Basic Properties of a New Polymer Gel for 3D-Dosimetry at High Dose-Rates Typical for FFF Irradiation Based on Dithiothreitol and Methacrylic Acid (MAGADIT): Sensitivity, Range, Reproducibility, Accuracy, Dose Rate Effect and Impact of Oxygen Scavenger
Abstract
:1. Introduction
1.1. Principles of MR-Based Polymer Gel Dosimetry: An Introduction
1.2. Actual Status in Polymer Gel Dosimetry with Respect to Dose Rate and Motivation
2. Materials and Methods
2.1. Gel Manufacturing
2.2. Irradiation and MR-Dosimetric Evaluation
2.2.1. Low Energy 200 kV-Protocol (Yxlon) for Basic Properties of MAGADIT
Calibration
Reproducibility and Accuracy
Dose Rate Dependence
MRI Measurements on the Basic Properties
2.2.2. Dosimetry of a Small Sized 5 × 10 mm2 FFF Field of a LINAC Used for Clinical Radiation therapy
3. Results
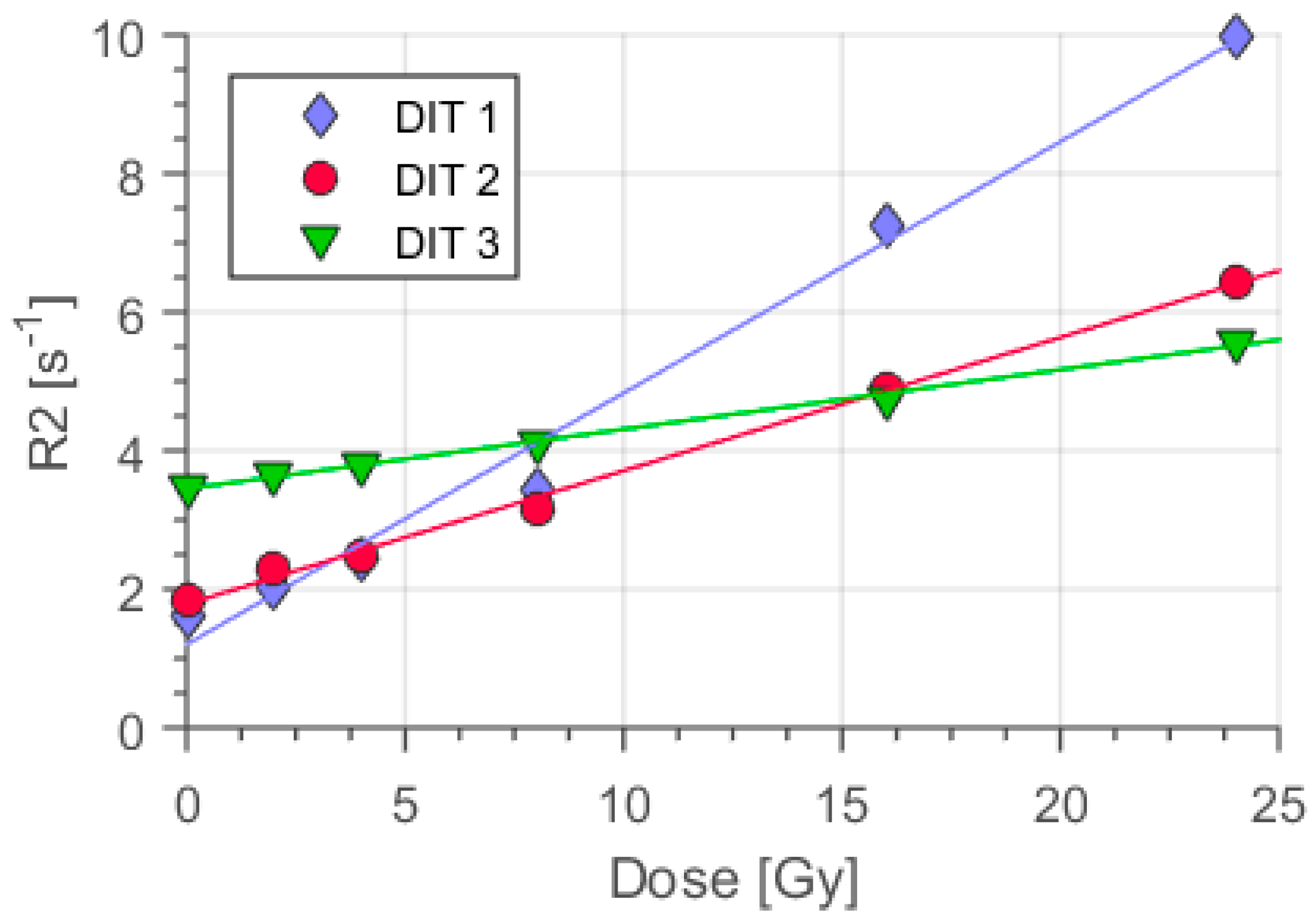
3.1. The Impact of the Oxygen Scavenger Concentration on Polymer Gel Sensitivity
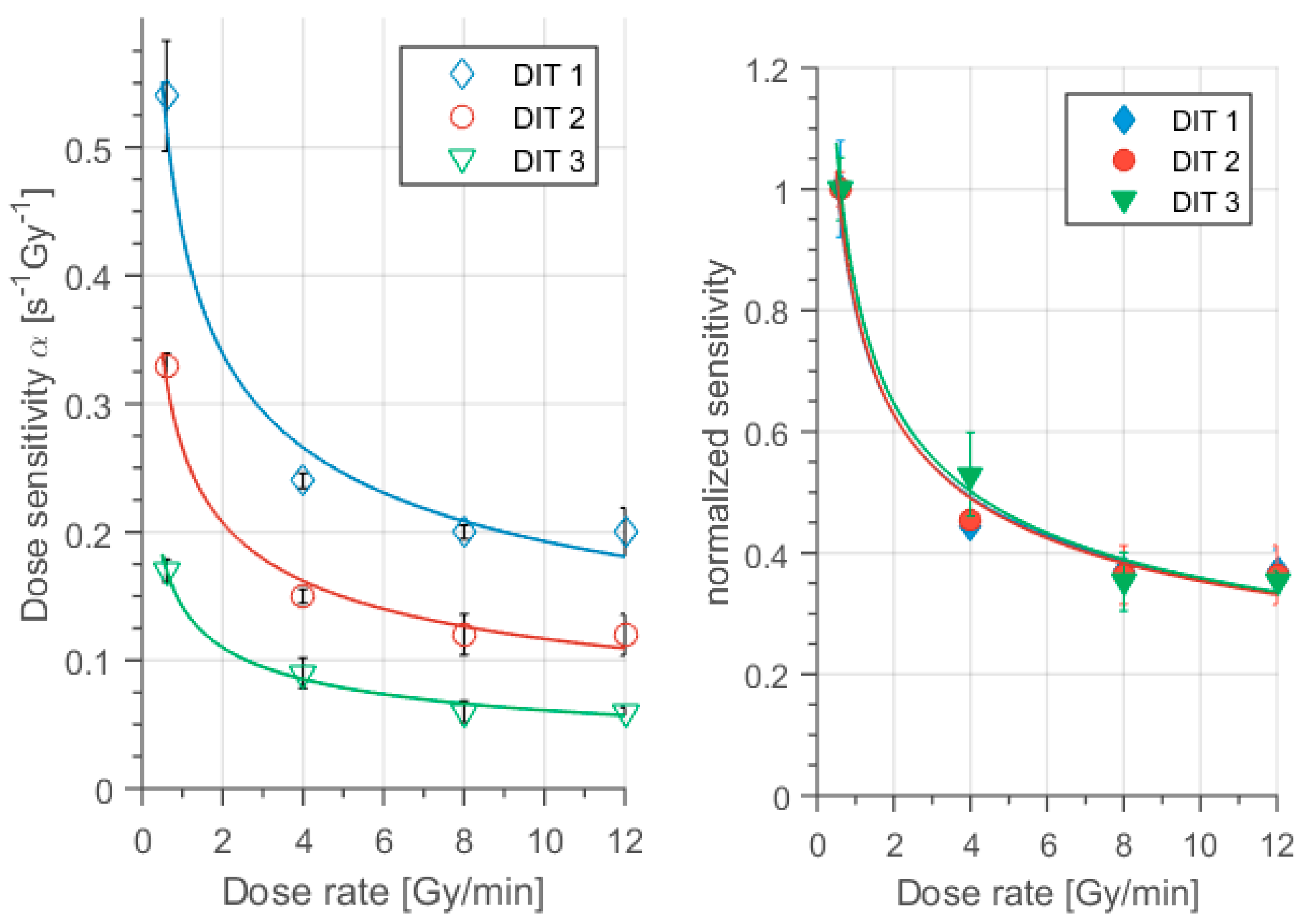
3.2. Dose Rate Dependence of the Dose Response in Dithio Gel Samples
3.3. Reproducibility and Accuracy
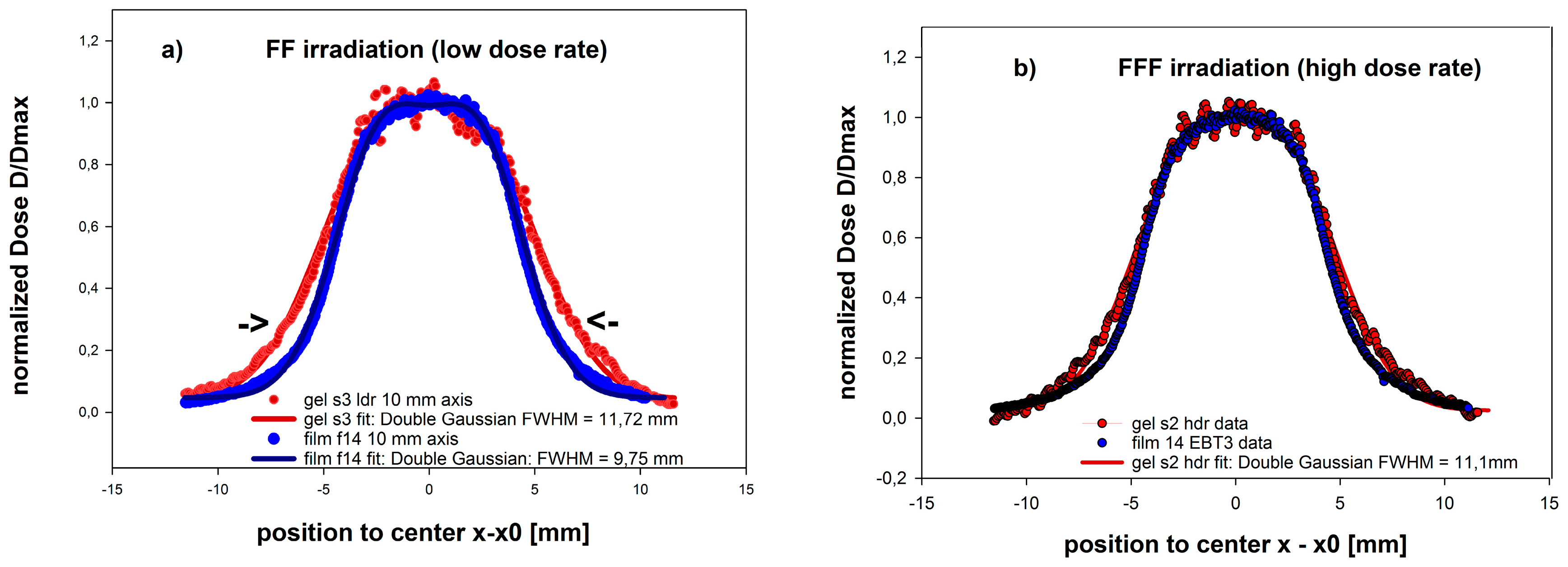
3.4. Dosimetry of a Small Sized 5 × 10 mm2 FFF Field of a LINAC used for Clinical Radiation Therapy
- (a)
- The FFF type irradiation protocol at a dose rate of 15.3 Gy/min (FFF) and;
- (b)
- A standard high dose rate (4 Gy/min) protocol (FF).
4. Discussion
4.1. MAGADIT in Comparison to Existing Methacrylic Acid Based Polymer Gels (MAG)
- (1).
- There was a strong dose rate-effect for dose rates in between = 0.6 Gy/min and about = 8 Gy/min for all investigated dithio concentrations.
- (2).
- This dose rate dependence of sensitivity almost disappeared within the accuracy of the results in the high dose rate range ≥ 8 Gy/min (Figure 5).
- (3).
- The change of normalized dose sensitivities with dose rate appeared to be independent of the oxygen scavenger concentration for MAGADIT dosimeters. This suggests a more universal correlation of the impact of oxygen scavengers (or presumably other additives) on dose rate and sensitivity evaluated as the slope of the dose response.
