Facile Synthesis of Nitrogen-Doped Carbon Quantum Dots with Chitosan for Fluorescent Detection of Fe3+
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of N-CQDs
2.3. Material Characterization
2.4. Detections of Fe3+
2.5. Real Sample Analysis
3. Results and Discussion
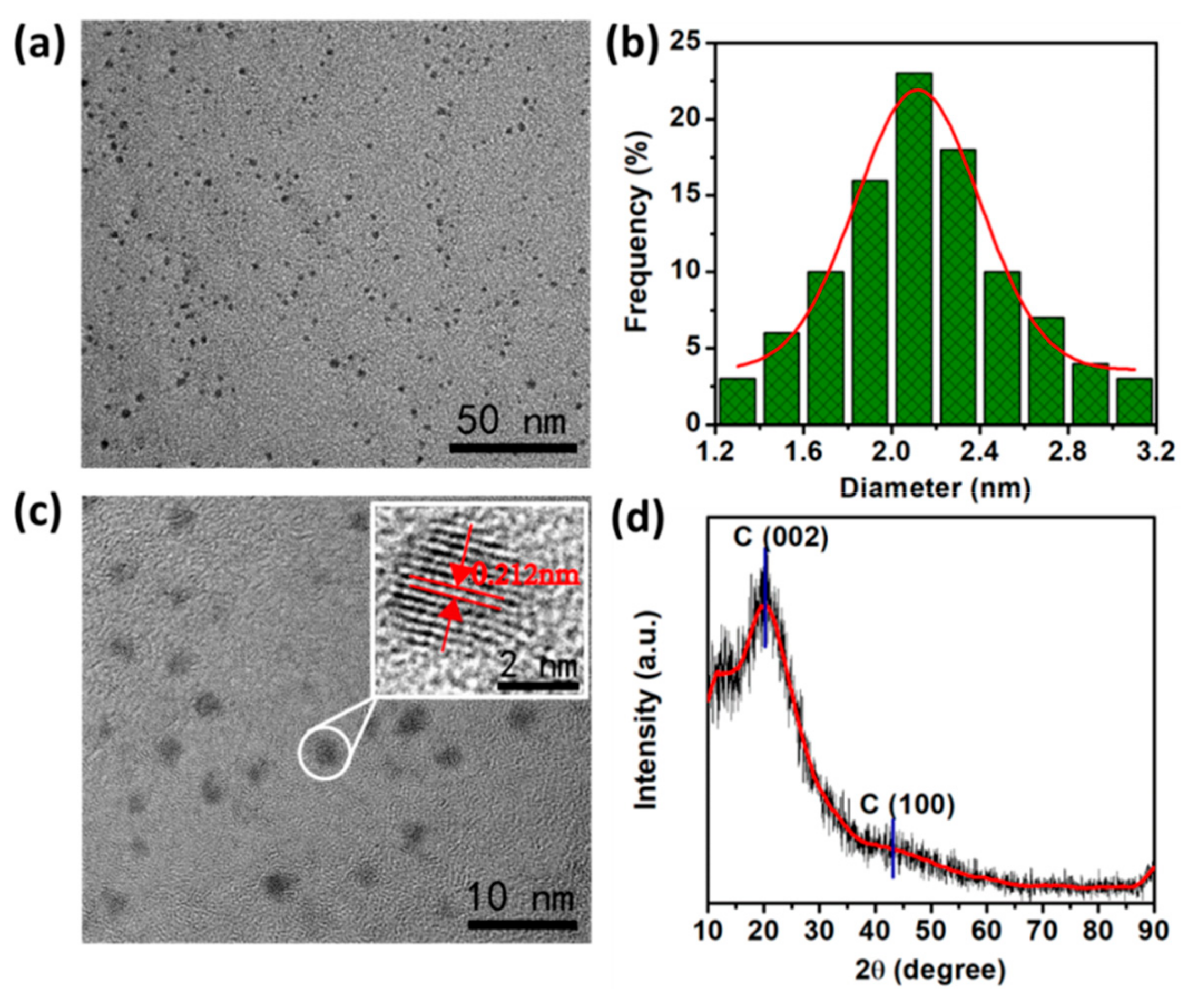
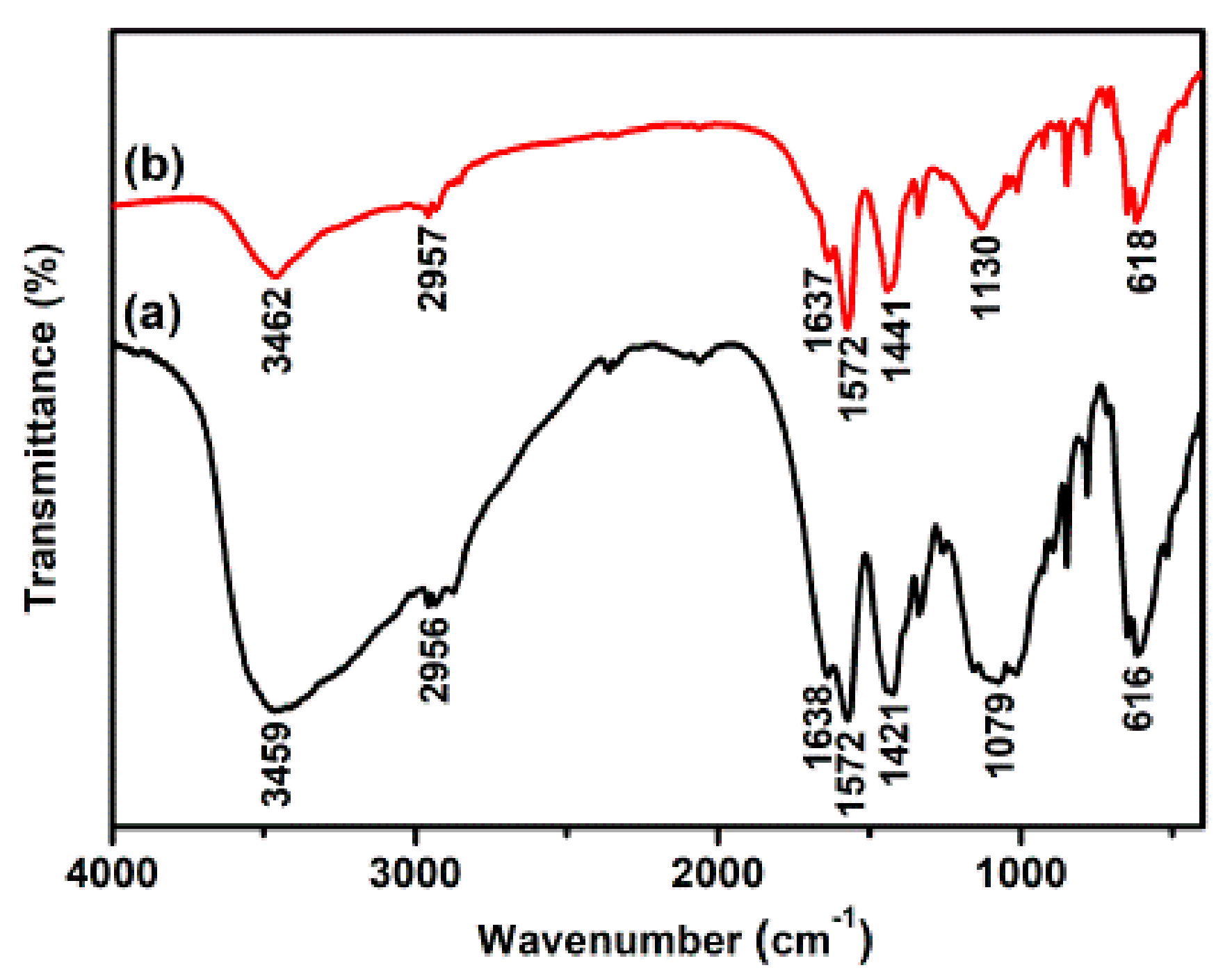
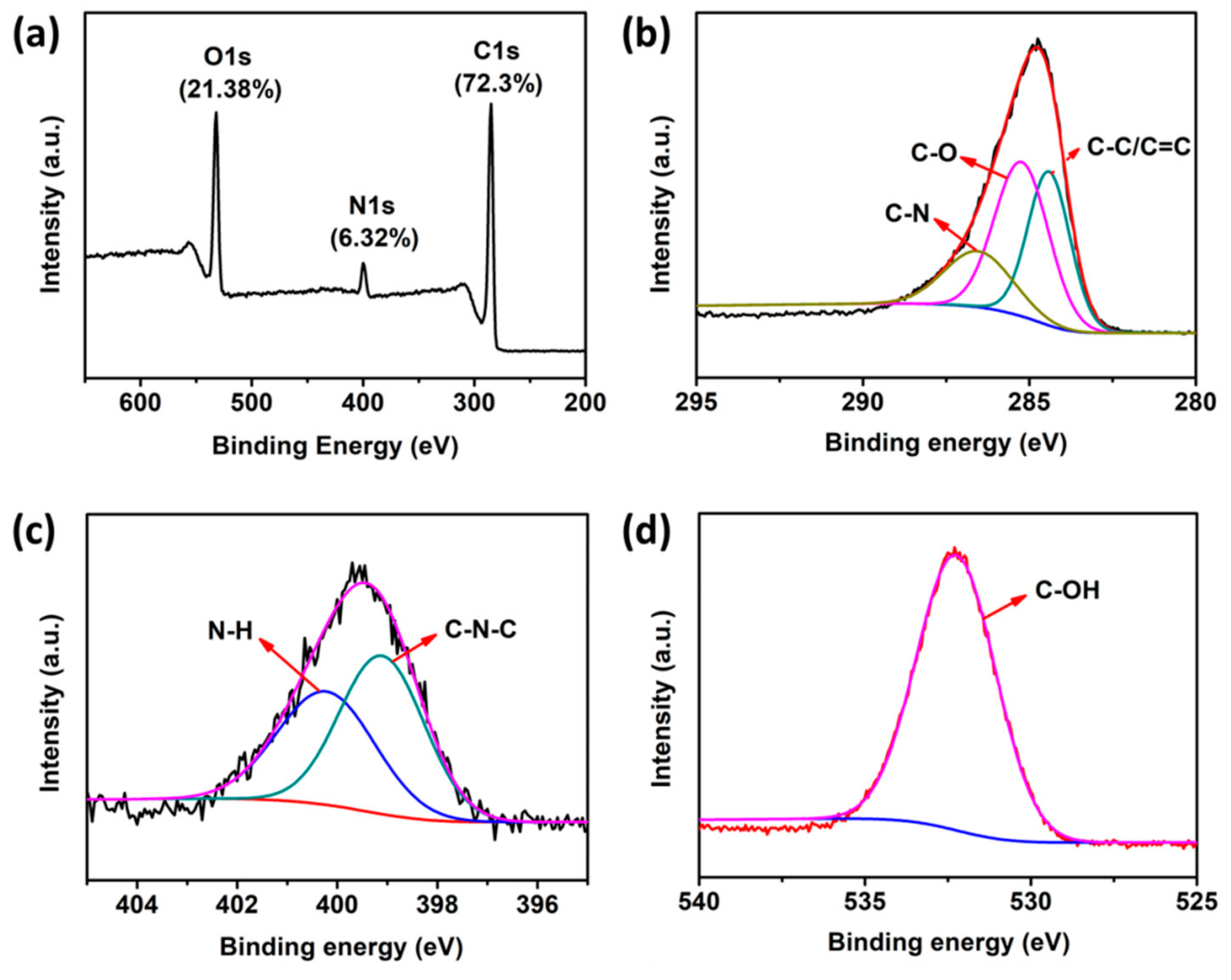
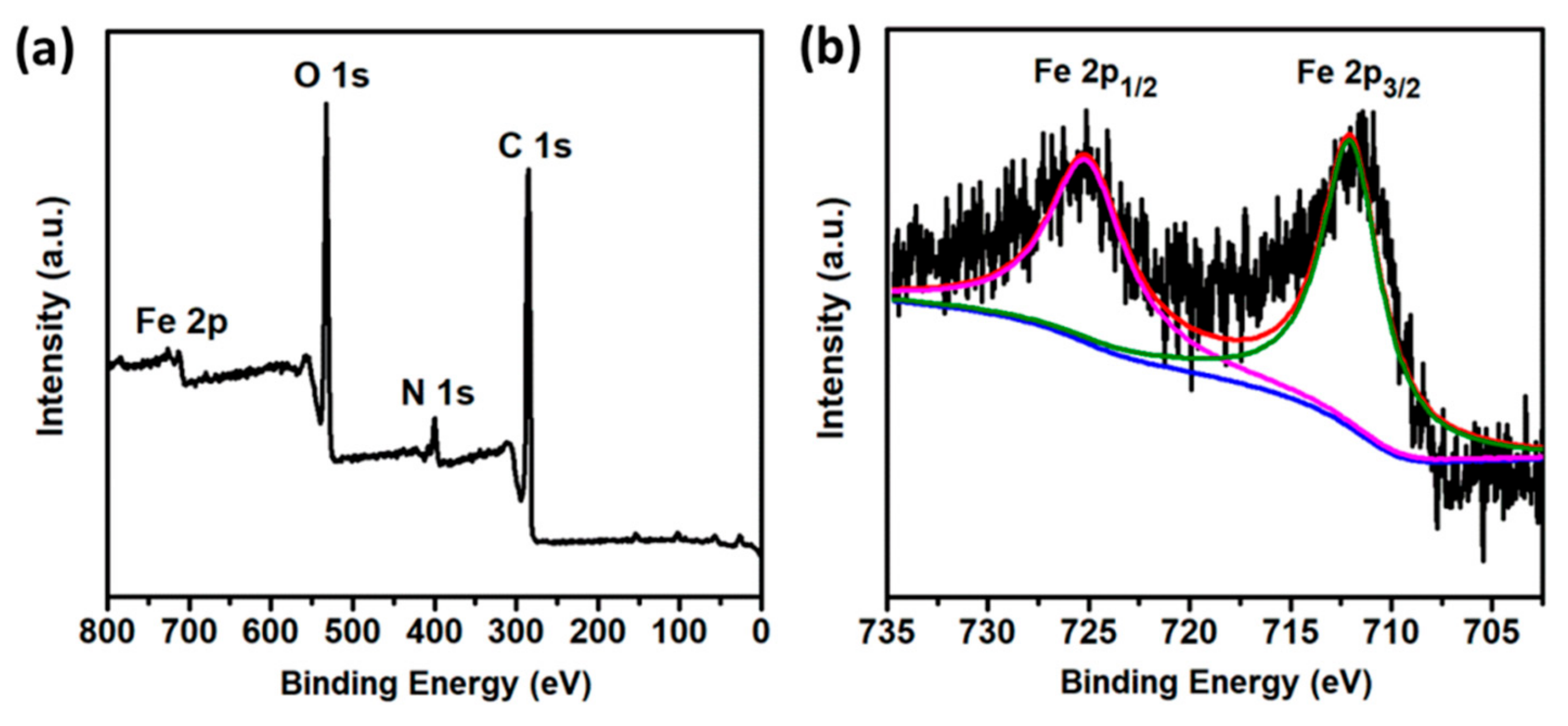
3.1. Preparation and Characterization of N-CQDs
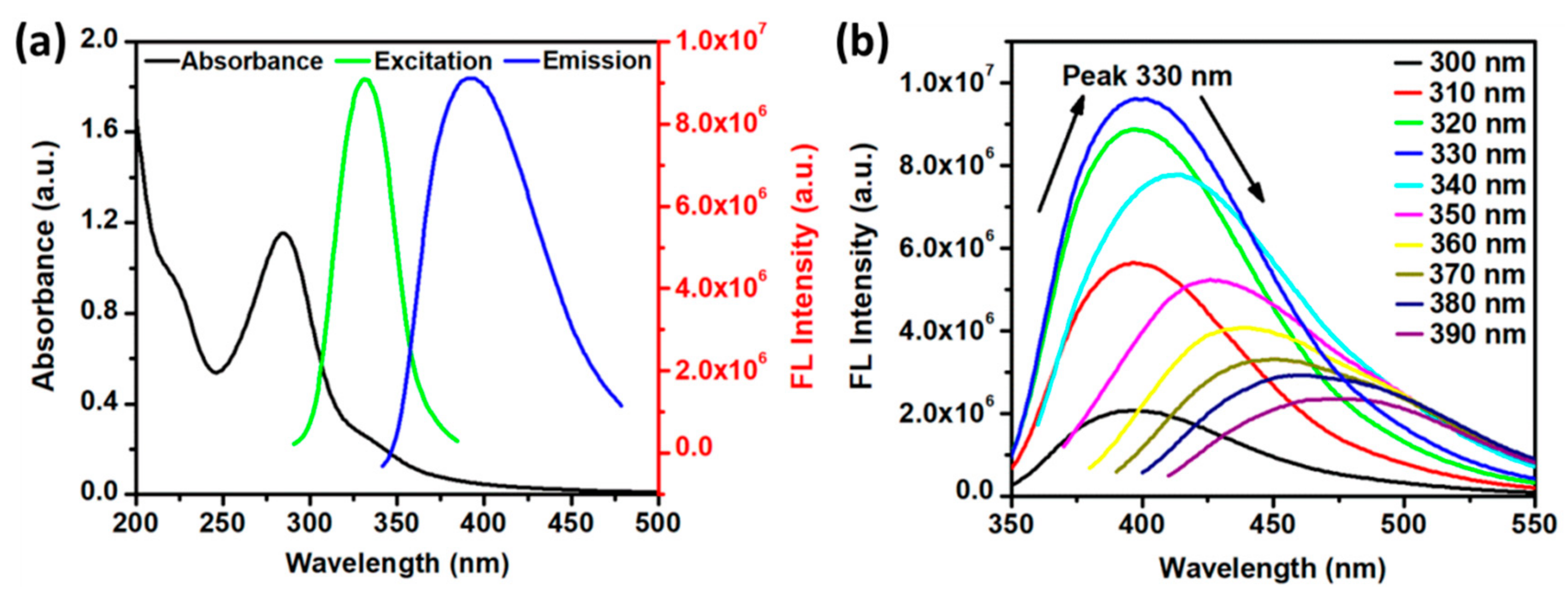
3.2. Optical Properties of N-CQDs
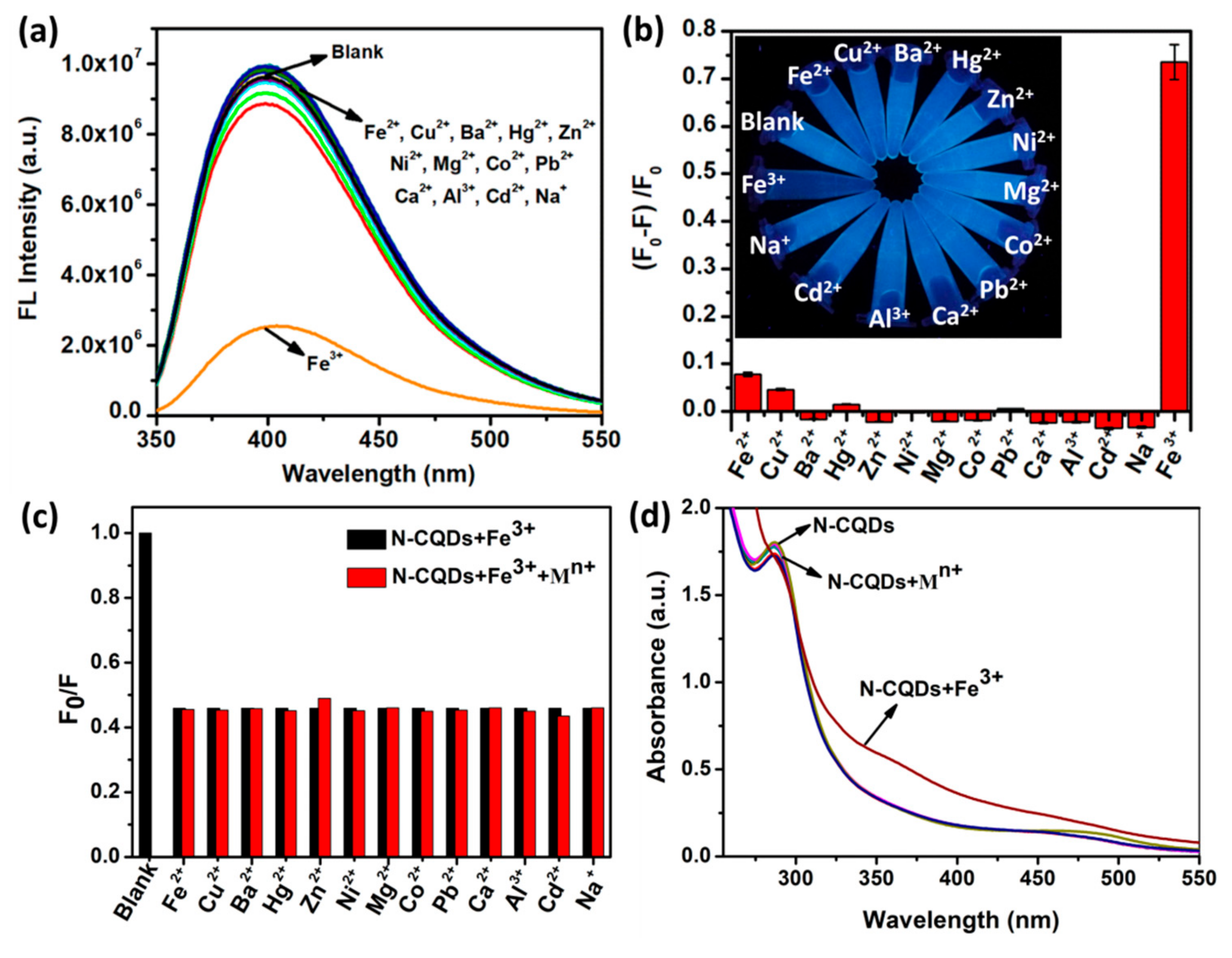
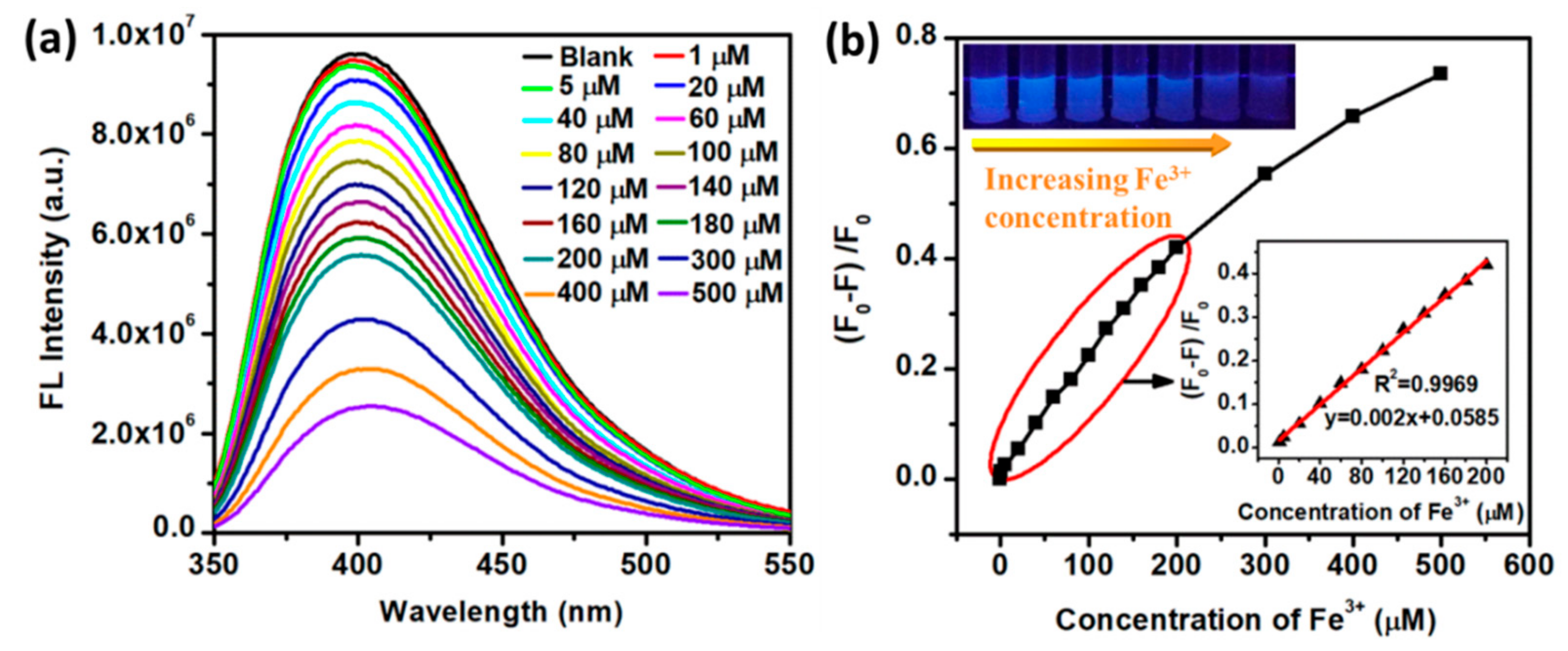
3.3. Detection of Metal Ions
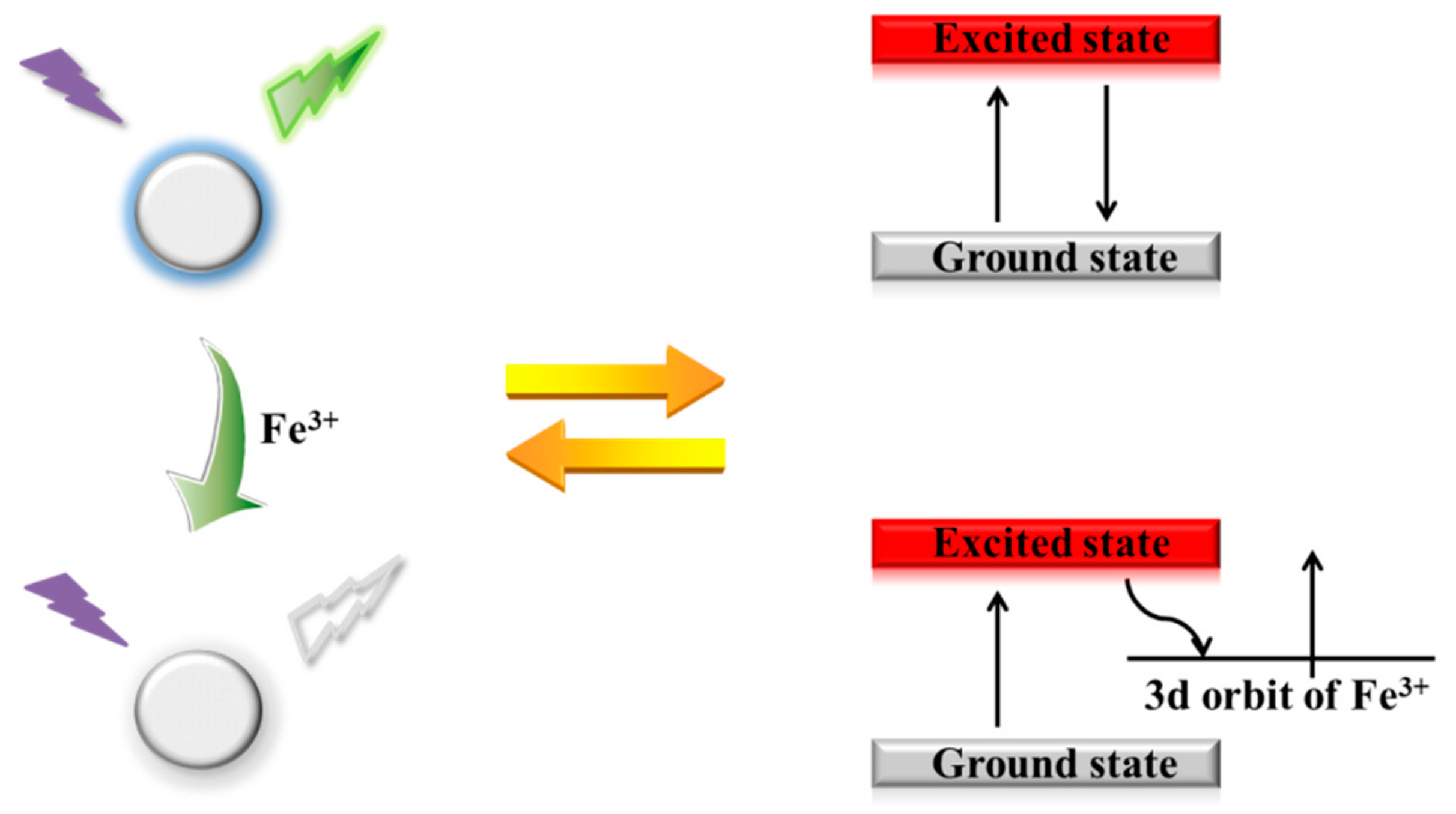
3.4. Fluorescence Quenching Mechanism of the N-CQD-Fe3+ System
3.5. Application to Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huo, B.; Liu, B.; Chen, T.; Cui, L.; Xu, G.; Liu, M.; Liu, J. One-step synthesis of fluorescent boron nitride quantum dots via a hydrothermal strategy using melamine as nitrogen source for the detection of ferric ions. Langmuir 2017, 33, 10673–10678. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Nesakumar, T.; Immanuel, J.; Raja, K. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Diao, H.; Chang, H.; Wang, H.; Li, T.; Wei, W. Green synthesis of carbon dots from rose-heart radish and application for Fe3+ detection and cell imaging. Sens. Actuat. B Chem. 2017, 241, 190–198. [Google Scholar] [CrossRef]
