New Triphenylamine-Based Oligomeric Schiff Bases Containing Tetraphenylsilane Moieties in the Backbone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis and Spectroscopic Characterization of Silylated Precursors and Monomers
2.1.2. Synthesis and Structural Characterization of PolySBs
3. Results and Discussion
3.1. Synthesis and Characterization of Diamines
3.2. Synthesis and Structural Characterization of Oligomers
3.3. Solubility
3.4. Thermal Properties and Molecular Weight
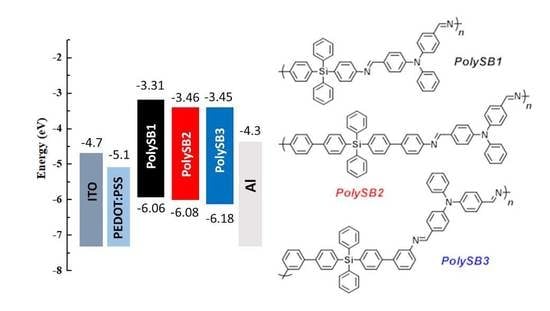
3.5. Optical and Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Tan, H.-S.; Guo, X.; Facchetti, A.; Yan, H. Material insights and challenges for non-fullerene organic solar cells based on small molecular acceptors. Nat. Energy 2018, 3, 720–731. [Google Scholar] [CrossRef]
- Ye, L.; Hu, H.; Ghasemi, M.; Wang, T.; Collins, B.A.; Kim, J.-H.; Jiang, K.; Carpenter, J.H.; Li, H.; Li, Z.; et al. Quantitative relations between interaction parameter, miscibility and function in organic solar cells. Nat. Mater. 2018, 17, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, G.; Kim, J.; Kwon, S.; Kim, H.; Lee, K. Bulk-Heterojunction Organic Solar Cells: Five Core Technologies for Their Commercialization. Adv. Mater. 2016, 28, 7821–7861. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, M.; Catanescu, C.O. Imine Oligomers and Polymers. J. Macromol. Sci. Part C Polym. Rev. 2004, 44, 131–173. [Google Scholar] [CrossRef]
- Iwan, A.; Schab-Balcerzak, E.; Korona, K.P.M.; Grankowska, S.; Kaminska, M. Investigation of optical and electrical properties of new aromatic polyazomethine with thiophene and cardo moieties toward application in organic solar cells. Synth. Met. 2013, 185–186, 17–24. [Google Scholar] [CrossRef]
- Kamacı, M.; Avcı, A.; Kaya, I. The crosslinked poly(azomethine-urethane)s containing o-hydroxyazomethine: Tunable multicolor emission, photophysical and thermal properties. Prog. Org. Coat. 2015, 88, 325–336. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Wen, H.; Niu, H.; Wang, C.; Qin, C.; Bai, X.; Lei, L.; Wang, W. Immobilized polyazomethines containing triphenylamine groups on ITO: Synthesis and acidochromic, electrochemical, electrochromic and photoelectronic propertie. RSC Adv. 2016, 6, 4564–4575. [Google Scholar] [CrossRef]
- Iwan, A. An overview of LC polyazomethines with aliphatic–aromatic moieties: Thermal, optical, electrical and photovoltaic properties. Renew. Sustain. Energy Rev. 2015, 52, 65–79. [Google Scholar] [CrossRef]
- Yea, H.; Jiang, F.; Li, H.; Xu, Z.; Yin, J.; Zhu, H. Facile synthesis of conjugated polymeric Schiff base as negative electrodes for lithium ion batteries. Electrochim. Acta 2017, 253, 319–323. [Google Scholar] [CrossRef]
- Verde-Sesto, E.; Maya, E.M.; Lozano, A.E.; de la Campa, J.G.; Sanchez, F.; Iglesias, M. Novel efficient catalysts based on imine-linked mesoporous polymers for hydrogenation and cyclopropanation reactions. J. Mater. Chem. 2012, 22, 24637–24643. [Google Scholar] [CrossRef]
- Petrus, M.L.; Bouwer, R.K.M.; Lafont, U.; Athanasopoulos, S.; Greenham, N.C.; Dingemans, T.J. Small-Molecule Azomethines: Organic Photovoltaics via Schiff Base Condensation Chemistry. J. Mater. Chem. A 2014, 2, 9474–9477. [Google Scholar] [CrossRef]
- Iwan, A.; Sek, D. Processible polyazomethines and polyketanils:from aerospace to light-emitting diodes and other advanced applications. Prog. Polym. Sci. 2008, 33, 289–345. [Google Scholar] [CrossRef]