4.2. Limitations of the Study and Possible Future Investigations
4.2.1. Edge Enhancement Effect
4.2.2. Temperature Dependence
4.2.3. Stability of the Dose Response with Time after Manufacturing
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
MESH Terms
Abbreviations
| AMPS | 2-acrylamido-2-methyl-propane sulfonic acid |
| BIS | N,N-methylene-bis-acrylamide |
| α | Dose sensitivity (ΔR2/ΔD) |
| ∆α/∆ | Absolute dose rate dependence (ΔR2/ΔD)/Δ) of the dose sensitivity |
| BANG | Bis acryl amide nitrogen gel |
| c | Concentration of the oxygen scavenger |
| CT | Computer tomography |
| D | Dose |
| Dose rate: ( = ∆D/∆t) | |
| Dithio | Dithiothreitol (IUPAC name: 1,4-bis(sulfanyl)butane-2.3-diol) |
| DIT1 | Polymer gel with dithiothreitol at lowest concentration (cdit1 = 2 mmol/kg) |
| DIT2 | Polymer gel with dithiothreitol at medium concentration (cdit2 = 10 mmol/kg) |
| DIT3 | Polymer gel with dithiothreitol at highest concentration (cdit3 = 50 mmol/kg) |
| EBT3 | Classification specification of a commercial radiochromic film |
| FFF | Flattening filter free |
| FWHM | Full width at half maximum |
| IMRT | Intensity modulated radiation therapy |
| iVIPET | Vinylpyrrolidone and THPC with inorganic add-on |
| LINAC | Linear accelerator |
| MA | Methacrylic acid (IUPAC name: 2-methylprop-2-enoic acid) |
| MAGIC | Methacrylic and ascorbic acid in gelatin initiated by copper |
| MAGAS | Methacrylic acid gel and ascorbic acid |
| MAGAT | Methacrylic acid gel and THPC |
| MRI | Magnetic resonance imaging |
| MRPD | Magnetic resonance imaging based polymer gel dosimetry |
| NIPAM | N-isopropylacrylamide |
| NVP | N-vinylpyrrolidone |
| PAG | Polyacrylamide gel |
| PAGAT | Polyacrylamide gel and THPC |
| PTV | Planning target volume |
| ROI | Region of interest, frame in the image to be investigated |
| R2 | Transverse relaxation rate (R2 = 1/T2) |
| R20 | Transverse relaxation rate of the polymer gel at Dose D = 0 Gy |
| T2 | Transverse relaxation time (spin-spin relaxation time) |
| SSD | Source to surface distance |
| THPC | Tetrakis(hydroxymethyl)phosphonium chloride |
| TPS | Treatment planning system |
| VIC | Vinylpyrrolidone with computed tomography |
| VIPAR | VIPAR N-vinylpyrrolidone argon |
| VIPET | Vinylpyrrolidone and THPC |
| VMAT | Volumetric modulated arc therapy |
References
- Lee, N.; Xia, P.; Quivey, J.M.; Sultanem, K.; Poon, I.; Akazawa, C.; Akawzawa, P.; Weinberg, V.; Fu, K.K. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: An update of the UCSF experience. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 12–22. [Google Scholar] [CrossRef]
- Purdy, J.A. Dose to normal tissues outside the radiation therapy patient’s treated volume: A review of different radiation therapy techniques. Health Phys. 2008, 95, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.P.; Ceizyk, M.; Vos, P.; Vinh-Hung, V.; Davis, R.; Desai, A.; Abraham, D.; Krafft, S.P.; Jang, S.; Watchman, C.J.; et al. Effectiveness of image-guided radiotherapy for laryngeal sparing in head and neck cancer. Oral Oncol. 2010, 46, 283–286. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Fuks, Z.; Happersett, L.; Lee, H.J.; Ling, C.C.; Burman, C.M.; Hunt, M.; Wolfe, T.; Venkatraman, E.S.; Jackson, A.; et al. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother. Oncol. 2000, 55, 241–249. [Google Scholar] [CrossRef]
- Teh, B.S.; Woo, S.Y.; Butler, E.B. Intensity modulated radiation therapy (IMRT): A new promising technology in radiation oncology. Oncologist 1999, 4, 433–442. [Google Scholar] [PubMed]
- Cheung, K. Intensity modulated radiotherapy: Advantages, limitations and future developments. Biomed. Imaging Interv. J. 2006, 2, e19. [Google Scholar] [CrossRef] [PubMed]
- Bortfeld, T. IMRT: A review and preview. Phys. Med. Biol. 2006, 51, R363–R379. [Google Scholar] [CrossRef] [PubMed]