- Guo, X.; Wang, C.F.; Yu, Z.Y.; Chen, L.; Chen, S. Facile access to versatile fluorescent carbon dots toward light-emitting diodes. Chem. Commun. 2012, 48, 2692–2694. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, P.; Zhai, X.; Tian, F.; Li, W.; Yang, J.; Liu, Y.; Wang, H.; Wang, W.; Liu, W. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 2012, 33, 3604–3613. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem. Commun. 2012, 48, 1147–1149. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014, 55, 83–90. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Edit. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, L.; Zhang, H.C.; Liu, Y.; Wang, H.Y.; Kang, Z.H.; Lee, S.T. Graphitic carbon quantum dots as a fluorescent sensing platform for highly efficient detection of Fe3+ ions. RSC Adv. 2013, 3, 3733–3738. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, L.; Jiang, K.; Wu, A.; Lin, H. Toward High-Efficient Red Emissive Carbon Dots: Facile Preparation, Unique Properties, and Applications as Multifunctional Theranostic Agents. Chem. Mater. 2016, 28, 8659–8668. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhang, C.; Qiao, Y.; Liu, F.; Wang, D.; Wu, M.; Wang, K.; Lv, X.; Kong, X.; Wang, H. Polyhydric polymer-functionalized fluorescent probe with enhanced aqueous solubility and specific ion recognition: A test strips-based fluorimetric strategy for the rapid and visual detection of Fe3+ ions. Talanta 2017, 170, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.; Feng, Y.; Wang, Y.; Chen, X. One-pot solid phase pyrolysis synthesis of nitrogen-doped carbon dots for Fe3+ sensing and bioimaging. Sens. Actuat. B Chem. 2017, 245, 868–874. [Google Scholar] [CrossRef]
