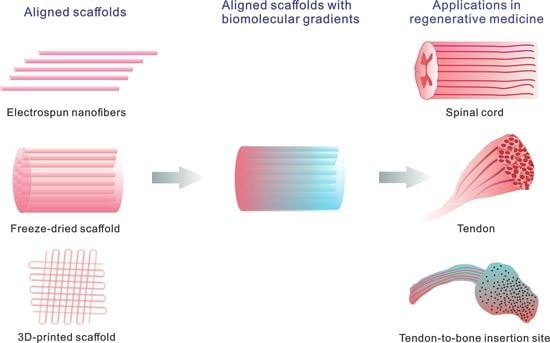
Aligned Scaffolds with Biomolecular Gradients for Regenerative Medicine
Abstract
:1. Introduction
2. Aligned Scaffolds
2.1. Electrospinning
2.2. Freeze Drying
2.3. 3D Printing
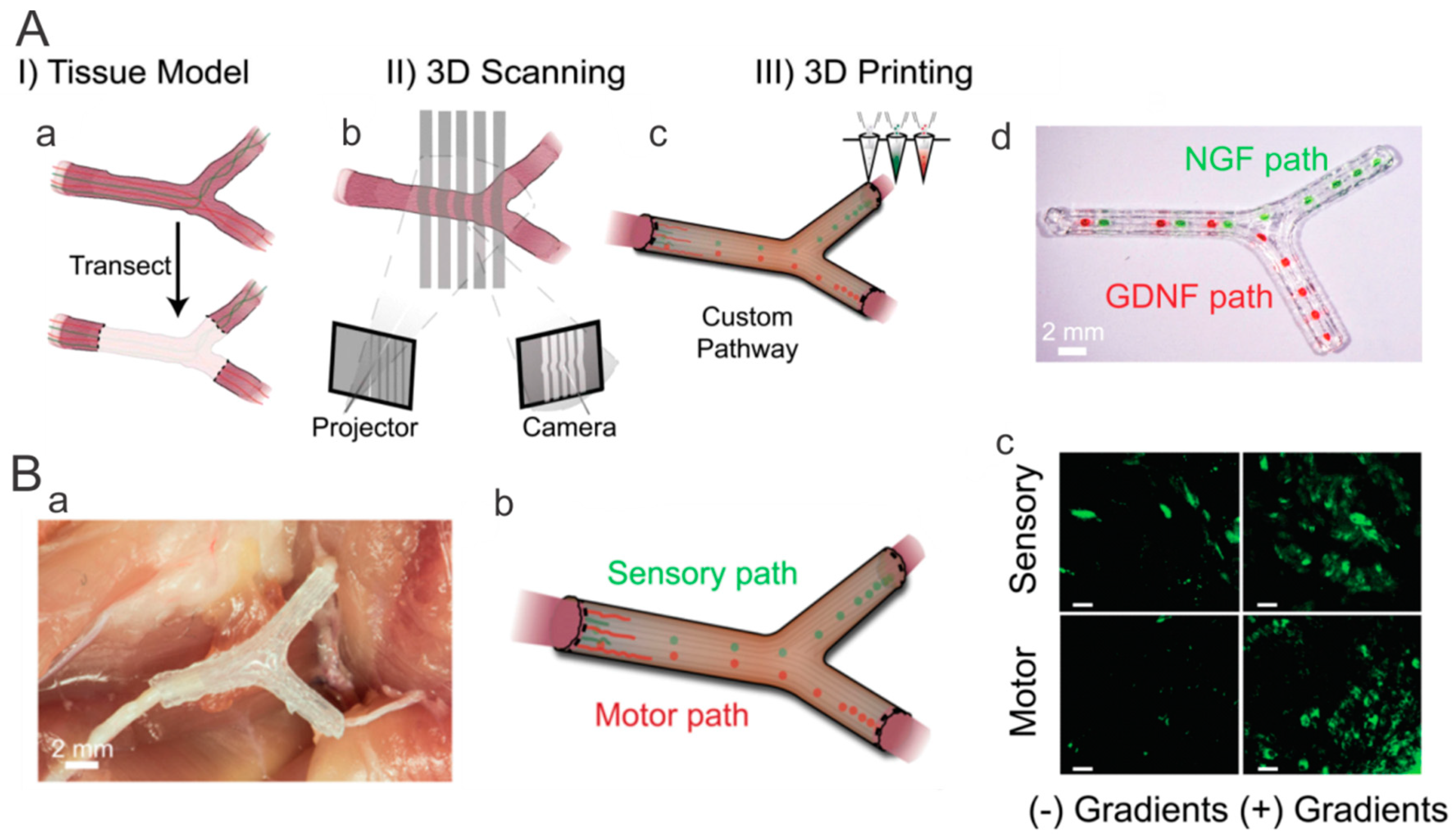
3. Aligned Scaffolds with Biomolecular Gradients
3.1. Direct Electrospinning
3.2. Direct 3D Printing
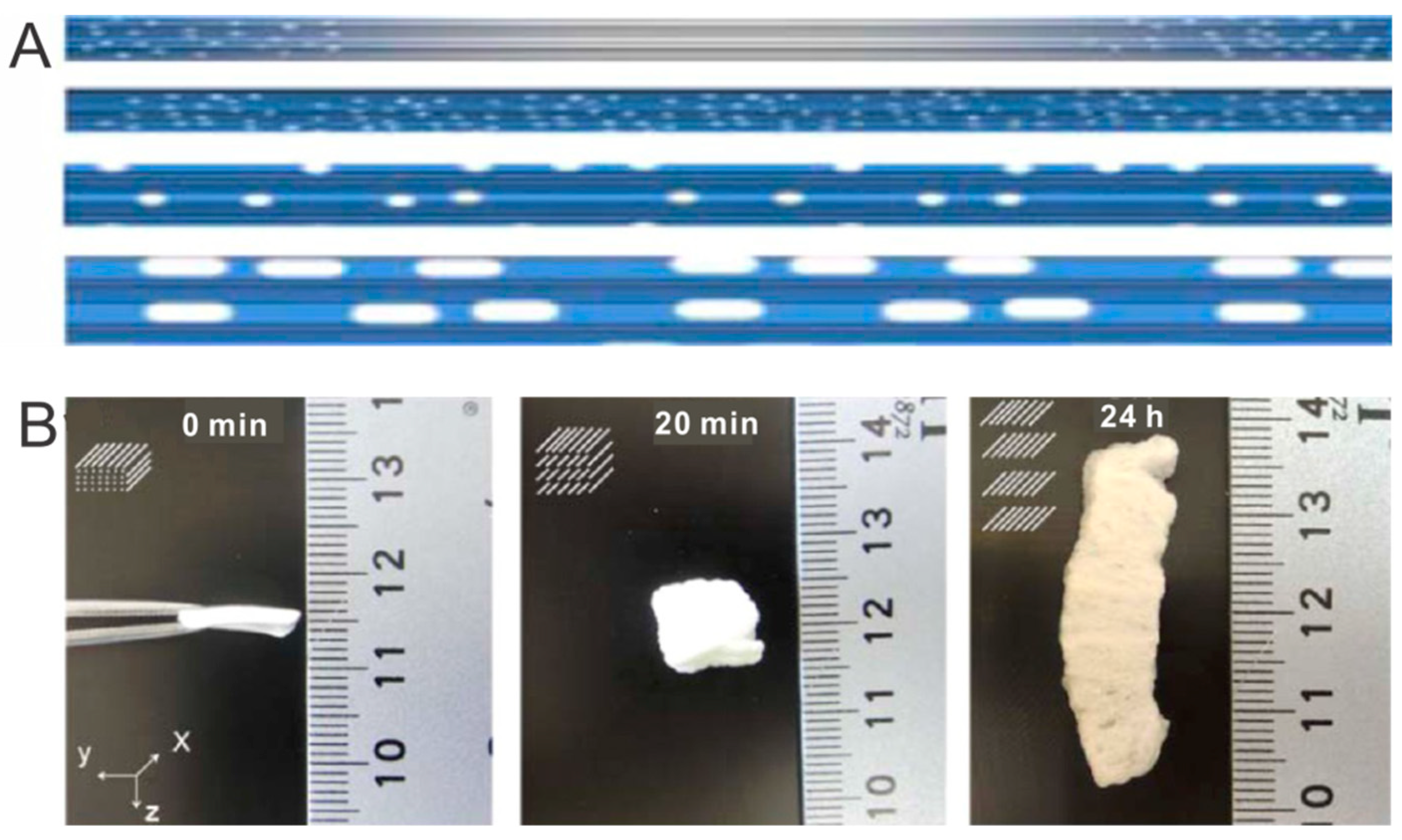
3.3. Post-Processing Treatment on Aligned Scaffolds–Inkjet Printing
3.4. Post-Processing Treatment on Aligned Scaffolds–Gradual Infusion
4. Applications in Regenerative Medicine
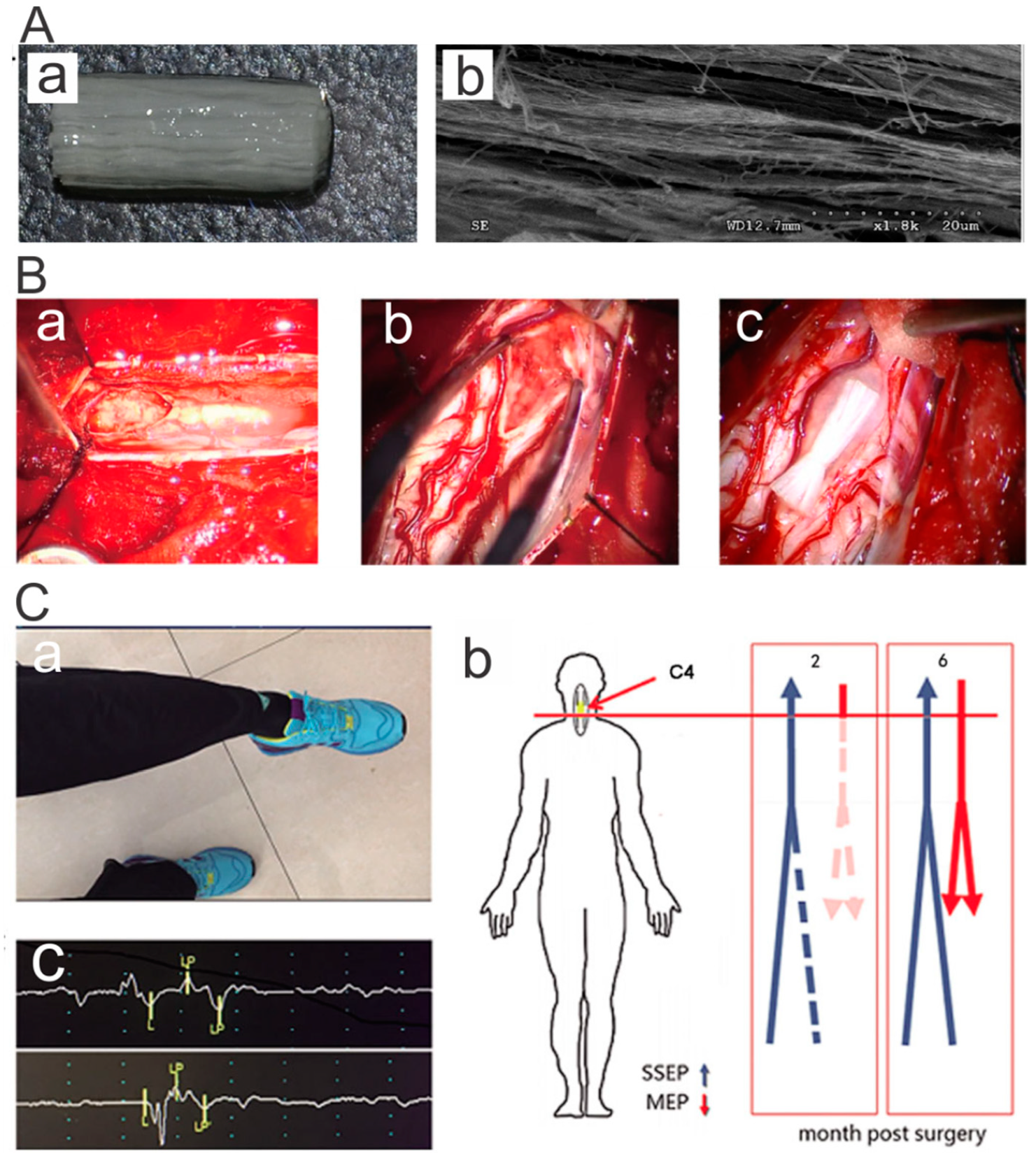
4.1. Nerve
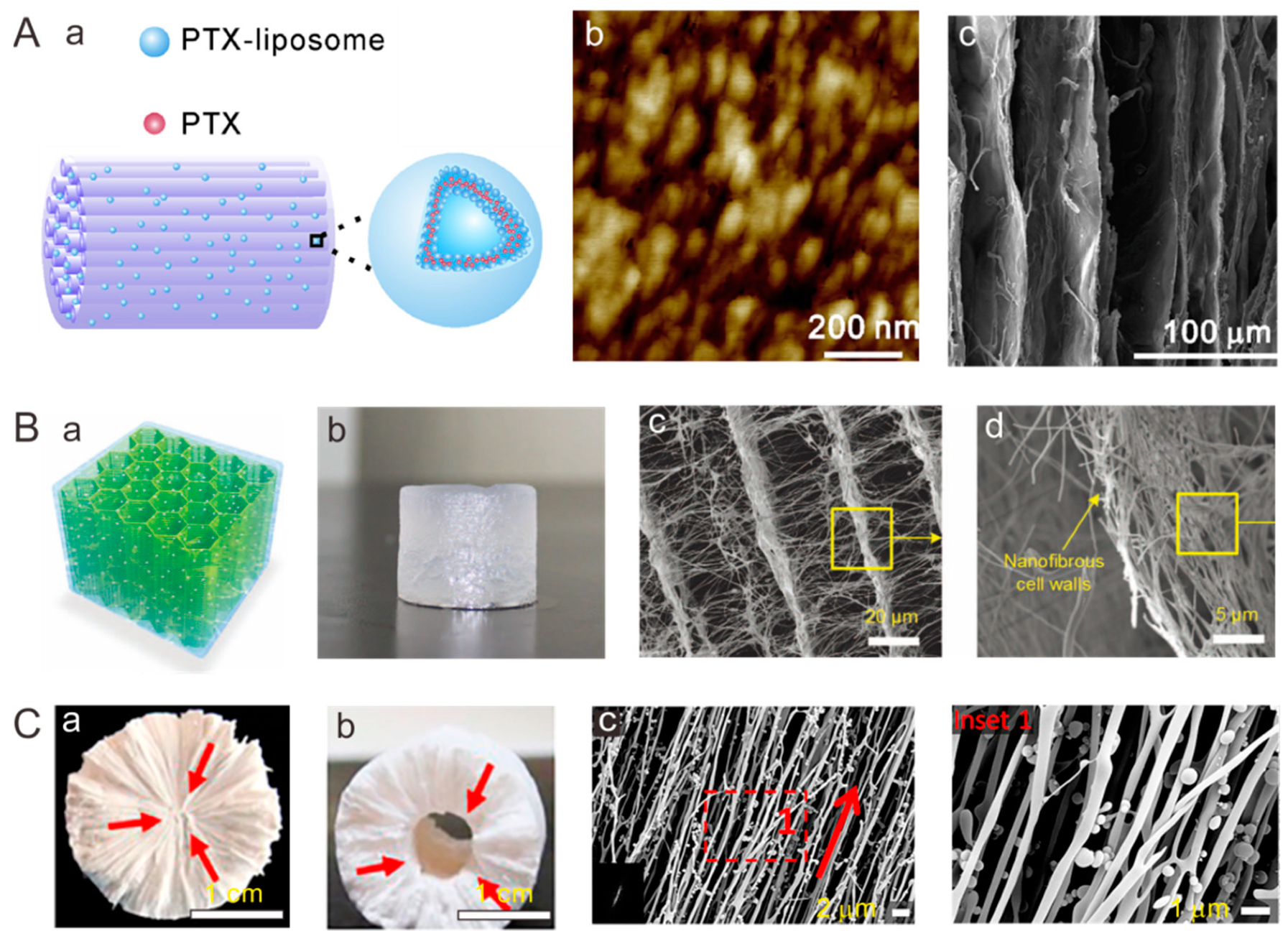
4.2. Tendon
4.3. Tendon/Ligament to Bone Insertion Site
5. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Guven, S.; Chen, P.; Inci, F.; Tasoglu, S.; Erkmen, B.; Demirci, U. Multiscale assembly for tissue engineering and regenerative medicine. Trends Biotechnol. 2015, 33, 269–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schell, J.Y.; Wilks, B.T.; Patel, M.; Franck, C.; Chalivendra, V.; Cao, X.; Shenoy, V.B.; Morgan, J.R. Harnessing cellular-derived forces in self-assembled microtissues to control the synthesis and alignment of ecm. Biomaterials 2016, 77, 120–129. [Google Scholar] [CrossRef]
- Shimozono, S.; Iimura, T.; Kitaguchi, T.; Higashijima, S.; Miyawaki, A. Visualization of an endogenous retinoic acid gradient across embryonic development. Nature 2013, 496, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Blood vessels and nerves: common signals, pathways and diseases. Nat. Rev. Genet. 2003, 4, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.R.; Granja, P.L.; Bártolo, P.J. Advances in electrospun skin substitutes. Prog. Mater. Sci. 2016, 84, 314–334. [Google Scholar] [CrossRef]
- Grayson, W.L.; Bunnell, B.A.; Martin, E.; Frazier, T.; Hung, B.P.; Gimble, J.M. Stromal cells and stem cells in clinical bone regeneration. Nat. Rev. Endocrinol. 2015, 11, 140–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Vashaghian, M.; Diedrich, C.M.; Zandieh-Doulabi, B.; Werner, A.; Smit, T.H.; Roovers, J.P. Gentle cyclic straining of human fibroblasts on electrospun scaffolds enhances their regenerative potential. Acta Biomater. 2019, 84, 159–168. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.L.; Xia, Y.N. Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv. Mater. 2004, 16, 361–366. [Google Scholar] [CrossRef]
- Shimomura, K.; Rothrauff, B.B.; Hart, D.A.; Hamamoto, S.; Kobayashi, M.; Yoshikawa, H.; Tuan, R.S.; Nakamura, N. Enhanced repair of meniscal hoop structure injuries using an aligned electrospun nanofibrous scaffold combined with a mesenchymal stem cell-derived tissue engineered construct. Biomaterials 2019, 192, 346–354. [Google Scholar] [CrossRef] [PubMed]