- Adamovics, J.; Maryanski, M.J. Characterisation of PRESAGE: A new 3-D radiochromic solid polymer dosemeter for ionising radiation. Radiat. Prot. Dosim. 2006, 120, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Avvakumov, N. Radiochromic leuco dye micelle hydrogels: I. Initial Investigation. Phys. Med. Biol. 2009, 54, 6773. [Google Scholar] [CrossRef] [PubMed]
- Babic, S.; Battista, J.; Jordan, K. Radiochromic leuco dye micelle hydrogels: II. Low diffusion rate leuco crystal violet gel. Phys. Med. Biol. 2009, 54, 6791–6808. [Google Scholar] [CrossRef]
- Penev, K.I.; Wang, M.; Mequanint, K. Tetrazolium salt monomers for gel dosimetry I: Principles. J. Phys. Conf. Ser. 2017, 847. [Google Scholar] [CrossRef]
- De Deene, Y.; Skyt, P.S.; Hil, R.; Booth, J.T. FlexyDos3D: A deformable anthropomorphic 3D radiation dosimeter: Radiation properties. Phys. Med. Biol. 2015, 60, 1543. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, L.J. Review of Fricke gel dosimeters. J. Phys. Conf. Ser. 2004, 3, 9–21. [Google Scholar] [CrossRef]
- d’Errico, F.; Lazzeri, L.; Dondi, D.; Mariani, M.; Marrale, M.; Souza, S.O.; Gambarini, G. Novel GTA-PVA Fricke gels for three-dimensional dose mapping in radiotherapy. Radiat. Meas. 2017, 106, 612–617. [Google Scholar] [CrossRef]
- Gore, J.C.; Ranade, M.; Maryansky, M.J.; Schulz, R.J. Radiation dose distributions in three dimensions from tomographic optical density scanning of polymer gels: I. Development of an optical scanner. Phys. Med. Biol. 1996, 41, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.; Venning, A.J.; Baldock, C. The dose response of normoxic polymer gel dosimeters measured using X-ray CT. Br. J. Radiol. 2005, 78, 623–630. [Google Scholar] [CrossRef]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef]
- Maryanski, M.J.; Schulz, R.J.; Ibbott, G.S.; Gatenby, J.C.; Xie, J.; Horton, D.; Gore, J.C. Magnetic resonance imaging of radiation dose distributions using a polymer-gel dosimeter. Phys. Med. Biol. 1994, 39, 1437. [Google Scholar] [CrossRef]
- Gore, J.C.; Kang, Y.S. Measurement of radiation dose distributions by nuclear magnetic resonance (NMR) imaging. Phys. Med. Biol. 1984, 29, 1189. [Google Scholar] [CrossRef]
- De Deene, Y.; De Wagter, C.; Van Duyse, B.; Derycke, S.; De Neve, W.; Achten, E. Three-dimensional dosimetry using polymer gel and magnetic resonance imaging applied to the verification of conformal radiation therapy in head-and-neck cancer. Radiother. Oncol. 1998, 48, 283–291. [Google Scholar] [CrossRef]
- Baldock, C.; Burford, R.P.; Billingham, N.; Wagner, G.S.; Patval, S.; Badawi, R.D.; Keevil, S.F. Experimental procedure for the manufacture and calibration of polyacrylamide gel (PAG) for magnetic resonance imaging (MRI) radiation dosimetry. Phys. Med. Biol. 1998, 43, 695–702. [Google Scholar] [CrossRef]
- Fong, M.P.; Derek, C.K.; Mark, D.D.; Gore, J.C. Polymer gels for magnetic resonance imaging of radiation dose distributions at normal room atmosphere. Phys. Med. Biol. 2001, 46, 3105. [Google Scholar] [CrossRef]
- Berg, A.; Ertl, A.; Moser, E. High-resolution polymer gel dosimetry by parameter selective MR-microimaging on a whole body scanner at 3T. Med. Phys. 2001, 28, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Senden, R.J.; De Jean, P.; McAuley, K.B.; Schreiner, L.J. Polymer gel dosimeters with reduced toxicity: A preliminary investigation of the NMR and optical dose response using different monomers. Phys. Med. Biol. 2006, 51, 3301–3314. [Google Scholar] [CrossRef] [PubMed]
- Pappas, E.; Maris, T.; Angelopoulos, A.; Paparigopoulou, M.; Sakelliou, L.; Sandilos, P.; Voyiatzi, S.; Vlachos, L. A new polymer gel for magnetic resonance imaging (MRI) radiation dosimetry. Phys. Med. Biol. 1999, 44, 2677. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Dudek, M.; Cłapa, M. On the development of a VIPARnd radiotherapy 3D polymer gel dosimeter. Phys. Med. Biol. 2017, 62, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Mizukami, S.; Eguchi, K.; Maeyama, T.; Hayashid, S.; Muraishi, H.; Terazaki, T.; Gomi, T. Dose distribution verification in high-dose-rate brachytherapy using a highly sensitive norrnoxic N-vinylpyrrolidone polymer gel dosimeter. Phys. Med. 2019, 57, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Pernkopf, M.; Waldhäusl, C.; Schmidt, W.; Moser, E. High resolution MR based polymer dosimetry versus film densitometry: A systematic study based on the modulation transfer function approach. Phys. Med. Biol. 2004, 49, 4087–4108. [Google Scholar] [CrossRef]
