Lipase-Catalyzed Synthesis of Renewable Plant Oil-Based Polyamides
Abstract
:1. Introduction
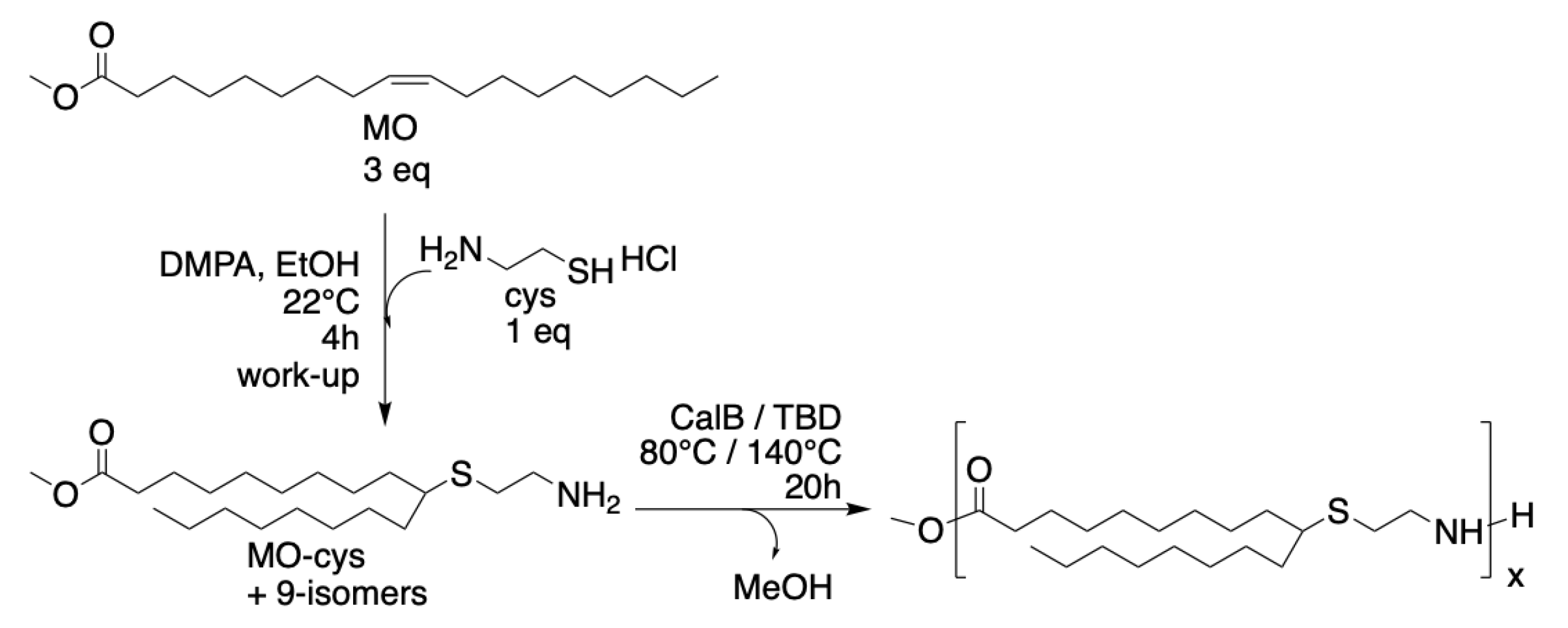
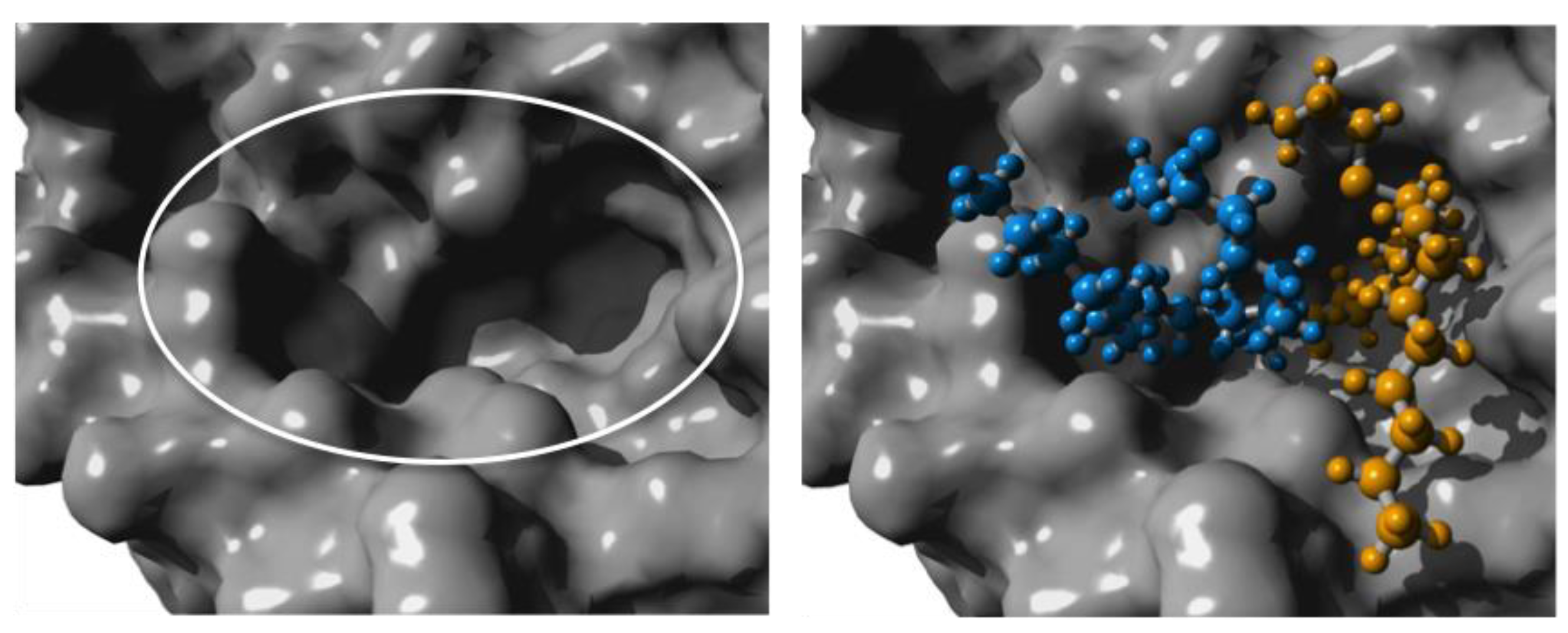
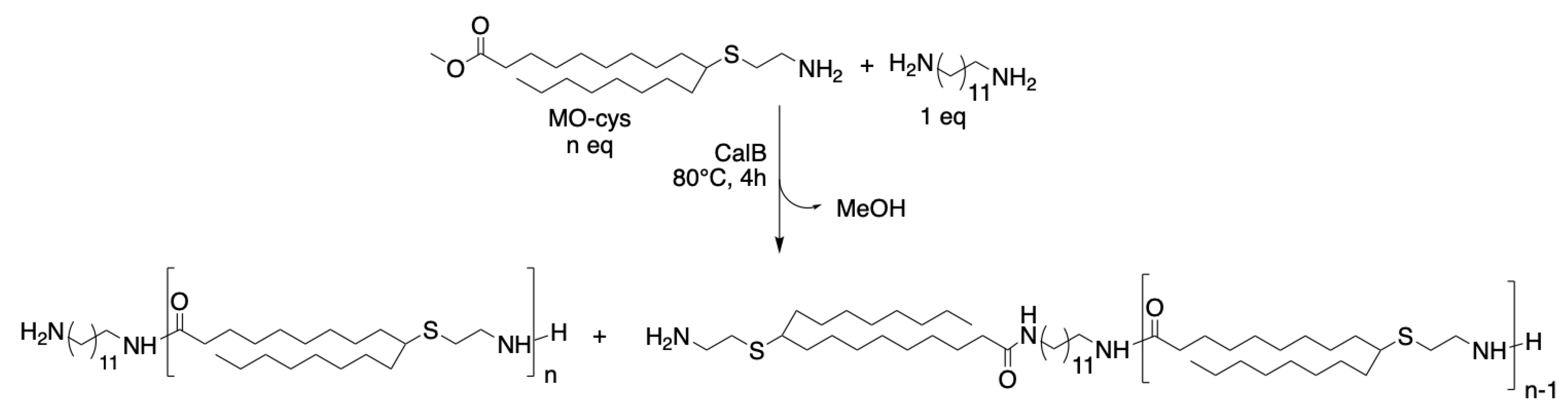
2. Results
3. Materials and Methods
3.1. Monomer Synthesis: Thiol-ene Addition of Cysteamine to Methyl Oleate
3.2. General Procedure for Polymerizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Kirchhoff, M.M.; Williamson, T.C. Catalysis as a foundational pillar of green chemistry. Appl. Catal. A-Gen. 2001, 221, 3–13. [Google Scholar] [CrossRef]
- Llevot, A.; Meier, M.A. Renewability–a principle of utmost importance! Green Chemistry. 2016, 18, 4800–4803. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Wu, D.; Xing, Z.; Zhou, Y.; Shi, W.; Li, Q. Chemoenzymatic synthesis of polymeric materials using lipases as catalysts: A review. Biotechnol. Adv. 2014, 32, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Douka, A.; Vouyiouka, S.; Papaspyridi, L.M.; Papaspyrides, C.D. A review on enzymatic polymerization to produce polycondensation polymers: The case of aliphatic polyesters, polyamides and polyesteramides. Prog. Polym. Sci. 2018, 79, 1–25. [Google Scholar] [CrossRef]
- Shoda, S.I.; Uyama, H.; Kadokawa, J.I.; Kimura, S.; Kobayashi, S. Enzymes as green catalysts for precision macromolecular synthesis. Chem. Rev. 2016, 116, 2307–2413. [Google Scholar] [CrossRef]