- González de Torre, I.; Ibáñez-Fonseca, A.; Quintanilla, L.; Alonso, M.; Rodríguez-Cabello, J.-C. Random and oriented electrospun fibers based on a multicomponent, in situ clickable elastin-like recombinamer system for dermal tissue engineering. Acta Biomater. 2018, 72, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.; Sun, J.; Levengood, S.; Zhang, M. Hyaluronic acid coated aligned nanofibers for promotion of glioblastoma migration. ACS Appl. Bio Mater. 2019. [Google Scholar] [CrossRef]
- Xie, J.W.; MacEwan, M.R.; Ray, W.Z.; Liu, W.Y.; Siewe, D.Y.; Xia, Y.N. Radially aligned, electrospun nanofibers as dural substitutes for wound closure and tissue regeneration applications. ACS Nano 2010, 4, 5027–5036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Barreto-Ortiz, S.F.; Yu, Y.; Ginn, B.P.; DeSantis, N.A.; Hutton, D.L.; Grayson, W.L.; Cui, F.Z.; Korgel, B.A.; et al. Creating polymer hydrogel microfibres with internal alignment via electrical and mechanical stretching. Biomaterials 2014, 35, 3243–3251. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Carlson, M.A.; Teusink, M.J.; Wang, H.J.; MacEwan, M.R.; Xie, J.W. Expanding two-dimensional electrospun nanofiber membranes in the third dimension by a modified gas-foaming technique. ACS Biomater. Sci. Eng. 2015, 1, 991–1001. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Z.; Wang, H.; Wang, Y.; Carlson, M.A.; Teusink, M.J.; MacEwan, M.R.; Gu, L.; Xie, J. Expanded 3D nanofiber scaffolds: Cell penetration, neovascularization, and host response. Adv. Healthc. Mater. 2016, 5, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Carlson, M.A.; Zhang, Y.S.; Hu, Y.; Xie, J.W. Fabrication of injectable and superelastic nanofiber rectangle matrices (“peanuts”) and their potential applications in hemostasis. Biomaterials 2018, 179, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Jia, X.; Yang, Y.; Yang, Q.; Gao, C.; Hu, S.; Zhao, Y.; Fan, Y.; Yuan, X. Nanofiber-mediated microRNA-126 delivery to vascular endothelial cells for blood vessel regeneration. Acta Biomater. 2016, 43, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Boda, S.K.; Batra, S.K.; Li, X.; Xie, J. Emerging roles of electrospun nanofibers in cancer research. Adv. Healthc. Mater. 2018, 7, e1701024. [Google Scholar] [CrossRef]
- Jiang, J.; Xie, J.; Ma, B.; Bartlett, D.E.; Xu, A.; Wang, C.H. Mussel-inspired protein-mediated surface functionalization of electrospun nanofibers for pH-responsive drug delivery. Acta Biomater. 2014, 10, 1324–1332. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.Z.; Sun, H.; Hostler, S.; Schiraldi, D.A. Effects of feather-fiber reinforcement on poly(vinyl alcohol)/clay aerogels: Structure, property and applications. Polymer 2018, 137, 201–208. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.X.; Alhassan, S.; Sun, H.; Choi, K.; Yu, C.; Schiraldi, D. Clay-facilitated aqueous dispersion of graphite and poly(vinyl alcohol) aerogels filled with binary nanofillers. Gels 2018, 4, 8. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Sun, L.; Kim, H.J.; Hu, X.; Rice, W.; Kluge, J.; Reis, R.L.; Kaplan, D.L. Aligned silk-based 3-D architectures for contact guidance in tissue engineering. Acta Biomater. 2012, 8, 1530–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Chen, Y.; Delattre, B.; Tomsia, A.P.; Ritchie, R.O. Bioinspired large-scale aligned porous materials assembled with dual temperature gradients. Sci. Adv. 2015, 1, e1500849. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wang, D.; Delattre, B.; Gao, W.; De Coninck, J.; Li, S.; Tomsia, A.P. Biomimetic gradient scaffold from ice-templating for self-seeding of cells with capillary effect. Acta Biomater. 2015, 20, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Han, J.; Zhao, Y.; Ding, W.; Wei, J.; Li, J.; Han, S.; Shang, X.; Wang, B.; Chen, B.; et al. Functionalized collagen scaffold implantation and cAMP administration collectively facilitate spinal cord regeneration. Acta Biomater. 2016, 30, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, C.; Xiao, Z.; Zhao, Y.; Zhang, H.; Sun, J.; Zhuang, Y.; Wu, X.; Shi, J.; Chen, Y.; et al. A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/beta-catenin signaling for spinal cord injury repair. Biomaterials 2018, 183, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Yu, J.; Tang, X.; Ge, J.; Ding, B. Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality. Nat. Commun. 2014, 5, 5802. [Google Scholar] [CrossRef] [Green Version]
- Si, Y.; Wang, L.; Wang, X.; Tang, N.; Yu, J.; Ding, B. Ultrahigh-water-content, superelastic, and shape-memory nanofiber-assembled hydrogels exhibiting pressure-responsive conductivity. Adv. Mater. 2017, 29, 1700339. [Google Scholar] [CrossRef]
- Fan, L.; Li, J.L.; Cai, Z.; Wang, X. Creating biomimetic anisotropic architectures with co-aligned nanofibers and macrochannels by manipulating ice crystallization. ACS Nano 2018. [Google Scholar] [CrossRef]
- Capel, A.J.; Rimington, R.P.; Lewis, M.P.; Christie, S.D.R. 3D printing for chemical, pharmaceutical and biological applications. Nat. Rev. Chem. 2018, 2, 422–436. [Google Scholar] [CrossRef]