- Hu, Y.C.; Chen, J.C.; Yen, H.J.; Lin, K.Y.; Yeh, J.M.; Chen, W.C.; Liou, G.S. Novel triphenylamine-containing ambipolar polyimides with pendant anthraquinone moiety for polymeric memory device, electrochromic and gas separation applications. J. Mater. Chem. 2012, 22, 20394–20402. [Google Scholar] [CrossRef]
- Nowak, E.M.; Sanetra, J.; Grucela, M.; Schab-Balcerzak, E. Azomethine naphthalene diimides as component of active layers in bulk heterojunction solar cells. Mater. Lett. 2015, 157, 93–98. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.S. Solution-processable triarylamine-based electroactive high performance polymers for anodically electrochromic applications. Polym. Chem. 2012, 3, 255–264. [Google Scholar] [CrossRef]
- Hacioglu, S.O.; Toksabay, S.; Sendur, M.; Toppare, L. Synthesis and electrochromic properties of triphenylamine containing copolymers: Effect of π-bridge on electrochemical properties. J. Polym. Sci. A Polym. Chem. 2014, 52, 537–544. [Google Scholar] [CrossRef]
- Xiong, J.; Wei, Z.; Xu, T.; Zhang, Y.; Xiong, C.; Dong, L. Polytriphenylamine derivative with enhanced electrochemical performance as the organic cathode material for rechargeable batteries. Polymer 2017, 130, 135–142. [Google Scholar] [CrossRef]
- Wang, X.; Lv, L.; Gu, W.; Wang, X.-I.; Dong, T.; Yang, Z.; Cao, H.; Huang, H. Novel triphenylamine-based copolymers for all-polymer solar cells. Dyes Pigm. 2017, 140, 141–149. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Z.-K.; Ye, T.-L.; Dai, Y.-F.; Ma, D.-G.; Ma, Z.; Liu, Q.-D.; Chen, Y. Novel fluorene-based light-emitting copolymers containing cyanophenyl pendants and carbazole-triphenylamines: Synthesis, characterization and their PLED application. Polymer 2010, 51, 1270–1278. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Hou, J.; Hayden, A.E.; Yang, H.; Houk, K.N.; Yang, Y. Silicon Atom Substitution Enhances Interchain Packing in a Thiophene-Based Polymer System. Adv. Mater. 2009, 21, 1–5. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, J.Y.L.; Chen, S.; Li, Y.; Jiang, K.; Zhao, J.; Li, Z.; Hu, H.; Ma, T.; Lin, H.; et al. Efficient non-fullerene polymer solar cells enabled by tetrahedron-shaped core based 3D-structure small-molecular electron acceptors. J. Mater. Chem. A 2015, 3, 13632–13636. [Google Scholar] [CrossRef]
- Moncada, J.; Terraza, C.A.; Tagle, L.H.; Coll, D.; Ortiz, P.; Pérez, G.; de la Campa, J.G.; Álvarez, C.; Tundidor-Camba, A. Synthesis, characterization and studies of properties of six polyimides derived from two new aromatic diamines containing a central silicon atom. Eur. Polym. J. 2017, 91, 354–367. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ren, H.; Yuan, Y.; Yu, G.; Zhu, G. Construction and adsorption properties of porous aromatic frameworks via AlCl3-triggered coupling polymerization. J. Mater. Chem. A 2014, 2, 11091–11098. [Google Scholar] [CrossRef]
- Kim, G.W.; Yang, D.R.; Kim, Y.C.; Yang, H.I.; Fan, J.G.; Lee, C.-H.; Chai, K.I.; Kwon, J.H. Di(biphenyl)silane and carbazole based bipolar host materials for highly efficient blue phosphorescent OLEDs. Dyes Pigm. 2017, 136, 8–16. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, G.; Hu, D.; Shen, F.; Lv, Y.; Sun, G.; Yang, B.; Lu, P.; Ma, Y. A Highly Efficient, Blue-Phosphorescent Device Based on a Wide-Bandgap Host/FIrpic: Rational Design of the Carbazole and Phosphine Oxide Moieties on Tetraphenylsilane. Adv. Funct. Mater. 2012, 22, 2830–2836. [Google Scholar] [CrossRef]