- Jiang, Y.; Loos, K. Enzymatic synthesis of biobased polyesters and polyamides. Polymers 2016, 8, 243. [Google Scholar] [CrossRef]
- Ajima, A.; Yoshimoto, T.; Takahashi, K.; Tamaura, Y.; Saito, Y.; Inada, Y. Polymerization of 10-hydroxydecanoic acid in benzene with polyethylene glycol-modified lipase. Biotechnol. Lett. 1985, 7, 3–6. [Google Scholar] [CrossRef]
- Okumura, S.; Iwai, M.; Tominaga, Y. Synthesis of ester oligomer by Aspergillus niger lipase. Agric. Biol Chem. 1984, 48, 2805–2808. [Google Scholar] [CrossRef]
- Ragupathy, L.; Ziener, U.; Dyllick-Brenzinger, R.; von Vacano, B.; Landfester, K. Enzyme-catalyzed polymerizations at higher temperatures: Synthetic methods to produce polyamides and new poly (amide-co-ester)s. J. Mol. Catal. B-Enzym. 2012, 76, 94–105. [Google Scholar] [CrossRef]
- Stavila, E.; Arsyi, R.; Petrovic, D.; Loos, K. Fusarium solani pisi cutinase-catalyzed synthesis of polyamides. Eur. Polym. J. 2013, 49, 34–42. [Google Scholar] [CrossRef]
- Jiang, Y.; Maniar, D.; Woortman, A.J.; Loos, K. Enzymatic synthesis of 2, 5-furandicarboxylic acid-based semi-aromatic polyamides: Enzymatic polymerization kinetics, effect of diamine chain length and thermal properties. RSC Adv. 2016, 6, 41–53. [Google Scholar] [CrossRef]
- Jiang, Y.; Maniar, D.; Woortman, A.J.; Alberda van Ekenstein, G.O.; Loos, K. Enzymatic polymerization of furan-2, 5-dicarboxylic acid-based furanic-aliphatic polyamides as sustainable alternatives to polyphthalamides. Biomacromolecules. 