- Jang, J.; Park, J.Y.; Gao, G.; Cho, D.-W. Biomaterials-based 3d cell printing for next-generation therapeutics and diagnostics. Biomaterials 2018, 156, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, M.; Kim, G.H. 3d-printed biomimetic scaffold simulating microfibril muscle structure. Adv. Funct. Mater. 2018, 28, 1800405. [Google Scholar] [CrossRef]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X. 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv. Mater. 2015, 27, 4035–4040. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Pitacco, P.; Nulty, J.; Cunniffe, G.M.; Kelly, D.J. 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials 2018, 162, 34–46. [Google Scholar] [CrossRef]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and functionally optimized silk-fibroin-gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3d printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef]
- Hong, J.; Shin, Y.; Kim, S.; Lee, J.; Cha, C. Complex tuning of physical properties of hyperbranched polyglycerol-based bioink for microfabrication of cell-laden hydrogels. Adv. Funct. Mater. 2019, 1808750. [Google Scholar] [CrossRef]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3d printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef]
- Dinis, T.M.; Elia, R.; Vidal, G.; Auffret, A.; Kaplan, D.L.; Egles, C. Method to form a fiber/growth factor dual-gradient along electrospun silk for nerve regeneration. ACS Appl. Mater. Interfaces 2014, 6, 16817–16826. [Google Scholar] [CrossRef] [PubMed]
- Kishan, A.P.; Robbins, A.B.; Mohiuddin, S.F.; Jiang, M.; Moreno, M.R.; Cosgriff-Hernandez, E.M. Fabrication of macromolecular gradients in aligned fiber scaffolds using a combination of in-line blending and air-gap electrospinning. Acta Biomater. 2017, 56, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Sun, J.; Zhuang, Y.; Shi, J.; Guan, D.; Chen, Y.; Dai, J. Radially aligned electrospun fibers with continuous gradient of SDF1alpha for the guidance of neural stem cells. Small 2016, 12, 5009–5018. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, L.G.; Smith, B.T.; Watson, E.; Arumugasaamy, N.; Mikos, A.G.; Fisher, J.P. 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater. 2017, 56, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xu, Z.Y.; Liang, Q.F.; Liu, B.; Li, H.F.; Wu, Y.H.; Zhang, Y.Y.; Lin, Z.F.; Wu, M.M.; Ruan, C.S.; et al. Direct 3D printing of high strength biohybrid gradient hydrogel scaffolds for efficient repair of osteochondral defect. Adv. Funct. Mater. 2018, 28, 1706644. [Google Scholar] [CrossRef]
- Guo, J.; Ling, S.J.; Li, W.Y.; Chen, Y.; Li, C.M.; Omenetto, F.G.; Kaplan, D.L. Coding cell micropatterns through peptide inkjet printing for arbitrary biomineralized architectures. Adv. Funct. Mater. 2018, 28, 1800228. [Google Scholar] [CrossRef]
- Choi, M.; Park, H.H.; Choi, D.; Han, U.; Park, T.H.; Lee, H.; Park, J.; Hong, J. Multilayer nanofilms via inkjet printing for stabilizing growth factor and designing desired cell developments. Adv. Healthc. Mater. 2017, 6, 1701089. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Yoon, S.; Kwon, J.; Now, H.; Kim, Y.K.; Kim, W.J.; Yoo, J.Y.; Jung, S. Freeform micropatterning of living cells into cell culture medium using direct inkjet printing. Sci. Rep. 2017, 7, 14610. [Google Scholar] [CrossRef]
