Sulfur-Based Copolymeric Polyamidoamines as Efficient Flame-Retardants for Cotton
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of Homopolymeric PAAs
2.4. Synthesis of Copolymeric PAAs
2.5. Treatment of Cotton Fabrics
2.6. Combustion Tests
3. Results and Discussion
3.1. Synthesis of Homopolymeric and Copolymeric PAAs
3.2. Thermal Stability of PAAs
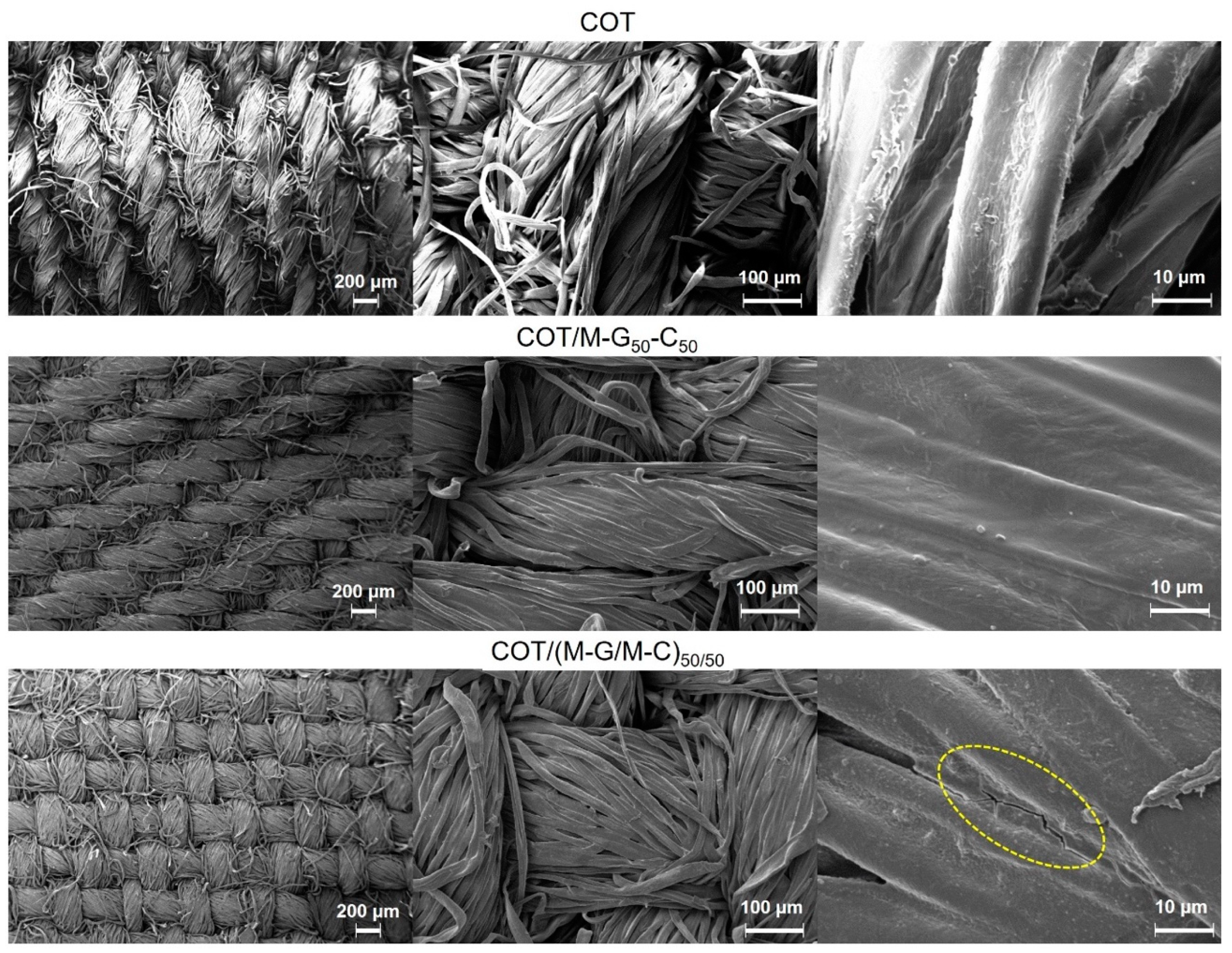
3.3. FT-IR and Morphological Characterization of PAA-Treated Cotton Fabrics
3.4. Thermal Characterization of PAA-Treated Cotton Fabrics
3.5. Combustion Characterization of PAA-Treated Cotton Fabrics
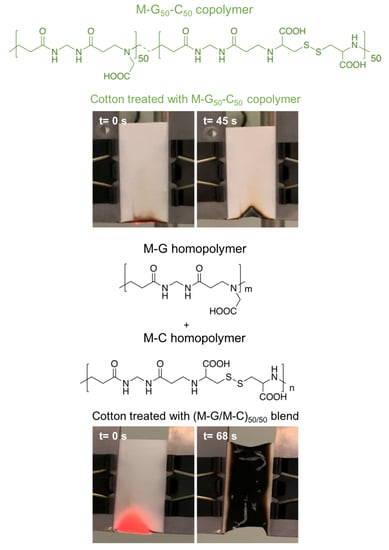
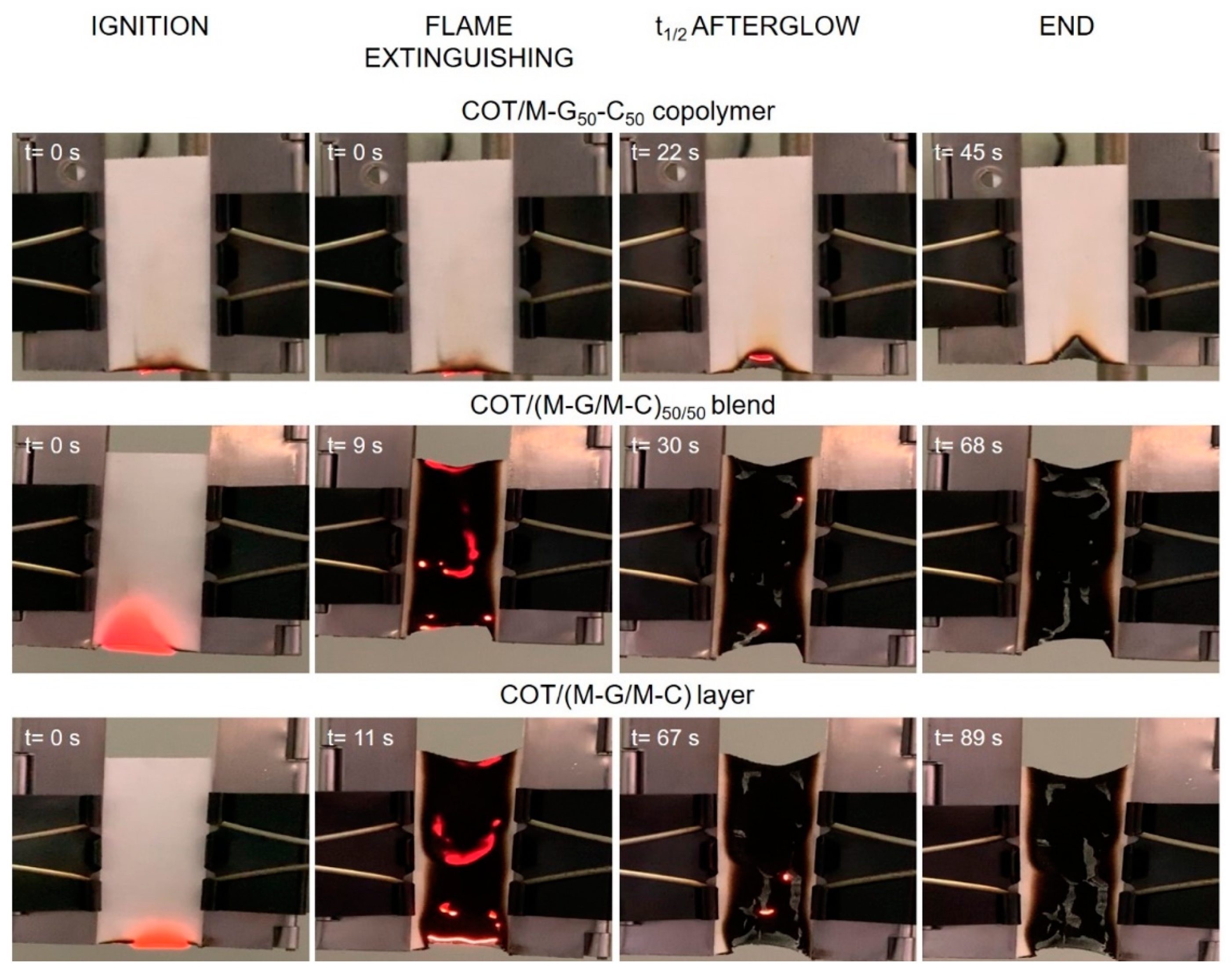
3.5.1. Vertical Flame Spread Tests
3.5.2. Horizontal Flame Spread Tests (HFST)
3.5.3. Cone Calorimetry Tests
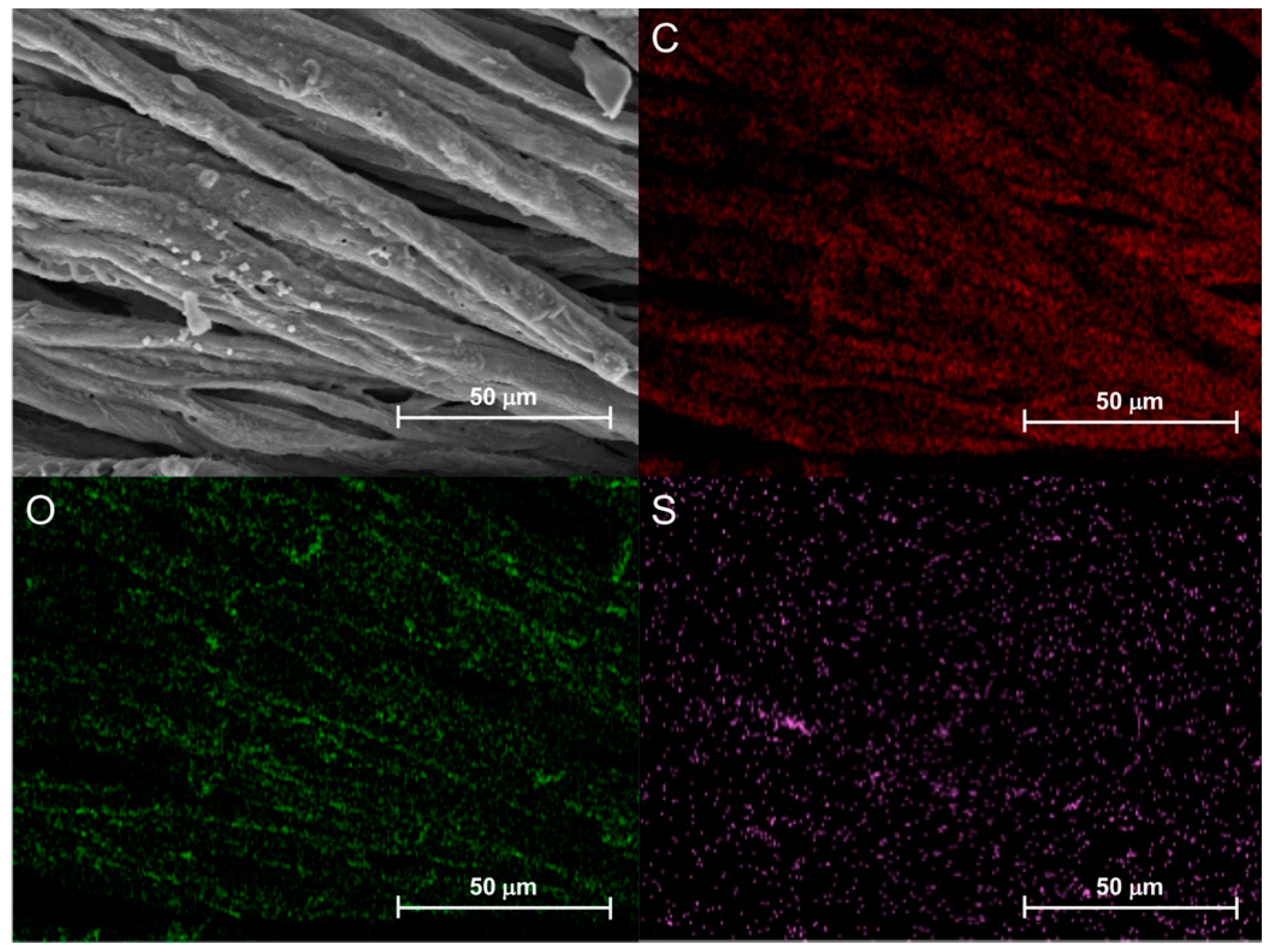
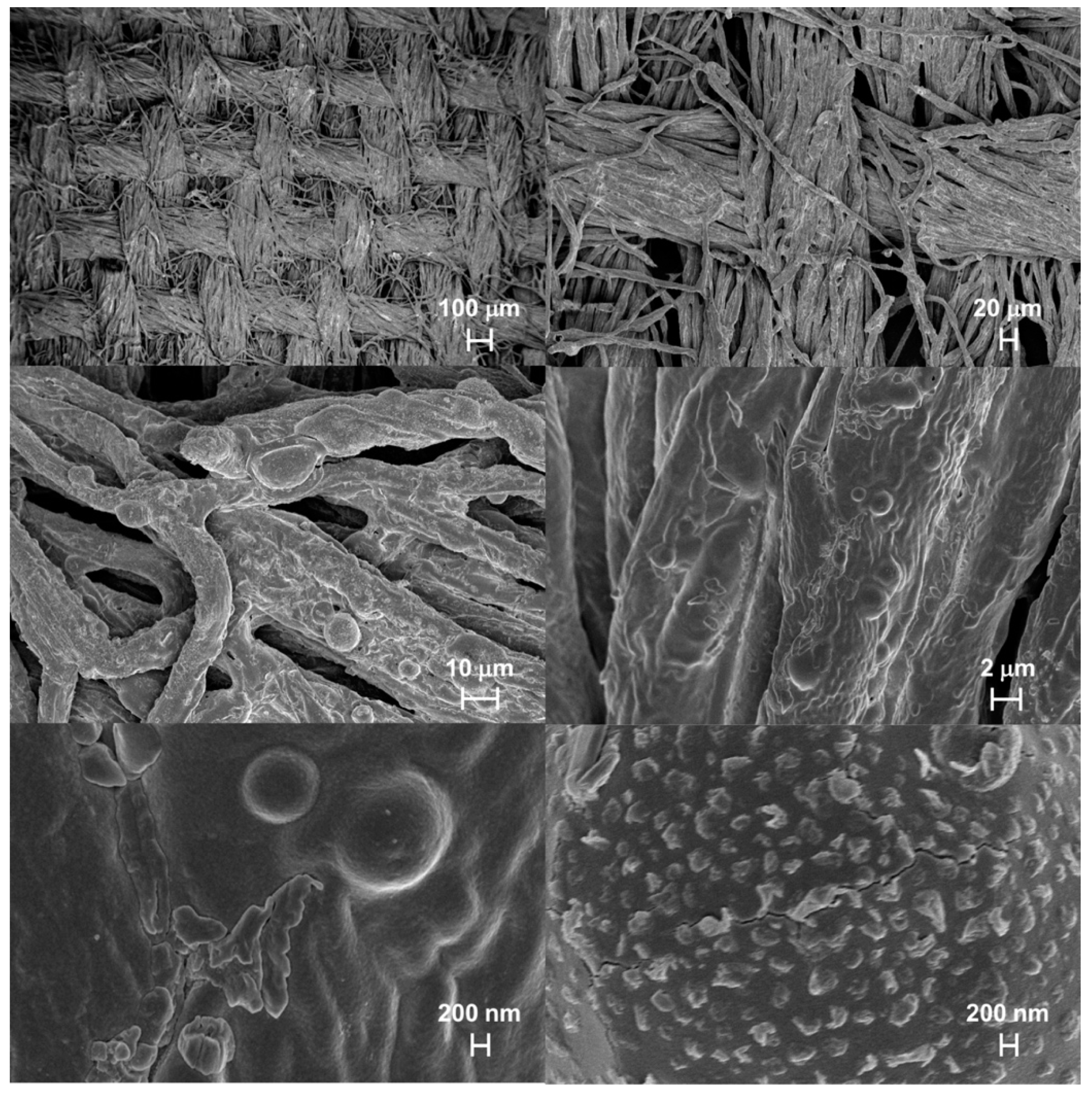
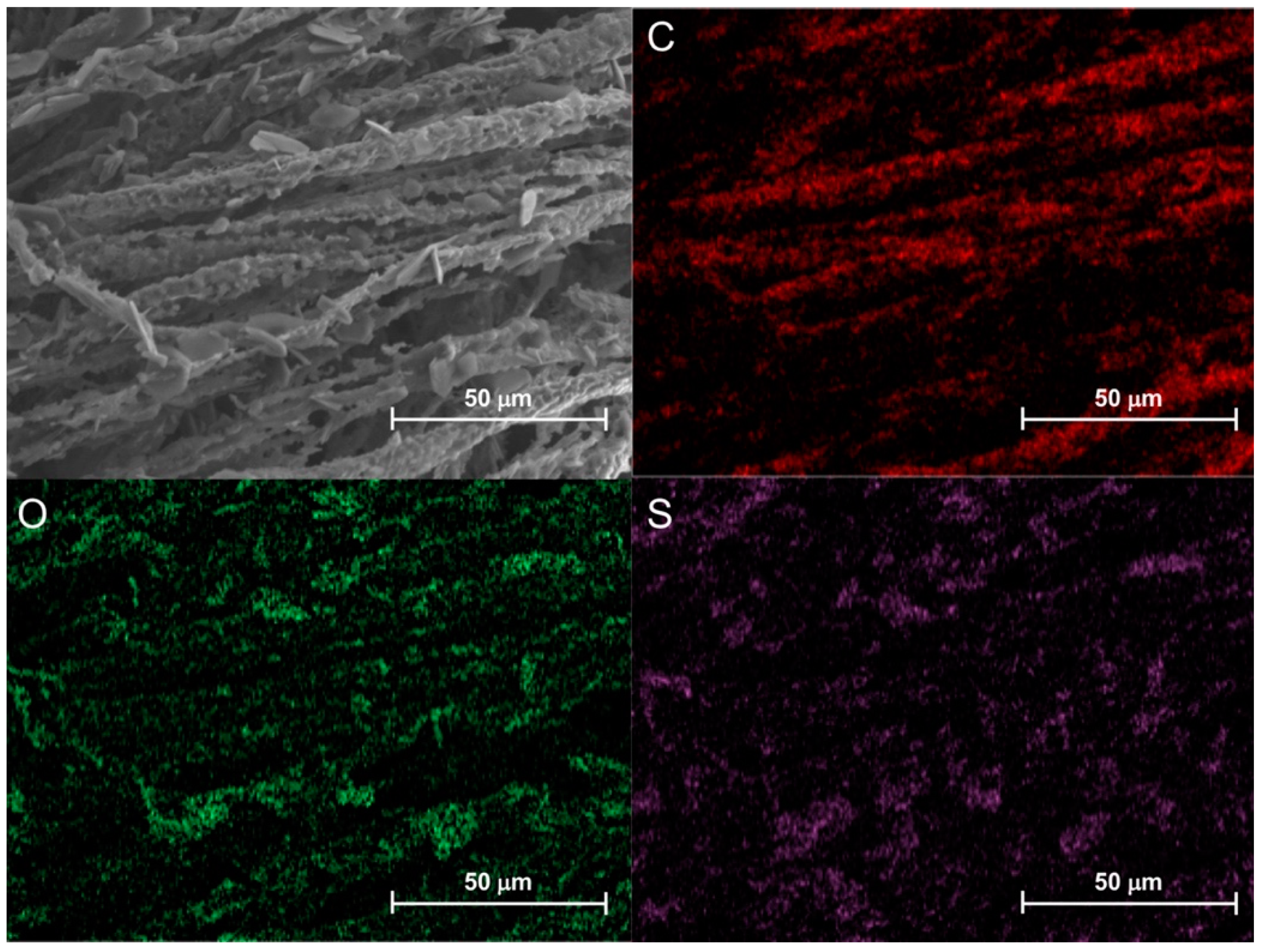
3.5.4. Morphological Characterization and EDX Elemental Analysis of Combustion Residues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horrocks, A.R. Developments in flame retardants for heat and fire resistant textiles e the role of char formation and intumescence. Polym. Degrad. Stabil. 1996, 54, 143–154. [Google Scholar] [CrossRef]
- Horrocks, A.R. Flame retardant challenges for textiles and fibres: New chemistry versus innovatory solutions. Polym. Degrad. Stabil. 2011, 96, 377–392. [Google Scholar] [CrossRef]
- Horrocks, R.A.; Wang, M.Y.; Hall, M.E.; Sunmonu, F.; Pearson, J.S. Flame retardant textile back-coatings. Part 2. Effectiveness of phosphorus-containing flame retardants in textile back-coating formulations. Polym. Int. 2000, 49, 1079–1091. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S.; Malucelli, G. Recent Advances for Flame Retardancy of Textiles Based on Phosphorus Chemistry. Polymers 2016, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Bourbigot, S.; Devaux, E.; Flambard, X. Flammability of polyamide-6/clay hybrid nanocomposite textiles. Polym. Degrad. Stabil. 2002, 75, 397–402. [Google Scholar] [CrossRef]