- De Deene, Y.D. Essential characteristics of polymer gel dosimeters. J. Phys. Conf. Ser. 2004, 3, 34–57. [Google Scholar] [CrossRef]
- Bayreder, C.; Schon, R.; Wieland, M.; Georg, D.; Moser, E.; Berg, A. The spatial resolution in dosimetry with normoxic polymer-gels investigated with the dose modulation transfer approach. Med. Phys. 2008, 35, 1756–1769. [Google Scholar] [CrossRef]
- Olding, T.; Holmes, O.; Dejean, P.; McAuley, K.B.; Nkongchu, K.; Santyr, G.; Schreiner, L.J. Small field dose delivery evaluations using cone beam optical computed tomography-based polymer gel dosimetry. Med. Phys. 2011, 36, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Razak, N.; Rahman, A.; Kandaiya, S.; Mustafa, I.; Yahaya, N.; Mahmoud, A.; Maizan, R. Accuracy and Precision of Magat Gel As a Dosimeter. Mater. Sci. Res. India 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Watanabe, Y.; Warmington, L.; Gopishankar, N. Three-dimensional radiation dosimetry using polymer gel and solid radiochromic polymer: From basics to clinical applications. World J. Radiol. 2017, 9, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Baldock, C. Historical overview of the development of gel dosimetry: Another personal perspective. J. Phys. Conf. Ser. 2009, 164. [Google Scholar] [CrossRef] [Green Version]
- Jirasek, A.; McAuley, K.B.; Lepage, M. How does the chemistry of polymer gel dosimeters affect their performance? J. Phys. Conf. Ser. 2009, 164. [Google Scholar] [CrossRef]
- De Deene, Y.; Reynaert, N.; De Wagter, C. On the accuracy of monomer/polymer gel dosimetry in the proximity of a high-dose-rate 192Ir source. Phys. Med. Biol. 2001, 46, 2801–2825. [Google Scholar] [CrossRef]
- Spevacek, V.; Novotny, J., Jr.; Dvorak, P.; Novotny, J.; Vymazal, J.; Cechak, T. Temperature dependence of polymer-gel dosimeter nuclear magnetic resonance response. Med. Phys. 2001, 28, 2370–2378. [Google Scholar] [CrossRef]
- Berg, A.; Bayreder, C.; Georg, D.; Bankamp, A.; Wolber, G. Aspects of radiation beam quality and their effect on the dose response of polymer gels: Photons, electrons and fast neutrons. J. Phys. Conf. Ser. 2009, 164. [Google Scholar] [CrossRef]
- De Deene, Y.; Vergote, K.; Claeys, C.; De Wagter, C. The fundamental radiation properties of normoxic polymer gel dosimeters: A comparison between a methacrylic acid based gel and acrylamide based gels. Phys. Med. Biol. 2006, 51, 653–673. [Google Scholar] [CrossRef]
- Crescenti, R.A.; Scheib, S.G.; Schneider, U.; Gianolini, S. Introducing gel dosimetry in a clinical environment: Customization of polymer gel composition and magnetic resonance imaging parameters used for 3D dose verifications in radiosurgery and intensity modulated radiotherapy. Med. Phys. 2007, 34, 1286–1297. [Google Scholar] [CrossRef]
- Vandecasteele, J.; De Deene, Y. On the validity of 3D polymer gel dosimetry: I. reproducibility study. Phys. Med. Biol. 2013, 58, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Kipouros, P.; Pappas, E.; Baras, P.; Hatzipanayoti, D.; Karaiskos, P.; Sakelliou, L.; Sandilos, P.; Seimenis, I. Wide dynamic dose range of VIPAR polymer gel dosimetry. Phys. Med. Biol. 2001, 46, 2143–2159. [Google Scholar] [CrossRef] [PubMed]
- Grebe, G.; Pfaender, M.; Roll, M.; Luedemann, L.; Wurm, R.E. Dynamic arc radiosurgery and radiotherapy: Commissioning and verification of dose distributions. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1451–1460. [Google Scholar] [CrossRef]