- Azadbakht, R.; Hakimi, M.; Khanabadi, J. Fluorescent organic nanoparticles with enhanced fluorescence by self-aggregation and their application for detection of Fe3+ ions. New J. Chem. 2018, 42, 5929–5936. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.; Wu, F.; Hao, G.F.; Chen, Y.; Tan, C.; Tan, Y.; Jiang, Y. A dual-response quinoline-based fluorescent sensor for the detection of Copper(II) and Iron(III) ions in aqueous medium. Sens. Actuat. B Chem. 2017, 243, 765–774. [Google Scholar] [CrossRef]
- Biller, D.V.; Bruland, K.W. Analysis of Mn, Fe, Co, Ni, Cu, Zn, Cd, and Pb in seawater using the Nobias-chelate PA1 resin and magnetic sector inductively coupled plasma mass spectrometry (ICP-MS). Mar. Chem. 2012, 130–131, 12–20. [Google Scholar] [CrossRef]
- Lunvongsa, S.; Oshima, M.; Motomizu, S. Determination of total and dissolved amount of iron in water samples using catalytic spectrophotometric flow injection analysis. Talanta 2006, 68, 969–973. [Google Scholar] [CrossRef]
- Ghaedi, M.; Shokrollahi, A.; Kianfar, A.H.; Mirsadeghi, A.S.; Pourfarokhi, A.; Soylak, M. The determination of some heavy metals in food samples by flame atomic absorption spectrometry after their separation-preconcentration on bis salicyl aldehyde, 1,3 propan diimine (BSPDI) loaded on activated carbon. J. Hazard. Mater. 2008, 154, 128–134. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, D.; Hu, X.; Han, H.; Lin, M.; Wang, C. An electrochemical sensor based on reduced graphene oxide/gold nanoparticles modified electrode for determination of iron in coastal waters. Sens. Actuat. B Chem. 2017, 243, 1–7. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Cao, J.; Zhu, J.; Fan, L.; Li, X. Sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of Fe3+. Analyt. Chem. 2014, 86, 10201–10207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, Y.; Lin, X.; Lu, F.; Zhang, Z.; Hu, Z. Lanthanum loaded graphitic carbon nitride nanosheets for highly sensitive and selective fluorescent detection of iron ions. Sens. Actuat. B Chem. 2018, 255, 2218–2222. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Cai, J.; Zhong, L.; Ren, G.; Ma, Q. One pot synthesis of gold nanoparticles using chitosan with varying degree of deacetylation and molecular weight. Carbohydr. Polym. 2017, 178, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Sureshkumar, S.; Sandhyarani, N. Synthesis and characterization of gold-chitosan nanocomposite and application of resultant nanocomposite in sensors. Colloids Surf. B Biointerf. 2012, 93, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, G.; Regiel-Futyra, A.; Andreu, V.; Sebastián, V.; Kyzioł, A.; Stochel, G.; Arruebo, M. Bactericidal Effect of Gold-Chitosan Nanocomposites in Coculture Models of Pathogenic Bacteria and Human Macrophages. ACS Appl. Mater. Interf. 2017, 9, 17693–17701. [Google Scholar] [CrossRef]
- Bao, J.; Hou, C.; Chen, M.; Li, J.; Huo, D.; Yang, M.; Luo, X.; Lei, Y. Plant Esterase-Chitosan/Gold Nanoparticles-Graphene Nanosheet Composite-Based Biosensor for the Ultrasensitive Detection of Organophosphate Pesticides. J. Agric. Food Chem. 2015, 63, 10319–10326. [Google Scholar] [CrossRef]