- Yeh, H.-C.; Chien, C.-H.; Shih, P.-I.; Yuan, M.-C.; Shu, C.-F. Polymers Derived from 3,6-Fluorene and Tetraphenylsilane Derivatives: Solution-Processable Host Materials for Green Phosphorescent OLEDs. Macromolecules 2008, 41, 3801–3807. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, X.; Li, H.; Sun, X.; Zheng, Y.; Ren, Z.; Ma, D.; Bryce, M.R.; Yan, S. A versatile hybrid polyphenylsilane host for highly efficient solution-processed blue and deep blue electrophosphorescence. J. Mater. Chem. C 2014, 2, 8277–8284. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Bai, Q.; Li, J.; Lu, F.; Sun, X.; Lu, P. Synthesis and properties of wide bandgap polymers based on tetraphenylsilane and their applications as hosts in electrophosphorescent devices. New J. Chem. 2018, 42, 3344–3349. [Google Scholar] [CrossRef]
- Tundidor-Camba, A.; González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Tagle, L.H.; Hauyón, R.A.; Sobarzo, P.A.; González, A.; Ortiz, P.A.; Maya, E.M.; et al. Silylated oligomeric poly(ether-azomethine)s from monomers containing biphenyl moieties: Synthesis and characterization. RSC Adv. 2018, 8, 1296–1312. [Google Scholar] [CrossRef]
- Cai, M.; Xiao, T.; Hellerich, E.; Chen, Y.; Shinar, R.; Shinar, J. High-Effi ciency Solution-Processed Small Molecule Electrophosphorescent Organic Light-Emitting Diodes. Adv. Mater. 2011, 23, 3590–3596. [Google Scholar] [CrossRef]
- Tundidor-Camba, A.; González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Tagle, L.H.; Sobarzo, P.A.; González, A.; Hauyón, R.A.; Mariman, A.P.; Terraza, C.A. Diphenylsilane-containing linear and rigid whole aromatic poly(azomethine)s. Structural and physical characterization. Polymer 2018, 150, 232–243. [Google Scholar] [CrossRef]
- Fei, T.; Cheng, G.; Hu, D.H.; Lu, P.; Ma, Y.G. A wide band gap polymer derived from 3,6-carbazole and tetraphenylsilane as host for green and blue phosphorescent complexes. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4784–4792. [Google Scholar] [CrossRef]
- Jung, S.O.; Kim, Y.-H.; Kwon, S.K.; Oh, H.-Y.; Yang, J.-H. New hole blocking material for green-emitting phosphorescent organic electroluminescent devices. Org. Electron. 2007, 8, 349–356. [Google Scholar] [CrossRef]
- Pratt, J.R.; Massey, W.D.; Pinkerton, F.H.; Thames, S.F. Organosilicon compounds. XX. Synthesis of aromatic diamines via trimethylsilyl-protecting aniline intermediates. J. Org. Chem. 1975, 40, 1090–1094. [Google Scholar] [CrossRef]
- Ueno, A.; Kitawaki, T.; Chida, N. Total Synthesis of (±)-Murrayazoline. Org. Lett. 2008, 10, 1999–2002. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.E.; Hughes, G.; Batsanov, A.S.; Bryce, M.R.; Parry, P.R.; Tarbit, B. Palladium-Catalyzed Cross-Coupling Reactions of Pyridylboronic Acids with Heteroaryl Halides Bearing a Primary Amine Group: Synthesis of Highly Substituted Bipyridines and Pyrazinopyridines. J. Org. Chem. 2005, 70, 388–390. [Google Scholar] [CrossRef] [PubMed]