- Ker, E.D.; Nain, A.S.; Weiss, L.E.; Wang, J.; Suhan, J.; Amon, C.H.; Campbell, P.G. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials 2011, 32, 8097–8107. [Google Scholar] [CrossRef]
- Jia, C.; Luo, B.; Wang, H.; Bian, Y.; Li, X.; Li, S.; Wang, H. Precise and arbitrary deposition of biomolecules onto biomimetic fibrous matrices for spatially controlled cell distribution and functions. Adv. Mater. 2017, 29, 1701154. [Google Scholar] [CrossRef]
- Li, X.; Liang, H.; Sun, J.; Zhuang, Y.; Xu, B.; Dai, J. Electrospun collagen fibers with spatial patterning of SDF1alpha for the guidance of neural stem cells. Adv. Healthc. Mater. 2015, 4, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, J.; Lipner, J.; Yuan, X.; Thomopoulos, S.; Xia, Y. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett. 2009, 9, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Liu, Y.; Luo, X.; Chen, F.; Guo, X.; Li, X. Electrospun fibrous scaffolds with continuous gradations in mineral contents and biological cues for manipulating cellular behaviors. Acta Biomater. 2012, 8, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Yu, S.; Zhou, N.; Deng, J.; Gao, C. Controlling the selective and directional migration of hepatocytes by a complementary density gradient of glycosylated hyperbranched polymers and poly(ethylene glycol) molecules. Acta Biomater. 2017, 56, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xue, J.; Li, H.; Zhu, C.; Mo, X.; Xia, Y. General method for generating circular gradients of active proteins on nanofiber scaffolds sought for wound closure and related applications. ACS Appl. Mater. Interfaces 2018, 10, 8536–8545. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, F.; Xiao, Z.; Han, G.; Wang, N.; Yin, N.; Chen, B.; Jiang, X.; Yun, C.; Han, W.; et al. Clinical study of NeuroRegen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 2017, 26, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Gallo, V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018, 21, 9–15. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Xiao, Z.; Zhao, Y.; Han, S.; Chen, B.; Dai, J. Scaffold-facilitated locomotor improvement post complete spinal cord injury: motor axon regeneration versus endogenous neuronal relay formation. Biomaterials 2019, 197, 20–31. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Z.; Han, J.; Chen, L.; Xiao, H.; Ma, F.; Hou, X.; Li, X.; Sun, J.; Ding, W.; et al. Promotion of neuronal differentiation of neural progenitor cells by using EGFR antibody functionalized collagen scaffolds for spinal cord injury repair. Biomaterials 2013, 34, 5107–5116. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Zhao, Y.; Ding, W.; Wei, J.; Han, S.; Shang, X.; Wang, B.; Chen, B.; Xiao, Z.; et al. Functionalized collagen scaffold neutralizing the myelin-inhibitory molecules promoted neurites outgrowth in vitro and facilitated spinal cord regeneration in vivo. ACS Appl. Mater. Interfaces 2015, 7, 13960–13971. [Google Scholar] [CrossRef]