- Ramirez, O.; Bonardd, S.; Saldías, C.; Radic, D.; Leiva, Á. Biobased Chitosan Nanocomposite Films Containing Gold Nanoparticles: Obtainment, Characterization, and Catalytic Activity Assessment. ACS Appl. Mater. Interf. 2017, 9, 16561–16570. [Google Scholar] [CrossRef]
- Zhou, S.F.; Han, X.J.; Liu, Y.Q. SWASV performance toward heavy metal ions based on a high-activity and simple magnetic chitosan sensing nanomaterials. J. Alloys Compd. 2016, 684, 1–7. [Google Scholar] [CrossRef]
- Abdullah, S.; Azeman, N.H.; Mobarak, N.N.; Zan, M.S.D.; Ahmad, A.A. Sensitivity enhancement of localized SPR sensor towards Pb(II) ion detection using natural bio-polymer based carrageenan. Optik 2018, 168, 784–793. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, X.; Li, K.; Liu, R.; Peng, D.; He, L.; Wang, M.; Zhang, H.; Zhou, L. One-step fabrication of electrochemical biosensor based on DNA-modified three-dimensional reduced graphene oxide and chitosan nanocomposite for highly sensitive detection of Hg(II). Sens. Actuat. B Chem. 2016, 225, 453–462. [Google Scholar] [CrossRef]
- Song, J.; Zhao, L.; Wang, Y.; Xue, Y.; Deng, Y.; Zhao, X. Carbon Quantum Dots Prepared with Chitosan for Synthesis of CQDs/AuNPs for Iodine Ions Detection. Nanomaterials 2018, 8, 1043. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, Y.; Wang, C.; Wei, W.; Ma, S.; Sun, X.; He, J. Green synthesis of carbon dots functionalized silver nanoparticles for the colorimetric detection of phoxim. Talanta 2018, 185, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, R.; Li, H.; Shao, J.; Chi, Y.; Lin, X.; Chen, G. Polyamine-functionalized carbon quantum dots for chemical sensing. Carbon 2012, 50, 2810–2815. [Google Scholar] [CrossRef]
