics 2012, 31, 6834–6842. [Google Scholar] [CrossRef]
- Onishi, N.; Shiotsuki, M.; Masuda, T.; Sano, N.; Sanda, F. Polymerization of phenylacetylenes using rhodium catalysts coordinated by norbornadiene linked to a phosphino or amino group. Organometallics 2013, 32, 846–853. [Google Scholar] [CrossRef]
- Tabata, M.; Yang, W.; Yokota, K. 1H-NMR and UV studies of Rh complexes as a stereoregular polymerization catalysts for phenylacetylenes: Effects of ligands and solvents on its catalyst activity. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 1113–1120. [Google Scholar] [CrossRef]
- Lindgren, M.; Lee, H.-S.; Yang, W.; Tabata, M.; Yokota, K. Synthesis of soluble polyphenylacetylenes containing a strong donor function. Polymer 1991, 32, 1531–1534. [Google Scholar] [CrossRef]
- Nakazato, A.; Saeed, I.; Katsumata, T.; Shiotsuki, M.; Masuda, T.; Zednik, J.; Vohlidal, J. Polymerization of substituted acetylenes by various rhodium catalysts: Comparison of catalyst activity and effect of additives. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4530–4536. [Google Scholar] [CrossRef]
- Motoshige, A.; Mawatari, Y.; Yoshida, Y.; Seki, C.; Matsuyama, H.; Tabata, M. Irreversible helix rearrangement from cis-transoid to cis-cisoid in poly(p-n-hexyloxyphenylacetylene) induced by heat-treatment in solid phase. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3008–3015. [Google Scholar] [CrossRef]
- Motoshige, A.; Mawatari, Y.; Motoshige, R.; Yoshida, Y.; Tabata, M. Contracted helix to stretched helix Rearrangement of an aromatic polyacetylene prepared in n-hexane with [Rh(norbornadiene)Cl]2-triethylamine catalyst. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 5177–5183. [Google Scholar] [CrossRef]
- Motoshige, A.; Mawatari, Y.; Yoshida, Y.; Motoshige, R.; Tabata, M. Synthesis and solid state helix to helix rearrangement of poly(phenylacetylene) bearing n-octyl alkyl side chains. Polym. Chem. 2014, 5, 971–978. [Google Scholar] [CrossRef]
- Motoshige, R.; Mawatari, Y.; Motoshige, A.; Yoshida, Y.; Sasaki, T.; Yoshimizu, H.; Suzuki, T.; Tsujita, Y.; Tabata, M. Mutual conversion between stretched and contracted helices accompanied by a drastic change in color and spatial structure of poly(phenylacetylene) prepared with a [Rh(nbd)Cl]2-amine catalyst. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 752–759. [Google Scholar] [CrossRef]
- Mawatari, Y.; Yoshida, Y.; Motoshige, A.; Motoshige, R.; Sasaki, T.; Tabata, M. Solid phase helical and crystal structures of poly(phenylacetylene)s with para-oligo ethylene oxy methylether moieties prepared with an organorhodium catalyst in ethanol. Eur. Polym. J. 2014, 57, 213–220. [Google Scholar] [CrossRef]
- Mawatari, Y.; Motoshige, A.; Yoshida, Y.; Motoshige, R.; Sasaki, T.; Tabata, M. Structural determination of stretched helix and contracted helix having yellow and red colors of poly(2-ethynylnaphthalene) prepared with a [Rh(norbornadiene)Cl]2-triethylamine catalyst. Polymer 2014, 55, 2356–2361. [Google Scholar] [CrossRef]
- Sasaki, T.; Yoshida, Y.; Mawatari, Y.; Tabata, M. Remarkably stretched cis–transoid helices generated in solid phase and solution of poly(carbazole acetylene) prepared using an organorhodium catalyst in toluene. Macromolecules 2015, 48, 889–897. [Google Scholar] [CrossRef]
- Sasaki, T.; Mawatari, Y.; Tabata, M. Configuration and conformation of poly(3-carbazolylacetylene) including cis and trans radicals revealed by ESR spectroscopy. Polym. Chem. 2015, 6, 8012–8018. [Google Scholar] [CrossRef]
- Zhang, W.; Tabei, J.; Shiotsuki, M.; Masuda, T. Synthesis of poly(propargyl esters) with rhodium catalysts and their characterization. Polym. Bull. 2006, 57, 463–472. [Google Scholar] [CrossRef]
- Sanda, F.; Masuda, T. Synthesis and functions of optically active helical conjugated polymers. J. Synth. Org. Chem. Jpn. 2008, 66, 757–764. [Google Scholar] [CrossRef]
- Yoshida, Y.; Mawatari, Y.; Seki, C.; Hiraoki, T.; Matsuyama, H.; Tabata, M. Cis and trans radicals generated in helical poly(propargyl acetate)s prepared using a [Rh(norbornadiene)Cl]2 catalyst. Polymer 2011, 52, 646–651. [Google Scholar] [CrossRef]
- Yoshida, Y.; Mawatari, Y.; Seki, C.; Hiraoki, T.; Matsuyama, H.; Tabata, M. Geometrical structures in solution and solid phase of poly(propargyl ester)s prepared by using a [Rh(norbornadiene)Cl]2-cocatalyst. Polymer 2011, 52, 3917–3924. [Google Scholar] [CrossRef]