- Thomas, A.; Newton, J.; Adamovics, J.; Oldham, M. Commissioning and benchmarking a 3D dosimetry system for clinical use. Med. Phys. 2011, 38, 4846–4857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, J.; Oldham, M.; Thomas, A.; Li, Y.; Adamovics, J.; Kirsch, D.G.; Das, S. Commissioning a small-field biological irradiator using point, 2D, and 3D dosimetry techniques. Med. Phys. 2011, 38, 6754–6762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, H.; Hilts, M.; Jirasek, A. SU-E-T-70: Commissioning a Multislice CT Scanner for X-ray CT Polymer Gel Dosimetry. Med. Phys. 2014, 41, 238. [Google Scholar] [CrossRef]
- Khan, M.; Heilemann, G.; Kuess, P.; Georg, D.; Berg, A. The impact of the oxygen scavenger on the dose-rate dependence and dose sensitivity of MAGIC type polymer gels. Phys. Med. Biol. 2018, 63, 06NT1. [Google Scholar] [CrossRef]
- O’Neil, M.J.; Heckelman, P.E.; Koch, C.B.; Roman, K.J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. J. Am. Chem. Soc. 2007, 129, 2197. [Google Scholar]
- Fruton, J.S. Oxidation-reduction potentials of ascorbic acid. J. Biol. Chem. 1934, 105, 79–85. [Google Scholar]
- Katsumi, H.; Nishikawa, M.; Nishiyama, K.; Hirosaki, R.; Nagamine, N.; Okamoto, H.; Mizugichi, H.; Kusamori, K.; Yasui, H.; Yamashita, F.; et al. Development of PEGylated serum albumin with multiple reduced thiols as a long-circulating scavenger of reactive oxygen species for the treatment of fulminant hepatic failure in mice. Free Radic. Biol. Med. 2014, 69, 318–323. [Google Scholar] [CrossRef]
- Cleland, W.W. Dithiothreitol, A New Protective Reagent for SH Groups. Biochemistry 1964, 3, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Bayreder, C.; Georg, D.; Moser, E.; Berg, A. Basic investigations on the performance of a normoxic polymer gel with tetrakis-hydroxy-methyl-phosphonium chloride as an oxygen scavenger: Reproducibility, accuracy, stability, and dose rate dependence. Med. Phys. 2006, 33, 2506–2518. [Google Scholar] [CrossRef] [PubMed]
- Kuess, P.; Bozsaky, E.; Hopfgartner, J.; Seifritz, G.; Dorr, W.; Georg, D. Dosimetric challenges of small animal irradiation with a commercial X-ray unit. Z. Med. Phys. 2014, 24, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Farajollahi, A.R.; Bonnett, D.E.; Ratcliffe, A.J.; Aukett, R.J.; Mills, J.A. An investigation into the use of polymer gel dosimetry in low dose rate brachytherapy. Br. J. Radiol. 1999, 72, 1085–1092. [Google Scholar] [CrossRef]
- Milford, D.; Rosbach, N.; Bendszus, M.; Heiland, S. Mono-Exponential Fitting in T2-Relaxometry: Relevance of Offset and First Echo. PLoS ONE 2015, 10, e0145255. [Google Scholar] [CrossRef]
- Dreindl, R.; Georg, D.; Stock, M. Radiochromic film dosimetry: Considerations on precision and accuracy for EBT2 and EBT3 type films. Z. Med. Phys. 2014, 24, 153–163. [Google Scholar] [CrossRef]
- Deene, Y.D.; Hurley, C.; Venning, A.; Vergote, K.; Mather, M.; Healy, B.J.; Baldock, C. A basic study of some normoxic polymer gel dosimeters. Phys. Med. Biol. 2002, 47, 3441–3463. [Google Scholar] [CrossRef]
- Karlsson, A.; Gustavsson, H.; Mansson, S.; McAuley, K.B.; Back, S.A.J. Dose integration characteristics in normoxic polymer gel dosimetry investigated using sequential beam irradiation. Phys. Med. Biol. 2007, 52, 4697–4706. [Google Scholar] [CrossRef]
- Abtahi, S.M. Characteristics of a novel polymer gel dosimeter formula for MRI scanning: Dosimetry, toxicity and temporal stability of response. Phys. Med. 2016, 32, 1156–1161. [Google Scholar] [CrossRef]
- Georg, D.; Knoos, T.; McClean, B. Current status and future perspective of flattening filter free photon beams. Med. Phys. 2011, 38, 1280–1293. [Google Scholar] [CrossRef]
- Zehtabian, M.; Faghihi, R.; Zahmatkesh, M.H.; Meigooni, A.S.; Mosleh-Shirazi, M.A.; Mehdizadeh, S.; Sina, S.; Bagheri, S. Investigation of the dose rate dependency of the PAGAT gel dosimeter at low dose rates. Radiat. Meas. 2012, 47, 139–144. [Google Scholar] [CrossRef]