- Chen, D.Q.; Wang, Y.Z.; Hu, X.P.; Wang, D.Y.; Qu, M.H.; Yang, B. Flame-retardant and anti-dripping effects of a novel char-forming flame retardant for the treatment of poly(ethylene terephthalate) fabrics. Polym. Degrad. Stabil. 2005, 88, 349–356. [Google Scholar] [CrossRef]
- Zhang, S.; Horrocks, A.R.; Hull, T.R.; Kandola, B.K. Flammability, degradation and structural characterization of fibre-forming polypropylene containing nanoclay flame retardant combinations. Polym. Degrad. Stabil. 2006, 91, 719–725. [Google Scholar] [CrossRef]
- Morterra, C.; Low, M.J.D. IR Studies of Carbons II: The pyrolysis of cellulose. Carbon 1983, 21, 283–288. [Google Scholar] [CrossRef]
- Morterra, C.; Low, M.J.D.; Severdia, A.G. IR Studies of Carbons III: The oxidation of cellulose chars. Carbon 1984, 22, 5–12. [Google Scholar] [CrossRef]
- Morterra, C.; Low, M.J.D. An infrared spectroscopic approach to the characterization of intermediate chars. Mater. Lett. 1984, 2, 289–293. [Google Scholar] [CrossRef]
- Xing, W.; Jie, G.; Song, L.; Hu, S.; Lv, X.; Wang, X.; Hu, Y. Flame retardancy and thermal degradation of cotton textiles based on UV-curable flame retardant coatings. Thermochim. Acta 2011, 513, 75–82. [Google Scholar] [CrossRef]
- Edwards, B.; Hauser, P.; EI-Shafei, A. Nonflammable cellulosic substrates by application of novel radiation-curable flame retardant monomers derived from cyclotriphosphazene. Cellulose 2015, 22, 275–287. [Google Scholar] [CrossRef]
- Edwards, B.; Rudolf, S.; Hauser, P.; EI-Shafei, A. Preparation, Polymerization, and Performance Evaluation of Halogen-Free Radiation Curable Flame Retardant Monomers for Cotton Substrates. Ind. Eng. Chem. Res. 2015, 54, 577–584. [Google Scholar] [CrossRef]
- Mayer-Gall, T.; Knittel, D.; Gutmann, J.S.; Opwis, K. Permanent Flame Retardant Finishing of Textiles by Allyl-Functionalized Polyphosphazenes. ACS Appl. Mater. Interfaces 2015, 7, 9349–9363. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.-T.; Wang, X.; Acuña, P.; Zhu, P.; Wagenknecht, U.; Heinrich, G.; Zhang, X.-Q.; Wang, R.; Wang, D.-Y. Effect of phosphorus-containing inorganic–organic hybrid coating on the flammability of cotton fabrics: Synthesis, characterization and flammability. Chem. Eng. J. 2016, 294, 167–175. [Google Scholar] [CrossRef]