- Murugan, N.; Prakash, M.; Jayakumar, M.; Sundaramurthy, A.; Sundramoorthy, A.K. Green synthesis of fluorescent carbon quantum dots from Eleusine coracana and their application as a fluorescence ‘turn-off’ sensor probe for selective detection of Cu2+. Appl. Surf. Sci. 2019, 476, 468–480. [Google Scholar] [CrossRef]
- Song, Z.; Quan, F.; Xu, Y.; Liu, M.; Cui, L.; Liu, J. Multifunctional N,S co-doped carbon quantum dots with pH- and thermo-dependent switchable fluorescent properties and highly selective detection of glutathione. Carbon 2016, 104, 169–178. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Gao, Z.H.; Lin, Z.Z.; Chen, X.M.; Lai, Z.Z.; Huang, Z.Y. Carbon dots-based fluorescent probe for trace Hg2+ detection in water sample. Sens. Actuat. B Chem. 2016, 222, 965–971. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhang, Y. One-step green synthesized fluorescent carbon nanodots from bamboo leaves for copper(II) ion detection. Sens. Actuat. B Chem. 2014, 196, 647–652. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, X.; Ma, Y.; Qian, T.; Wang, C.; Chu, F. Facile and cost-effective preparation of carbon quantum dots for Fe3+ ion and ascorbic acid detection in living cells based on the “on-off-on” fluorescence principle. Appl. Surf. Sci. 2019, 469, 911–916. [Google Scholar] [CrossRef]
- Meng, A.; Xu, Q.; Zhao, K.; Li, Z.; Liang, J.; Li, Q. A highly selective and sensitive “on-off-on” fluorescent probe for detecting Hg(II) based on Au/N-doped carbon quantum dots. Sens Actuat. B Chem. 2018, 255, 657–665. [Google Scholar] [CrossRef]