- González-Henríquez, C.M.; Terraza, C.A.; Sarabia, M. Theoretical and Experimental Vibrational Spectroscopic Investigation of Two R1R2-Diphenylsilyl-Containing Monomers and Their Optically Active Derivative Polymer. J. Phys. Chem. A 2014, 118, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Terraza, C.A.; Tagle, L.H.; Concha, F.; Poblete, L. Synthesis and characterization of new bi-functional monomers based on germarylene or silarylene units: 4,4′-(R1R2-silylene)bis(phenyl chloroformates) and 4,4′-(R1R2-germylene)bis(phenyl chloroformates). Des. Monomers Polym. 2007, 10, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Iwan, A.; Boharewicz, B.; Parafiniuk, K.; Tazbir, I.; Gorecki, L.; Sikora, A.; Filapek, M.; Schab-Balcerzak, E. New Air-Stable Aromatic Polyazomethines with Triphenylamine or Phenylenevinylene Moieties towards Photovoltaic Application. Synth. Met. 2014, 195, 341–349. [Google Scholar] [CrossRef]
- Terraza, C.A.; Tagle, L.H.; Tundidor-Camba, A.; González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Coll, D. Synthesis and characterization of aromatic poly(ether-imide)s based on bis(4-(3,4-dicarboxyphenoxy)phenyl)-R,R-silane anhydrides (R = Me, Ph)–spontaneous formation of surface micropores from THF solutions. RSC Adv. 2016, 6, 49335–49347. [Google Scholar] [CrossRef]
- Wen, H.; Niu, H.; Li, B.; Ma, X.; Bai, X.; Zhang, Y.; Wang, W. Synthesis and Acidochromic, Electrochromic Properties of Schiff Bases Containing Furan and Triphenylamine Units. Synth. Met. 2015, 202, 89–97. [Google Scholar] [CrossRef]
- Liou, G.; Lin, H.; Hsieh, Y.; Yang, Y. Synthesis and Characterization of Wholly Aromatic Poly(azomethine)s Containing Donor–Acceptor Triphenylamine Moieties. Polym. Sci. Part A Polym. Chem. 2007, 45, 4921–4932. [Google Scholar] [CrossRef]
- Liu, H.; Bai, Q.; Yao, L.; Hu, D.; Tang, X.; Shen, F.; Zhang, H.; Gao, Y.; Lu, P.; Yang, B.; et al. Solution-Processable Hosts Constructed by Carbazole/PO Substituted Tetraphenylsilanes for Efficient Blue Electrophosphorescent Devices. Adv. Funct. Mater. 2014, 24, 5881–5888. [Google Scholar] [CrossRef]
- Sanchez, C.O.; Schott, E.; Zarate, X.; MacLeod-Carey, D.; Sobarzo, P.; Gatica, N. Effect of triphenylamine as electron-donor evenly spaced in 2, 4, 6 and 8 thiophene units of the main chain: Synthesis and properties. Polym. Bull. 2015, 72, 897–913. [Google Scholar] [CrossRef]
- Iwan, A.; Palewicz, M.; Chuchmała, A.; Gorecki, L.; Sikora, A.; Mazurek, B.; Pasciak, G. Opto(Electrical) Properties of New Aromatic Polyazomethines with Fluorene Moieties in the Main Chain for Polymeric Photovoltaic Devices. Synth. Met. 2012, 162, 143–153. [Google Scholar] [CrossRef]
- Marin, L.; Bejan, A.; Ailincai, D.; Belei, D. Poly(azomethine-phenothiazine)s with efficient emission in solid state. Eur. Polym. J. 2017, 95, 127–137. [Google Scholar] [CrossRef]
- Yıldırım, M.; Kaya, I. Electrochemical syntheses and characterizations of poly(2-aminobenzothiazole)s. Synth. Met. 2012, 162, 834–842. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, W.; Fan, Z.; Li, J.; Chen, X.; Luo, G.; Zhang, X. Synthesis and characterization of high triplet energy polyfluorene bearing m-tetraphenylsilane segment as a polymer host for green phosphorescent polymer light emitting diodes. Synth. Met. 2015, 204, 70–75. [Google Scholar] [CrossRef]
- Lee, J.; Rajeeva, B.B.; Yuan, T.; Guo, Z.-H.; Lin, Y.-H.; Al-Hashimi, M.; Zheng, Y.; Fang, L. Thermodynamic synthesis of solution processable ladder polymers. Chem. Sci. 2016, 7, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Niu, H.; Wen, H.; Wang, S.; Lian, Y.; Jiang, X.; Wang, C.; Bai, X.; Wang, W. Synthesis, electrochromic, halochromic and electro-optical properties of polyazomethines with a carbazole core and triarylamine units serving as functional groups. J. Mater. Chem. C 2015, 3, 3482–3493. [Google Scholar] [CrossRef]