- Yang, Z.; Fei, B.; Wang, X.; Xin, J.H. A novel halogen-free and formaldehyde-free flame retardant for cotton fabrics. Fire Mater. 2012, 36, 31–39. [Google Scholar] [CrossRef]
- Hu, S.; Hu, Y.; Song, L.; Lu, H. Effect of modified organic-inorganic hybrid materials on thermal properties of cotton fabrics. J. Therm. Anal. Calorim. 2011, 103, 423–427. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Cotton fabrics treated with hybrid organic-inorganic coatings obtained through dual-cure processes. Cellulose 2011, 18, 1335–1348. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Wang, W.; Liu, Y.; Zhao, P. Synthesis of a formaldehyde-free phosphorus–nitrogen flame retardant with multiple reactive groups and its application in cotton fabrics. Polym. Degrad. Stabil. 2015, 120, 193–202. [Google Scholar] [CrossRef]
- Nguyen, T.-M.D.; Chang, S.; Condon, B.; Slopek, R. Synthesis of a novel flame retardant containing phosphorus-nitrogen and its comparison for cotton fabric. Fiber Polym. 2012, 13, 963–970. [Google Scholar] [CrossRef]
- Chang, S.C.; Condon, B.; Graves, E.; Uchimiya, M.; Fortier, C.; Easson, M.; Wakelyn, P. Flame retardant properties of triazine phosphonates derivative with cotton fabric. Fibers Polym. 2011, 12, 334–339. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, F.-L.; Park, S.-J. Synthesis of a novel phosphorus-nitrogen-containing intumescent flame retardant and its application to fabrics. J. Ind. Eng. Chem. 2015, 27, 40–43. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Javeri, N.; Shekarriz, S. Flame retardant cotton fibers produced using novel synthesized gen-free phosphoramide nanoparticles. Carbohydr. Polym. 2015, 118, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-M.; Chang, S.; Condon, B. The comparison of differences in flammability and thermal degradation between cotton fabrics treated with phosphoramidate derivatives. Polym. Adv. Technol. 2014, 25, 665–672. [Google Scholar] [CrossRef]