2015, 16, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, B.; Li, Y.; Wang, Y.; Lin, X.; Wu, Q. Solvent-free lipase-catalyzed synthesis: Unique properties of enantiopure D-and L-polyaspartates and their complexation. Biomacromolecules. 2015, 17, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Totsingan, F.; Centore, R.; Gross, R.A. CAL-B catalyzed regioselective bulk polymerization of l-aspartic acid diethyl ester to α-linked polypeptides. Chem. Commun. 2017, 53, 4030–4033. [Google Scholar] [CrossRef] [PubMed]
- Türünç, O.; Firdaus, M.; Klein, G.; Meier, M.A. Fatty acid derived renewable polyamides via thiol–ene additions. Green Chem. 2012, 14, 2577–2583. [Google Scholar] [CrossRef]
- Pratt, R.C.; Lohmeijer, B.G.; Long, D.A.; Waymouth, R.M.; Hedrick, J.L. Triazabicyclodecene: A simple bifunctional organocatalyst for acyl transfer and ring-opening polymerization of cyclic esters. J. Am. Chem. Soc. 2006, 128, 4556–4557. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Odelius, K. Exploiting Ring-Opening Aminolysis–Condensation as a Polymerization Pathway to Structurally Diverse Biobased Polyamides. Biomacromolecules. 2018, 19, 1573–1581. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Claudino, M.; Johansson, M.; Jonsson, M. Thiol–ene coupling of 1, 2-disubstituted alkene monomers: The kinetic effect of cis/trans-isomer structures. Eur. Polym. J. 2010, 46, 2321–2332. [Google Scholar] [CrossRef]