- Sánchez, C.O.; Bèrnede, J.C.; Cattin, L.; Makha, M.; Gatica, N. Schiff base polymer based on triphenylamine moieties in the main chain. Characterization and studies in solar cells. Thin Solid Films 2014, 562, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Jessop, I.A.; Zamora, P.P.; Díaz, F.R.; del Valle, M.A.; Leiva, A.; Cattin, L.; Makha, M.; Bernede, J.C. New Polymers Based on 2,6-di(thiophen-2-yl)aniline and 2,2′-(thiophen-2,5-diyl)dianiline Monomers. Preparation, Characterization and Thermal, Optical, Electronic and Photovoltaic Properties. Int. J. Electrochem. Sci. 2012, 7, 9502–9517. [Google Scholar]
- Jo, J.W.; Jung, J.W.; Wang, H.-W.; Kim, P.; Russell, T.P.; Jo, W.H. Fluorination of Polythiophene Derivatives for High Performance Organic Photovoltaics. Chem. Mater. 2014, 26, 4214–4220. [Google Scholar] [CrossRef]
- Zhong, Z.; Wang, X.; Guo, T.; Cui, J.; Ying, L.; Peng, J.; Cao, Y. Crosslinkable triphenylamine-based hole-transporting polymers for solution-processed polymer light-emitting diodes. Org. Electron. 2018, 53, 35–42. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Wang, X. Semiconducting Polymer Composites: Principles, Morphologies, Properties and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Brabec, C.J.; Gowrisanker, S.; Halls, J.J.M.; Laird, D.; Jia, S.J.; Williams, S.P. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2010, 22, 3839–3856. [Google Scholar] [CrossRef] [PubMed]








| Polymer | T5% [°C] a | T10% [°C] a | Char Yield [%] b | Tg [°C] c |
|---|---|---|---|---|
| polySB1 | 341 | 411 | 50 | 266 |
| polySB2 | 478 | 502 | 64 | 334 |
| polySB3 | 442 | 475 | 52 | 257 |
| PolySBs | λmaxabs [nm] | λonsetabs [nm] | λmaxem [nm] | Egopt [eV] a | EHOMO [eV] b | ELUMO [eV] c | (M−1 cm−1) d |
|---|---|---|---|---|---|---|---|
| polySB1 | 394 | 451 | 493 | 2.75 | −6.06 | −3.31 | 18,257 |
| polySB2 | 403 | 473 | 498 | 2.62 | −6.08 | −3.46 | 15,047 |
| polySB3 | 397 | 457 | 499 | 2.71 | −6.18 | −3.45 | 13,667 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobarzo, P.A.; González, A.F.; Schott, E.; Tagle, L.H.; Tundidor-Camba, A.; González-Henríquez, C.; Jessop, I.A.; Terraza, C.A. New Triphenylamine-Based Oligomeric Schiff Bases Containing Tetraphenylsilane Moieties in the Backbone. Polymers 2019, 11, 216. https://doi.org/10.3390/polym11020216
Sobarzo PA, González AF, Schott E, Tagle LH, Tundidor-Camba A, González-Henríquez C, Jessop IA, Terraza CA. New Triphenylamine-Based Oligomeric Schiff Bases Containing Tetraphenylsilane Moieties in the Backbone. Polymers. 2019; 11(2):216. https://doi.org/10.3390/polym11020216
Chicago/Turabian StyleSobarzo, Patricio A., Alexis F. González, Eduardo Schott, Luis H. Tagle, Alain Tundidor-Camba, Carmen González-Henríquez, Ignacio A. Jessop, and Claudio A. Terraza. 2019. "New Triphenylamine-Based Oligomeric Schiff Bases Containing Tetraphenylsilane Moieties in the Backbone" Polymers 11, no. 2: 216. https://doi.org/10.3390/polym11020216
APA StyleSobarzo, P. A., González, A. F., Schott, E., Tagle, L. H., Tundidor-Camba, A., González-Henríquez, C., Jessop, I. A., & Terraza, C. A. (2019). New Triphenylamine-Based Oligomeric Schiff Bases Containing Tetraphenylsilane Moieties in the Backbone. Polymers, 11(2), 216. https://doi.org/10.3390/polym11020216