- Nguyen, T.-M.; Chang, S.; Condon, B.; Smith, J. Fire Self-Extinguishing Cotton Fabric: Development of Piperazine Derivatives Containing Phosphorous-Sulfur-Nitrogen and Their Flame Retardant and Thermal Behaviors. Mater. Sci. Appl. 2014, 5, 789–802. [Google Scholar] [CrossRef]
- Nguyen, T.-M.; Chang, S.; Condon, B.; Slopek, R.; Graves, E.; Yoshioka-Tarver, M. Structural Effect of Phosphoramidate Derivatives on the Thermal and Flame Retardant Behaviors of Treated Cotton Cellulose. Ind. Eng. Chem. Res. 2013, 52, 4715–4724. [Google Scholar] [CrossRef]
- Dong, C.; Lu, Z.; Zhang, F.; Zhu, P.; Wang, P.; Che, Y.; Sui, S. Combustion behaviors of cotton fabrics treated by a novel nitrogen- and phosphorus-containing polysiloxane flame retardant. J. Therm. Anal. Calorim. 2016, 123, 535–544. [Google Scholar] [CrossRef]
- Tawiah, B.; Yu, B.; Yang, W.; Yuen, R.K.K.; Fe, B. Facile flame retardant finishing of cotton fabric with hydrated sodium metaborate. Cellulose 2019, 26, 4629–4640. [Google Scholar] [CrossRef]
- Nine, M.J.; Tran, D.N.H.; Thanh Tung, T.; Kabiri, S.; Losic, D. Graphene-Borate as an Efficient Fire Retardant for Cellulosic Materials with Multiple and Synergetic Modes of Action. ACS Appl. Mater. Interfaces 2017, 9, 10160–10168. [Google Scholar] [CrossRef]
- Dong, C.; Lu, Z.; Zhu, P.; Zhang, F.; Zhang, X. Combustion behaviors of cotton fabrics treated by a novel guanidyl- and phosphorus-containing polysiloxane flame retardant. J. Therm. Anal. Calorim. 2015, 119, 349–357. [Google Scholar] [CrossRef]
- Dong, C.; Lu, Z.; Zhang, F. Preparation and properties of cotton fabrics treated with a novel guanidyl and phosphorus-containing polysiloxane antimicrobial and flame retardant. Mater. Lett. 2015, 142, 35–37. [Google Scholar] [CrossRef]
- Ferruti, P. Polyamidoamines: Past, present and perspectives. J. Polym. Sci. Polym. Chem. 2013, 51, 2319–2353. [Google Scholar] [CrossRef]