- Tabata, M.; Inaba, Y.; Yokota, K.; Nozaki, Y. Stereoregular polymerization of alkyl propiolate catalyzed by Rh complex. J. Macromol. Sci. Part A 1994, 31, 465–475. [Google Scholar] [CrossRef]
- Minakawa, H.; Tabata, M.; Yokota, K. Structural differences between polypentynoates bearing mesogenic moieties polymerized with Rh complex and WCl6 catalysts. A 13C-NMR and raman study. J. Macromol. Sci. Part. A 1996, 33, 291–303. [Google Scholar] [CrossRef]
- Nakako, H.; Nomura, R.; Tabata, M.; Masuda, T. Synthesis and structure in solution of poly[(−)-menthyl propiolate] as a new class of helical polyacetylene. Macromolecules 1999, 32, 2861–2864. [Google Scholar] [CrossRef]
- Kozuka, M.; Sone, T.; Sadahiro, Y.; Tabata, M.; Enoto, T. Columnar assemblies of aliphatic poly(acetylene ester)s prepared with a [Rh(norbornadiene)Cl]2 catalyst. 1H and 13C NMR, X-ray diffraction and AFM studies. Macromol. Chem. Phys. 2002, 203, 66–70. [Google Scholar] [CrossRef]
- Sato, E.; Mawatari, Y.; Sadahiro, Y.; Yamada, B.; Tabata, M.; Kashiwaya, Y. Geometrical structures of poly(haloalkyl propiolate)s prepared with a [Rh(norbornadiene)Cl]2 catalyst. Polymer 2008, 49, 1620–1628. [Google Scholar] [CrossRef]
- Nomura, R.; Fukushima, Y.; Nakako, H.; Masuda, T. Conformational study of helical poly(propiolic esters) in solution. J. Am. Chem. Soc. 2000, 122, 8830–8836. [Google Scholar] [CrossRef]
- Nomura, R.; Nakako, H.; Masuda, T. Design and synthesis of semiflexible substituted polyacetylenes with helical conformation. J. Mol. Catal. Chem. 2002, 190, 197–205. [Google Scholar] [CrossRef]
- Yoshida, Y.; Mawatari, Y.; Motoshige, A.; Motoshige, R.; Hiraoki, T.; Wagner, M.; Müllen, K.; Tabata, M. Accordion-like oscillation of contracted and stretched helices of polyacetylenes synchronized with the restricted rotation of side chains. J. Am. Chem. Soc. 2013, 135, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Mawatari, Y.; Motoshige, A.; Motodshige, R.; Hiraoki, T.; Tabata, M. Helix oscillation of polyacetylene esters detected by dynamic 1H NMR, IR, and UV-vis methods in solution. Polym. Chem. 2013, 4, 2982–2988. [Google Scholar] [CrossRef]
- Yoshida, Y.; Mawatari, Y.; Sasaki, T.; Hiraoki, T.; Wagner, M.; Müllen, K.; Tabata, M. Strictly alternating sequences when copolymerizing racemic and chiral acetylene monomers with an organo-rhodium catalyst. Macromolecules 2017, 50, 1291–1301. [Google Scholar] [CrossRef]
- Shao, Y.; Molnar, L.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.; Gilbert, A.; Slipchenko, L.; Levchenko, S.; O’Neill, D.P.; et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.A.; Cîrcu, V.; Weber, R.; Varnali, T.; Carlton, L. Tris(triphenylphosphine)rhodium cyano and triphenylcyanoborate complexes: Structures and a DFT study. J. Chem. Crystallogr. 2002, 32, 273–278. [Google Scholar] [CrossRef]





| Polymer | Yield 2 | Mn3 | Mw/Mn3 | Cis4 |
|---|---|---|---|---|
| (%) | (×10−5) | (%) | ||
| POP(1) | 74 | 1.3 | 3.1 | 84 |
| POP(2) | 73 | 2.1 | 6.8 | 82 |
| POP(3) | 71 | 0.9 | 4.4 | 83 |
| POP(4) | 42 | 0.7 | 2.5 | 85 |
| POP(5) | 58 | 3.5 | 3.7 | 84 |
| POP(6) | 53 | 0.9 | 3.5 | 83 |
| Solvent | Yield 2 | Mn3 | Mw/Mn3 | Cis4 |
|---|---|---|---|---|
| (%) | (×10−5) | (%) | ||
| EtOH | 56 | 2.37 | 7.30 | 73 |
| IPA | 20 | 1.31 | 17.5 | 71 |
| THF | 8 | 0.02 | 1.39 | 53 |
| EtOAc | trace | - | - | - |
| MeCN | 52 | 2.84 | 2.65 | 63 |
| DMF | 71 | 1.85 | 11.2 | 78 |
| Water | 51 | 2.17 | 7.60 | 60 |
| Toluene | trace | - | - | - |
| Acetone | 12 | 0.81 | 3.35 | 49 |
| CHCl3 | trace | - | - | - |
| DEGMA | trace | - | - | - |
| DEGME | 16 | 0.77 | 2.33 | 58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, Y.; Mawatari, Y.; Tabata, M. Proposed Mechanism for the High-Yield Polymerization of Oxyethyl Propiolates with Rh Complex Catalyst Using the Density Functional Theory Method. Polymers 2019, 11, 93. https://doi.org/10.3390/polym11010093
Yoshida Y, Mawatari Y, Tabata M. Proposed Mechanism for the High-Yield Polymerization of Oxyethyl Propiolates with Rh Complex Catalyst Using the Density Functional Theory Method. Polymers. 2019; 11(1):93. https://doi.org/10.3390/polym11010093
Chicago/Turabian StyleYoshida, Yoshiaki, Yasuteru Mawatari, and Masayoshi Tabata. 2019. "Proposed Mechanism for the High-Yield Polymerization of Oxyethyl Propiolates with Rh Complex Catalyst Using the Density Functional Theory Method" Polymers 11, no. 1: 93. https://doi.org/10.3390/polym11010093