- Chen, B.; Xiao, Z.F.; Zhao, Y.N.; Dai, J. Functional biomaterial-based regenerative microenvironment for spinal cord injury repair. Natl. Sci. Rev. 2017, 4, 530–532. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Tang, F.; Tang, J.; Yang, H.; Zhao, Y.; Chen, B.; Han, S.; Wang, N.; Li, X.; Cheng, S.; et al. One-year clinical study of NeuroRegen scaffold implantation following scar resection in complete chronic spinal cord injury patients. Sci. China Life Sci. 2016, 59, 647–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhao, Y.; Cheng, S.; Han, S.; Shu, M.; Chen, B.; Chen, X.; Tang, F.; Wang, N.; Tu, Y.; et al. Cetuximab modified collagen scaffold directs neurogenesis of injury-activated endogenous neural stem cells for acute spinal cord injury repair. Biomaterials 2017, 137, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhao, Y.; Xiao, Z.; Wang, B.; Liang, H.; Li, X.; Fang, Y.; Han, S.; Li, X.; Fan, C.; et al. A dual functional scaffold tethered with EGFR antibody promotes neural stem cell retention and neuronal differentiation for spinal cord injury repair. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xiao, Z.; Zhao, Y.; Wang, B.; Li, X.; Li, J.; Dai, J. Collagen scaffold combined with human umbilical cord-derived mesenchymal stem cells promote functional recovery after scar resection in rats with chronic spinal cord injury. J. Tissue Eng. Regen. Med. 2018, 12, e1154–e1163. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, J.; Xiao, Z.; Zhao, Y.; Han, S.; Liu, D.; Yin, W.; Li, J.; Li, J.; Wanggou, S.; et al. Transplantation of hUC-MSCs seeded collagen scaffolds reduces scar formation and promotes functional recovery in canines with chronic spinal cord injury. Sci. Rep. 2017, 7, 43559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Li, X.; Zhao, Y.; Xiao, Z.; Xue, W.; Sun, J.; Li, X.; Zhuang, Y.; Chen, Y.; Dai, J. Cetuximab and Taxol co-modified collagen scaffolds show combination effects for the repair of acute spinal cord injury. Biomater. Sci. 2018, 6, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, X.; Xiao, Z.; Zhao, Y.; Liang, H.; Wang, B.; Han, S.; Li, X.; Xu, B.; Wang, N.; et al. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017, 51, 304–316. [Google Scholar] [CrossRef]