- Shamsipur, M.; Molaei, K.; Molaabasi, F.; Alipour, M. Facile preparation and characterization of new green emitting carbon dots for sensitive and selective off/on detection of Fe3+ ion and ascorbic acid in water and urine samples and intracellular imaging in living cells. Talanta 2018, 183, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yu, H.; Jiang, X.; Luo, Z.; Zheng, Y. High sensitivity label-free detection of Fe3+ ion in aqueous solution using fl uorescent MoS2 quantum dots. Sens. Actuat. B. Chem. 2019, 281, 989–997. [Google Scholar] [CrossRef]
- Deng, M.; Wang, S.; Liang, C.; Shang, H.; Jiang, S. A FRET fluorescent nanosensor based on carbon dots for ratiometric detection of Fe3+ in aqueous. RSC Adv. 2016, 6, 26936–26940. [Google Scholar] [CrossRef]
- Ananthanarayanan, A.; Wang, X.; Routh, P.; Sana, B.; Lim, S.; Kim, D.; Lim, K.; Li, J.; Chen, P. Facile Synthesis of Graphene Quantum Dots from 3D Graphene and their Application for Fe3+ Sensing. Adv. Funct. Mater. 2014, 24, 3021–3026. [Google Scholar] [CrossRef]
- Vikneswaran, R.; Ramesh, S.; Yahya, R. Green synthesized carbon nanodots as a fluorescent probe for selective and sensitive detection of iron(III) ions. Mater. Lett. 2014, 136, 179–182. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, Z.; Gong, A.; Zhang, Y.; Li, Q. Synthesis of highly photoluminescent carbon dots via citric acid and Tris for iron(III) ions sensors and bioimaging. Talanta 2015, 143, 107–113. [Google Scholar] [CrossRef]