- Ranucci, E.; Manfredi, A. Polyamidoamines: Versatile bioactive polymers with potential for biotechnological applications. Chem. Afr. 2019, 2, 167–193. [Google Scholar] [CrossRef] [Green Version]
- Ferruti, P.; Mauro, N.; Falciola, L.; Pifferi, V.; Bartoli, C.; Gazzarri, M.; Chiellini, F.; Ranucci, E. Amphoteric, Prevailingly cationic L-arginine polymers of poly(amidoamino acid) structure: Synthesis, acid/base properties and preliminary cytocompatibility and cell-permeating characterizations. Macromol. Biosci. 2014, 14, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, A.; Mauro, N.; Terenzi, A.; Alongi, J.; Lazzari, F.; Ganazzoli, F.; Raffaini, G.; Ranucci, E.; Ferruti, P. Self-ordering secondary structure of D- and L-arginine-derived polyamidoamino acids. ACS Macro Lett. 2017, 6, 987–991. [Google Scholar] [CrossRef]
- Lazzari, F.; Manfredi, A.; Alongi, J.; Mendichi, R.; Ganazzoli, F.; Raffaini, G.; Ferruti, P.; Ranucci, E. Self-structuring in water of polyamidoamino acids with hydrophobic side chains deriving from natural α-amino acids. Polymers 2018, 10, 1261. [Google Scholar] [CrossRef] [Green Version]
- Lazzari, F.; Manfredi, A.; Alongi, J.; Marinotto, D.; Ferruti, P.; Ranucci, E. D-, L- and D,L-Tryptophan-Based polyamidoamino acids: pH-dependent structuring and fluorescent properties. Polymers 2019, 11, 543. [Google Scholar] [CrossRef] [Green Version]
- Ranucci, E.; Ferruti, P.; Lattanzio, E.; Manfredi, A.; Rossi, M.; Mussini, P.R.; Chiellini, F.; Bartoli, C. Acid-base properties of poly(amidoamine)s. J. Polym. Sci. A Polym. Chem. 2009, 47, 6977–6991. [Google Scholar] [CrossRef]
- Manfredi, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Linear polyamidoamines as novel biocompatible phosphorus-free surface confined intumescent flame retardants for cotton fabrics. Polym. Degrad. Stabil. 2018, 151, 52–64. [Google Scholar] [CrossRef]
- Manfredi, A.; Carosio, F.; Ferruti, P.; Alongi, J.; Ranucci, E. Disulfide-containing polyamidoamines with remarkable flame retardant activity for cotton fabrics. Polym. Degrad. Stabil. 2018, 156, 1–13. [Google Scholar] [CrossRef]