- Prendergast, B.M.; Fiveash, J.B.; Popple, R.A.; Clark, G.M.; Thomas, E.M.; Minnich, D.J.; Jacob, R.; Spencer, S.A.; Bonner, J.A.; Dobelbower, M.C. Flattening filter-free linac improves treatment delivery efficiency in stereotactic body radiation therapy. J. Appl. Clin. Med. Phys. 2013, 14, 64–71. [Google Scholar] [CrossRef]
- Jirasek, A.; Johnston, H.; Hilts, M. Dose rate properties of NIPAM-based x-ray CT polymer gel dosimeters. Phys. Med. Biol. 2015, 60, 4399–4411. [Google Scholar] [CrossRef] [PubMed]
- Massilon, J.L.; Minniti, R.; Soares, C.G.; Maryanski, M.J.; Robertson, S. Characteristics of a new polymer gel for high-dose gradient dosimetry using a micro optical CT scanner. Appl. Radiat. Isotopes 2009, 68, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Fuxman, A.M.; McAuley, K.B.; Schreiner, L.J. Modeling of polyacrylamide gel dosimeters with spatially non-uniform radiation dose distributions. Chem. Eng. Sci. 2005, 60, 1277–1293. [Google Scholar] [CrossRef]
- Vergote, K.; De Deene, Y.; Vanden Bussche, E.; De Wagter, C. On the relation between the spatial dose integrity and the temporal instability of polymer gel dosimeters. Phys. Med. Biol. 2004, 49, 4507–4522. [Google Scholar] [CrossRef] [Green Version]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys. Rev. 1948, 7, 679–746. [Google Scholar] [CrossRef]
- Jaszczak, M.; Kolesińska, B.; Wach, R.; Maras, P.; Dudek, M.; Kozicki, M. Examination of THPC as an oxygen scavenger impacting VIC dosimeter thermal stability and comparison of NVP-containing polymer gel dosimeters. Phys. Med. Biol. 2019, 64, 035019. [Google Scholar] [CrossRef]
- De Deene, Y.; Hanselaer, P.; De Wagter, C.; Achten, E.; De Neve, W. An investigation of the chemical stability of a monomer/polymer gel dosimeter. Phys. Med. Biol. 2000, 45, 859–878. [Google Scholar] [CrossRef]
- Khan, M. Magnetic Resonance Imaging Based Polymer Gel Dosimetry for Radiation Therapy: Basic Properties of New Normoxic Polymer Gels for High Dose Rates. Ph.D. Thesis, Medical University of Vienna, Vienna, Austria, May 2018. [Google Scholar]




| Ingredients | DIT 1 | DIT 2 | DIT 3 |
|---|---|---|---|
| Distilled water | 82% | 82% | 82% |
| Gelatin | 10% | 10% | 10% |
| Methacrylic acid | 8% | 8% | 8% |
| Dithio | 2 mmol/kg | 10 mmol/kg | 50 mmol/kg |
| Dose Rate (Gy/min) | Dose Sensitivity α (s−1 Gy−1) | ||
|---|---|---|---|
| DIT1 | DIT2 | DIT3 | |
| 0.6 | 0.54 ± 0.04 | 0.33 ± 0.01 | 0.17 ± 0.01 |
| 4 | 0.24 ± 0.01 | 0.15 ± 0.01 | 0.09 ± 0.01 |
| 8 | 0.20 ± 0.01 | 0.12 ± 0.02 | 0.06 ± 0.01 |
| 12 | 0.20 ± 0.02 | 0.12 ± 0.02 | 0.06 ± 0.00 |
| Gel Type | Relative Accuracy ar (%) | Reproducibility σr (%) at 5 Gy/min | Average R2 of 4 Samples (s−1) and Standard Deviation | |
|---|---|---|---|---|
| 5 Gy/min | 2 Gy/min | |||
| DIT1 | 3.5 | 20.2 | 3.3 | 8.5 ± 0.28 |
| DIT2 | 7.4 | 23.6 | 6 | 5.5 ± 0.33 |
| DIT3 | 7.9 | 16.0 | 3.6 | 5.3 ± 0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.; Heilemann, G.; Lechner, W.; Georg, D.; Berg, A.G. Basic Properties of a New Polymer Gel for 3D-Dosimetry at High Dose-Rates Typical for FFF Irradiation Based on Dithiothreitol and Methacrylic Acid (MAGADIT): Sensitivity, Range, Reproducibility, Accuracy, Dose Rate Effect and Impact of Oxygen Scavenger. Polymers 2019, 11, 1717. https://doi.org/10.3390/polym11101717
Khan M, Heilemann G, Lechner W, Georg D, Berg AG. Basic Properties of a New Polymer Gel for 3D-Dosimetry at High Dose-Rates Typical for FFF Irradiation Based on Dithiothreitol and Methacrylic Acid (MAGADIT): Sensitivity, Range, Reproducibility, Accuracy, Dose Rate Effect and Impact of Oxygen Scavenger. Polymers. 2019; 11(10):1717. https://doi.org/10.3390/polym11101717
Chicago/Turabian StyleKhan, Muzafar, Gerd Heilemann, Wolfgang Lechner, Dietmar Georg, and Andreas Georg Berg. 2019. "Basic Properties of a New Polymer Gel for 3D-Dosimetry at High Dose-Rates Typical for FFF Irradiation Based on Dithiothreitol and Methacrylic Acid (MAGADIT): Sensitivity, Range, Reproducibility, Accuracy, Dose Rate Effect and Impact of Oxygen Scavenger" Polymers 11, no. 10: 1717. https://doi.org/10.3390/polym11101717