- Claudino, M.; van der Meulen, I.; Trey, S.; Jonsson, M.; Heise, A.; Johansson, M. Photoinduced thiol–ene crosslinking of globalide/ε-caprolactone copolymers: Curing performance and resulting thermoset properties. J. Polym. Sci. Pol. Chem. 2012, 50, 16–24. [Google Scholar] [CrossRef]
- Hedfors, C.; Hult, K.; Martinelle, M. Lipase chemoselectivity towards alcohol and thiol acyl acceptors in a transacylation reaction. J. Mol. Catal. B-Enzym. 2010, 66, 120–123. [Google Scholar] [CrossRef]
- Montalbetti, C.A.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron. 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Mulalee, S.; Srisuwan, P.; Phisalaphong, M. Influences of operating conditions on biocatalytic activity and reusability of Novozym 435 for esterification of free fatty acids with short-chain alcohols: A case study of palm fatty acid distillate. Chin. J. Chem. Eng. 2015, 23, 1851–1856. [Google Scholar] [CrossRef]
- Åkerman, C.O.; Hagström, A.E.; Mollaahmad, M.A.; Karlsson, S.; Hatti-Kaul, R. Biolubricant synthesis using immobilised lipase: Process optimisation of trimethylolpropane oleate production. Process. Biochem. 2011, 46, 2225–2231. [Google Scholar] [CrossRef]
- Uppenberg, J.; Oehrner, N.; Norin, M.; Hult, K.; Kleywegt, G.J.; Patkar, S.; Jones, T.A. Crystallographic and molecular-modeling studies of lipase B from Candida antarctica reveal a stereospecificity pocket for secondary alcohols. Biochemistry. 1995, 34, 16838–16851. [Google Scholar] [CrossRef]