- Han, S.; Wang, B.; Jin, W.; Xiao, Z.; Li, X.; Ding, W.; Kapur, M.; Chen, B.; Yuan, B.; Zhu, T.; et al. The linear-ordered collagen scaffold-BDNF complex significantly promotes functional recovery after completely transected spinal cord injury in canine. Biomaterials 2015, 41, 89–96. [Google Scholar] [CrossRef]
- Yin, W.; Li, X.; Zhao, Y.; Tan, J.; Wu, S.; Cao, Y.; Li, J.; Zhu, H.; Liu, W.; Tang, G.; et al. Taxol-modified collagen scaffold implantation promotes functional recovery after long-distance spinal cord complete transection in canines. Biomater. Sci. 2018, 6, 1099–1108. [Google Scholar] [CrossRef]
- Xiao, Z.; Tang, F.; Zhao, Y.; Han, G.; Yin, N.; Li, X.; Chen, B.; Han, S.; Jiang, X.; Yun, C.; et al. Significant improvement of acute complete spinal cord injury patients diagnosed by a combined criteria implanted with NeuroRegen scaffolds and mesenchymal stem cells. Cell Transplant. 2018, 27, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.A.; Krick, K.D.; Ju, A.; Meng, F.; et al. 3D printed anatomical nerve regeneration pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lipner, J.; Moran, C.H.; Feng, L.; Li, X.; Thomopoulos, S.; Xia, Y. Generation of electrospun nanofibers with controllable degrees of crimping through a simple, plasticizer-based treatment. Adv. Mater. 2015, 27, 2583–2588. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.B.; Chainani, A.; Hippensteel, K.J.; Kishan, A.; Gilchrist, C.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015, 24, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliari, S.R.; Harley, B.A. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 2011, 32, 5330–5340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younesi, M.; Islam, A.; Kishore, V.; Anderson, J.M.; Akkus, O. Tenogenic induction of human MSCs by anisotropically aligned collagen biotextiles. Adv. Funct. Mater. 2014, 24, 5762–5770. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, X.; Zhu, T.; Hu, J.J.; Song, H.X.; Shen, W.L.; Jiang, L.Y.; Heng, B.C.; Ji, J.F.; Ouyang, H.W. The effect of decellularized matrices on human tendon stem/progenitor cell differentiation and tendon repair. Acta Biomater. 2013, 9, 9317–9329. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, H.; Liu, H.; Chen, X.; Lu, P.; Zhu, T.; Yang, L.; Yin, Z.; Heng, B.C.; Zhang, Y.; et al. Well-aligned chitosan-based ultrafine fibers committed teno-lineage differentiation of human induced pluripotent stem cells for Achilles tendon regeneration. Biomaterials 2015, 53, 716–730. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Williams, G.R.; Gimbel, J.A.; Favata, M.; Soslowsky, L.J. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J. Orthop. Res. 2003, 21, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Deymier, A.C.; An, Y.; Boyle, J.J.; Schwartz, A.G.; Birman, V.; Genin, G.M.; Thomopoulos, S.; Barber, A.H. Micro-mechanical properties of the tendon-to-bone attachment. Acta Biomater. 2017, 56, 25–35. [Google Scholar] [CrossRef]