- Alongi, J.; Ferruti, P.; Manfredi, A.; Carosio, F.; Feng, Z.; Hakkarainen, M.; Ranucci, E. Superior flame retardancy of cotton by synergistic effect of cellulose derived nano-graphene oxide carbon dots and disulphide-containing polyamidoamines. Polym. Degrad. Stab. 2019, 169, 108993. [Google Scholar] [CrossRef]
- Emilitri, E.; Ferruti, P.; Annunziata, R.; Ranucci, E.; Rossi, M.; Falciola, L.; Mussini, P.; Chiellini, F.; Bartoli, C. Novel amphoteric cystine-based poly(amidoamine)s responsive to redox stimuli. Macromolecules 2007, 40, 4785–4793. [Google Scholar] [CrossRef]
- Tata, J.; Alongi, J.; Carosio, F.; Frache, A. Optimization of the procedure to burn textile fabrics by cone calorimeter: Part I. Combustion behavior of polyester. Fire Mater. 2011, 35, 397–409. [Google Scholar] [CrossRef]
- ISO 5660 Fire Test. Reaction to Fire, Rate of Heat Release (Cone Calorimeter Method); International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Vahabi, H.; Kandola, B.K.; Saeb, M.R. Flame Retardancy Index for Thermoplastic Composites. Polymers 2019, 11, 407. [Google Scholar] [CrossRef] [Green Version]
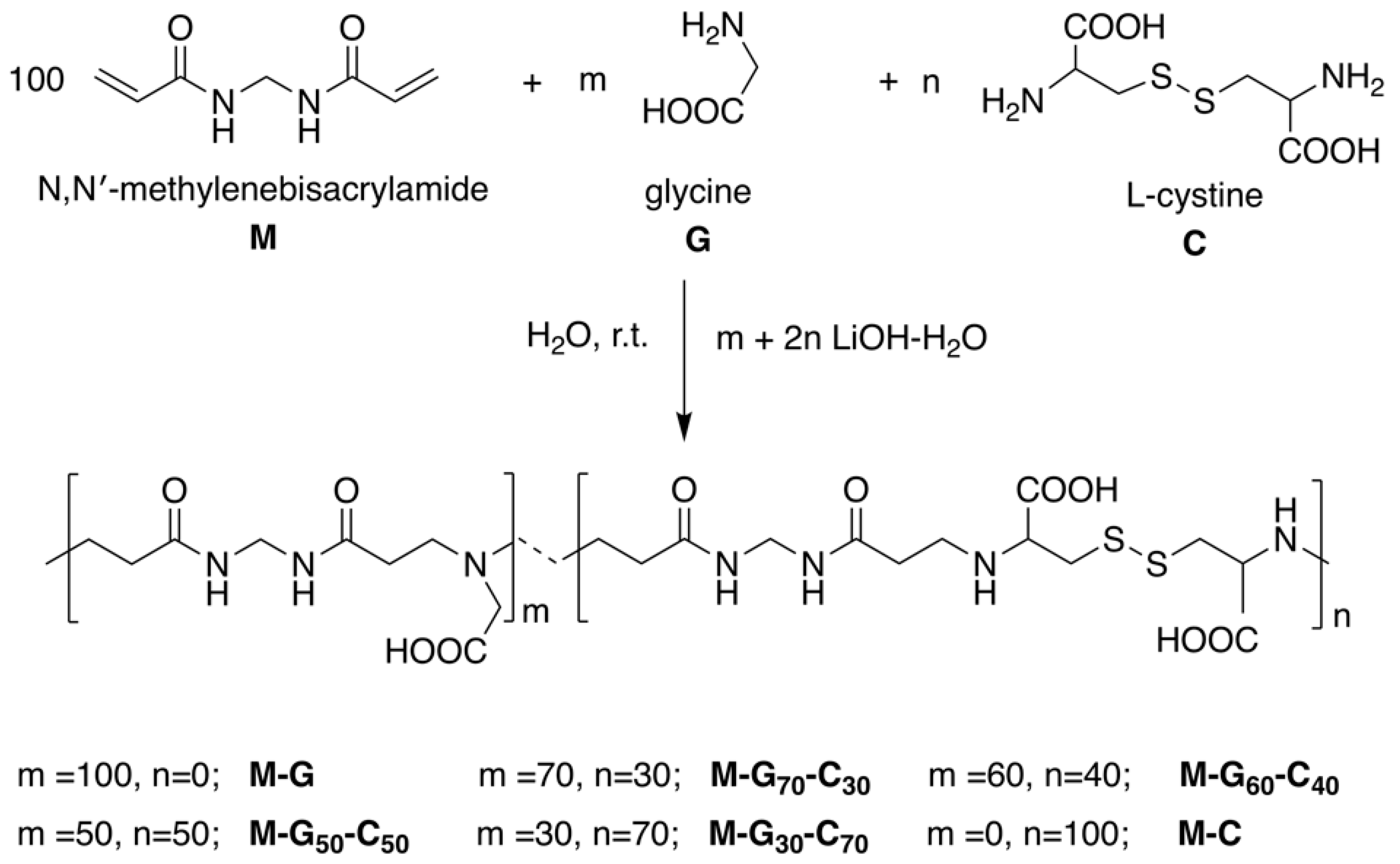









| PAA | G/C Molar Ratio | [M] (g, mmol) | [G] (g, mmol) | [C] (g, mmol) | LiOH·H2O (g, mmol) | H2O (mL) | N (%) | S (%) |
|---|---|---|---|---|---|---|---|---|
| M–G | - | 3.08, 19.98 | 1.49, 19.98 | - | 0.87, 20.07 | 8.10 | 18.3 | - |
| M–C | - | 1.54, 9.99 | - | 2.41, 9.99 | 0.86, 20.50 | 7.00 | 14.2 | 16.2 |
| M–G70–C30 | 0.7/0.3 | 1.04, 6.75 | 0.35, 4.72 | 0.49, 2.05 | 0.37, 8.82 | 3.30 | 16.6 | 6.9 |
| M–G60–C40 | 0.4/0.6 | 1.54, 9.99 | 0.45, 5.99 | 0.96, 3.99 | 0.60, 14.30 | 5.30 | 16.1 | 8.7 |
| M–G50–C50 | 0.5/0.5 | 1.01, 6.55 | 0.24, 3.20 | 0.77, 3.22 | 0.42, 10.00 | 3.67 | 15.7 | 10.3 |
| M–G30–C70 | 0.3/0.7 | 1.01, 6.55 | 0.15, 1.99 | 1.09, 4.54 | 0.48, 11.44 | 4.15 | 15.0 | 13.0 |
| Cotton Sample | Add-on 1 (%) |
|---|---|
| Copolymers | |
| COT/M–G70–C30 | 16.4 |
| COT/M–G60–C40 | 16.3 |
| COT/M–G50–C50 | 16.0 |
| COT/M–G30–C70 | 16.2 |
| Blends | |
| COT/(M–G/M–C)70/30 | 16.2 |