- Winnacker, M.; Rieger, B. Biobased polyamides: Recent advances in basic and applied research. Macromol. Rapid Comm. 2016, 37, 1391–1413. [Google Scholar] [CrossRef]

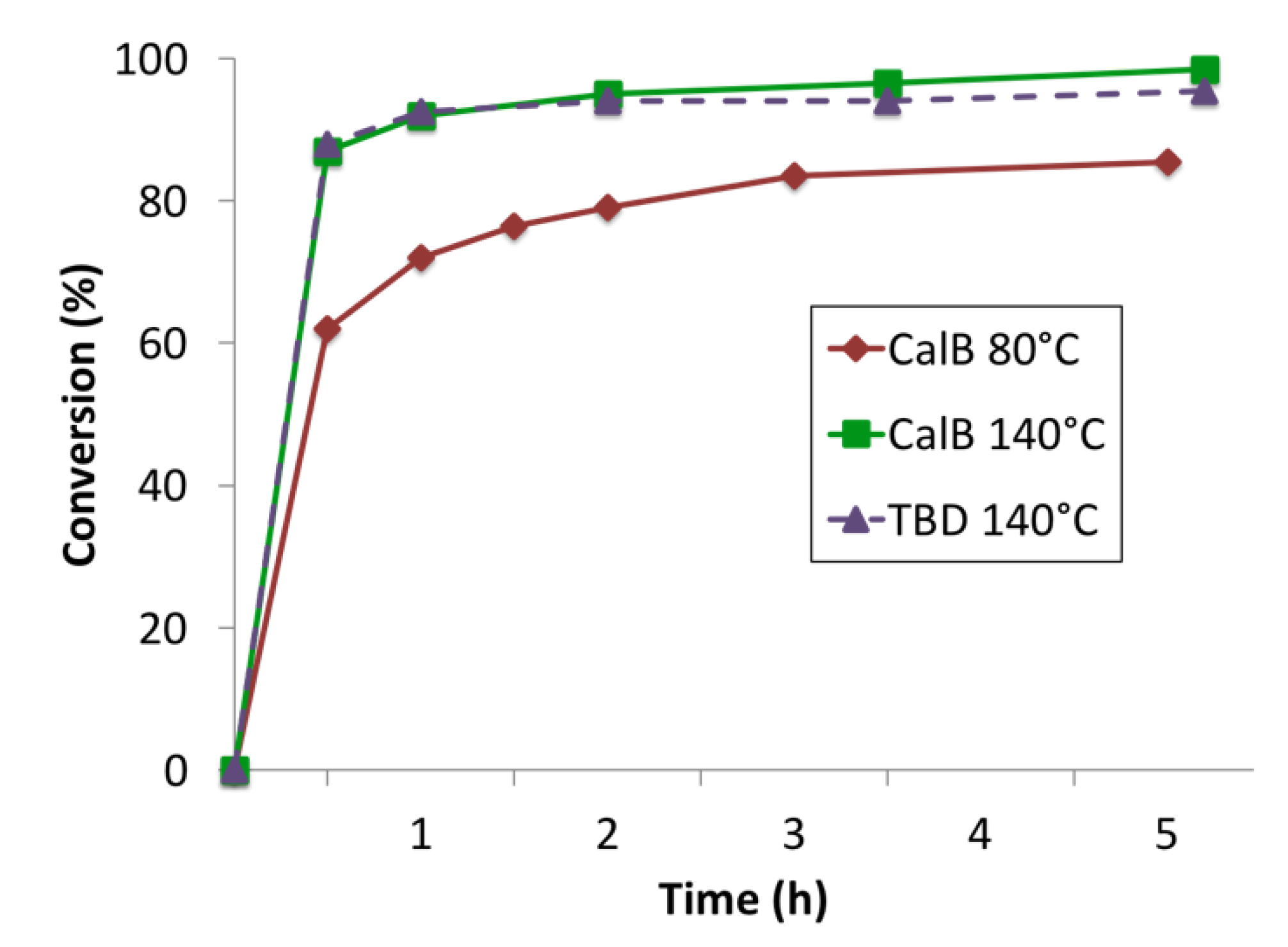


| Entry | Catalyst | mol% Catalyst a | Ratio MO-cys:DA | Temp (°C) | Time (h) | Conversion b (%) | DP b |
|---|---|---|---|---|---|---|---|
| 1 | CalB | 0.004 | 1:0 | 80 | 20 | >85 | >6 |
| 2 | CalB | 0.004 | 1:0 | 140 | 20 | >95 | >20 |
| 3 | TBD | 5 | 1:0 | 80 | 20 | >85 | >6 |
| 4 | TBD | 5 | 1:0 | 140 | 20 | >95 | >20 |
| 5 | CalB | 0.004 | 2:1 | 80 | 4 | >98 | 2 |
| 6 | CalB | 0.004 | 4:1 | 80 | 4 | >98 | 4 |
| 7 | - c | - | 1:0 | 80 | 20 | 20 | - |
| 8 | - c | - | 1:0 | 140 | 20 | 30 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finnveden, M.; Hendil-Forssell, P.; Claudino, M.; Johansson, M.; Martinelle, M. Lipase-Catalyzed Synthesis of Renewable Plant Oil-Based Polyamides. Polymers 2019, 11, 1730. https://doi.org/10.3390/polym11111730
Finnveden M, Hendil-Forssell P, Claudino M, Johansson M, Martinelle M. Lipase-Catalyzed Synthesis of Renewable Plant Oil-Based Polyamides. Polymers. 2019; 11(11):1730. https://doi.org/10.3390/polym11111730
Chicago/Turabian StyleFinnveden, Maja, Peter Hendil-Forssell, Mauro Claudino, Mats Johansson, and Mats Martinelle. 2019. "Lipase-Catalyzed Synthesis of Renewable Plant Oil-Based Polyamides" Polymers 11, no. 11: 1730. https://doi.org/10.3390/polym11111730