- Deng, X.; Feng, Y.; Li, H.; Du, Z.; Teng, Q.; Wang, H. N-doped carbon quantum dots as fluorescent probes for highly selective and sensitive detection of Fe3+ ions. Particuology 2018, 41, 94–100. [Google Scholar] [CrossRef]
- Chen, B.; Li, F.; Zou, L.; Chen, D. Intermolecular hydrogen bonding-mediated synthesis of high-quality photoluminescent carbon dots for label-free fluorometric detection of Fe3+ ions. J. Colloid Interf. Sci. 2019, 534, 381–388. [Google Scholar] [CrossRef]

| Fluorescent Probe | Linear Range/(µM) | LOD/(µM) | Ref. |
|---|---|---|---|
| G-CDs | 0.2–11 | 0.08 | [41] |
| MoS2 QDs | 0–50 | 1.00 | [42] |
| CDs | 0–50 | 0.73 | [43] |
| GQDs | 0–80 | 7.22 | [44] |
| N-C dots | 0–200 | 0.10 | [14] |
| CDs from banana peels | 2–16 | 0.21 | [45] |
| C-QDs | 2–50 | 1.30 | [46] |
| N-CQDs | 0–200 | 0.15 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Wang, Y.; Zhao, X.; Deng, Y.; Xia, Y. Facile Synthesis of Nitrogen-Doped Carbon Quantum Dots with Chitosan for Fluorescent Detection of Fe3+. Polymers 2019, 11, 1731. https://doi.org/10.3390/polym11111731
Zhao L, Wang Y, Zhao X, Deng Y, Xia Y. Facile Synthesis of Nitrogen-Doped Carbon Quantum Dots with Chitosan for Fluorescent Detection of Fe3+. Polymers. 2019; 11(11):1731. https://doi.org/10.3390/polym11111731
Chicago/Turabian StyleZhao, Li, Yesheng Wang, Xihui Zhao, Yujia Deng, and Yanzhi Xia. 2019. "Facile Synthesis of Nitrogen-Doped Carbon Quantum Dots with Chitosan for Fluorescent Detection of Fe3+" Polymers 11, no. 11: 1731. https://doi.org/10.3390/polym11111731