| COT/(M–G/M–C)50/50 | 16.5 |
| COT/(M–G/M–C)30/70 | 16.5 |
| Layers | |
| COT/M–G/M–C | 16.1 |
| COT/M–C/M–G | 16.2 |
| PAA | Add-on (%) | Tonset10% 1 (°C) | Tmax1 2 (°C) | Tmax2 3 (°C) | RMF at 750 °C 4 (%) |
|---|---|---|---|---|---|
| Nitrogen | |||||
| M–G | 16.0 | 146 | 144, 268 5 | - | 11.5 |
| M–C | 16.0 | 154 | 146, 208, 246 5 | - | 4.0 |
| M–G70–C30 | 16.4 | 152 | 2485, 322, 410 | - | 6.5 |
| M–G60–C40 | 16.3 | 159 | 2465, 386, 410 | - | 5.5 |
| M–G50–C50 | 16.0 | 158 | 213, 2535, 408 | - | 9.5 |
| M–G30–C70 | 16.2 | 149 | 213, 2435, 408 | - | 2.0 |
| Air | |||||
| M–G | 16.0 | 148 | 139, 272 5 | 468 | 8.5 |
| M–C | 16.0 | 170 | 221, 225, 250 5 | 449 | 22.0 |
| M–G70–C30 | 16.4 | 154 | 155, 251 5 | 445 | 11.5 |
| M–G60–C40 | 16.3 | 161 | 156, 243 | 444 | 18.0 |
| M–G50–C50 | 16.0 | 161 | 162, 253 5 | 449 | 12.0 |
| M–G30–C70 | 16.2 | 138 | 201, 240 5 | 449 | 9.0 |
| Sample | Add-on (%) | Tonset10% 1 (°C) | Tmax1 2 (°C) | Tmax2 3 (°C) | RMF at 750 °C 4 (%) |
|---|---|---|---|---|---|
| Nitrogen | |||||
| COT | - | 319 | 365 | - | 12.0 |
| COT/M–G70–C30 | 16.4 | 251 | 327 | - | 18.0 |
| COT/M–G60–C40 | 16.3 | 251 | 329 | - | 18.0 |
| COT/M–G50–C50 | 16.0 | 251 | 328 | - | 18.0 |
| COT/M–G30–C70 | 16.2 | 250 | 329 | - | 18.0 |
| Air | |||||
| COT | - | 304 | 345 | 480 | 0 |
| COT/M–G70–C30 | 16.4 | 256 | 319 | 487 | 1.5 |
| COT/M–G60–C40 | 16.3 | 256 | 319 | 492 | 2.0 |
| COT/M–G50–C50 | 16.0 | 264 | 322 | 444 | 2.0 |
| COT/M–G30–C70 | 16.2 | 242 | 317 | 500 | 4.0 |
| Sample | Add-on (%) | Note | Combustion Time 1 (s) | Extinguishment (YES/NO) | RMF 2 (%) |
|---|---|---|---|---|---|
| COT | - | Flaming | 33 | NO | 2 |
| Cotton fabrics treated with PAA copolymers | |||||
| COT/M–G70–C30 | 16.4 | Flaming | 76 | NO | 31 |
| COT/M–G60–C40 | 16.3 | Flaming | 191 | NO | 41 |
| COT/M–G50–C50 | 16.0 | No flaming, only afterglow | 24 | YES | 95 |
| COT/M–G30–C70 | 16.2 | No flaming, only afterglow | 23 | YES | 96 |
| Cotton fabrics treated with PAA blends | |||||
| COT/(M–G/M–C)70/30 | 16.2 | Flaming | 74 | NO | 27 |
| COT/(M–G/M–C)50/50 | 16.5 | Flaming | 58 | NO | 32 |
| COT/(M–G/M–C)30/70 | 16.5 | Flaming | 82 | NO | 35 |
| Cotton fabrics treated with PAA layers | |||||
| COT/M–G/M–C | 16.1 | Flaming | 73 | NO | 37 |
| COT/M–C/M–G | 16.2 | Flaming | 112 | NO | 29 |
| Sample | Add-on (%) | Combustion Time 1 (s) | Extinguishment (YES/NO) | RMF 2 (%) |
|---|---|---|---|---|
| COT | - | 50 | NO | 1 |
| Cotton fabrics treated with PAA copolymers | ||||
| COT/M–G70–C30 | 16.4 | 28 | YES | 94 |
| COT/M–G60–C40 | 16.3 | 27 | YES | 82 |
| COT/M–G50–C50 | 16.0 | 27 | YES | 85 |
| COT/M–G30–C70 | 16.2 | 21 | YES | 88 |
| Cotton fabric treated with PAA blend | ||||
| COT/(M–G/M–C)50/50 | 16.5 | 34 | YES | 85 |
| Cotton fabric treated with PAA layer | ||||
| COT/M–G/M–C | 16.2 | 23 | YES | 80 |
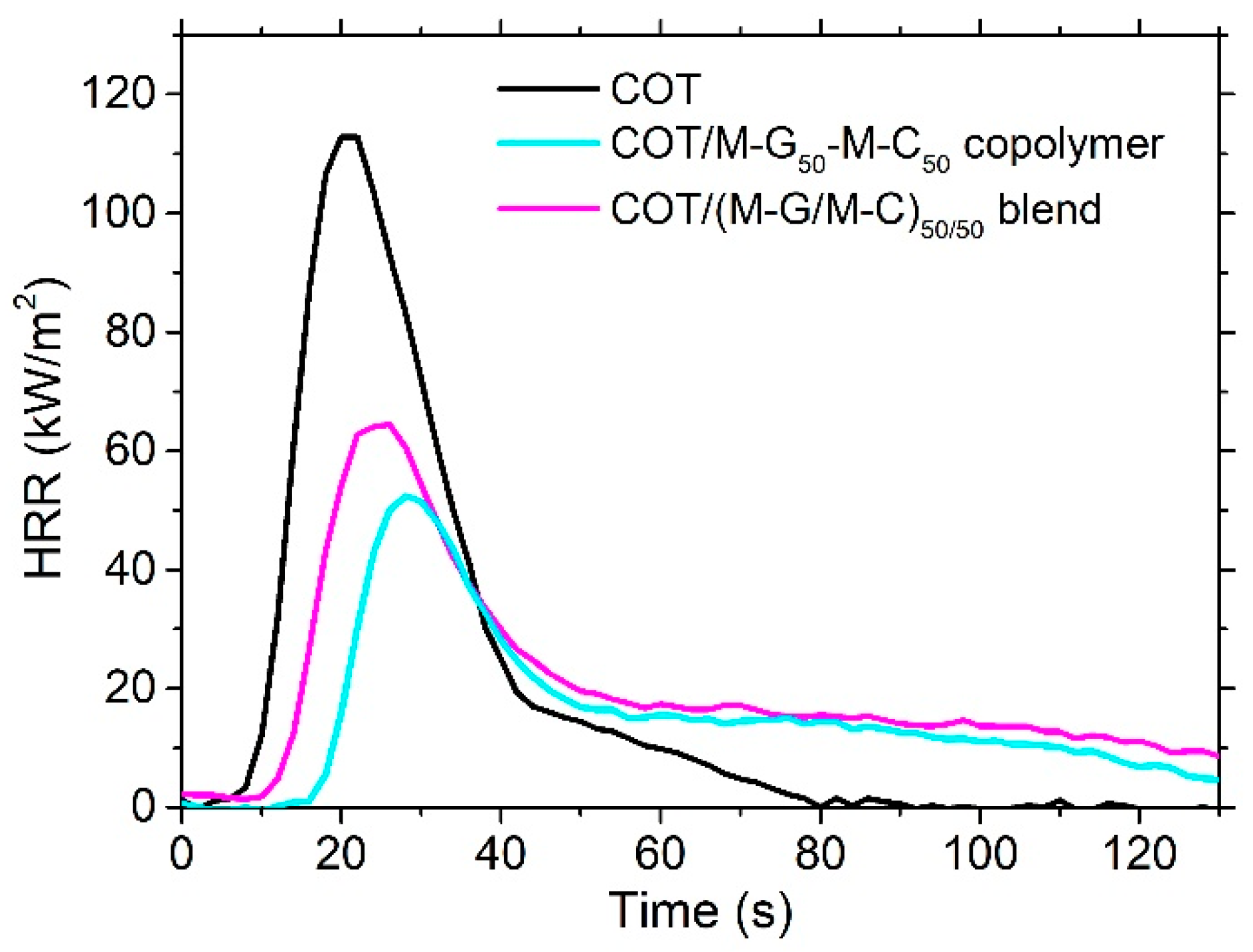
| Sample | Add-on (%) | TTI 1 (s) | PHRR 2 (kw m−2) | FRI 3 | THR 4 (MJ m−2) | [CO] (kg kg−1) | [CO2] (kg kg−1) | RMF 5 (%) |
|---|---|---|---|---|---|---|---|---|
| COT | - | 12 ±4 | 116 ±6 | - | 2.2 ±0.1 | 0.065 ±0.003 | 1.90 ±0.01 | - |
| COT/M–G50–C50 copolymer | 16.0 | 18 ±1 | 53 ± 3 (−55%) 6 | 3.6 | 2.0 ±0.1 | 0.052 ±0.003 (−20%) 6 | 1.35 ±0.01 (−29%) 6 | 8.0 ± 0.5 |
| COT/(M–G/M–C)50/50 blend | 16.5 | 14 ±1 | 66 ± 3 (−44%) 6 | 1.7 | 2.6 ±0.1 | 0.063 ±0.003 (−4%) 6 | 1.61 ±0.01 (−15%) 6 | 7.0 ± 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beduini, A.; Carosio, F.; Ferruti, P.; Ranucci, E.; Alongi, J. Sulfur-Based Copolymeric Polyamidoamines as Efficient Flame-Retardants for Cotton. Polymers 2019, 11, 1904. https://doi.org/10.3390/polym11111904
Beduini A, Carosio F, Ferruti P, Ranucci E, Alongi J. Sulfur-Based Copolymeric Polyamidoamines as Efficient Flame-Retardants for Cotton. Polymers. 2019; 11(11):1904. https://doi.org/10.3390/polym11111904
Chicago/Turabian StyleBeduini, Alessandro, Federico Carosio, Paolo Ferruti, Elisabetta Ranucci, and Jenny Alongi. 2019. "Sulfur-Based Copolymeric Polyamidoamines as Efficient Flame-Retardants for Cotton" Polymers 11, no. 11: 1904. https://doi.org/10.3390/polym11111904