- Ansari, S.; Khorshidi, S.; Karkhaneh, A. Engineering of gradient osteochondral tissue: From nature to lab. Acta Biomater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Yang, L.; Zhang, E.; Zhang, R.; Cai, D.D.; Zhu, S.; Ran, J.; Bunpetch, V.; Cai, Y.; Heng, B.C.; et al. Biomimetic tendon extracellular matrix composite gradient scaffold enhances ligament-to-bone junction reconstruction. Acta Biomater. 2017, 56, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lipner, J.; Xie, J.; Manning, C.N.; Thomopoulos, S.; Xia, Y. Nanofiber scaffolds with gradients in mineral content for spatial control of osteogenesis. ACS Appl. Mater. Interfaces 2014, 6, 2842–2849. [Google Scholar] [CrossRef]
- Madhurakkat Perikamana, S.K.; Lee, J.; Ahmad, T.; Kim, E.M.; Byun, H.; Lee, S.; Shin, H. Harnessing biochemical and structural cues for tenogenic differentiation of adipose derived stem cells (ADSCs) and development of an in vitro tissue interface mimicking tendon-bone insertion graft. Biomaterials 2018, 165, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Robinson, J.; Lu, H. Biomimetic strategies for engineering composite tissues. Curr. Opin. Biotechnol. 2016, 40, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Y.S.; Heinrich, M.A.; De Ferrari, F.; Jang, H.L.; Bakht, S.M.; Alvarez, M.M.; Yang, J.; Li, Y.C.; Trujillo-de Santiago, G.; et al. Rapid continuous multimaterial extrusion bioprinting. Adv. Mater. 2017, 29, 1604630. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv. Healthc. Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef]
- Ying, G.L.; Jiang, N.; Maharjan, S.; Yin, Y.X.; Chai, R.R.; Cao, X.; Yang, J.Z.; Miri, A.K.; Hassan, S.; Zhang, Y.S. Aqueous two-phase emulsion bioink-enabled 3D bioprinting of porous hydrogels. Adv. Mater. 2018, 30, e1805460. [Google Scholar] [CrossRef]
- Miri, A.K.; Nieto, D.; Iglesias, L.; Goodarzi Hosseinabadi, H.; Maharjan, S.; Ruiz-Esparza, G.U.; Khoshakhlagh, P.; Manbachi, A.; Dokmeci, M.R.; Chen, S.; et al. Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Adv. Mater. 2018, 30, e1800242. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, R.; Wu, J.; Song, J.; Bai, H.; Li, B.; Zhao, Q.; Xie, T. Ultrafast digital printing toward 4D shape changing materials. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, Z.; Zhang, H.; Zhuang, Y.; Shen, H.; Chen, Y.; Zhao, Y.; Chen, B.; Xiao, Z.; Dai, J. Aligned Scaffolds with Biomolecular Gradients for Regenerative Medicine. Polymers 2019, 11, 341. https://doi.org/10.3390/polym11020341
Li X, Chen Z, Zhang H, Zhuang Y, Shen H, Chen Y, Zhao Y, Chen B, Xiao Z, Dai J. Aligned Scaffolds with Biomolecular Gradients for Regenerative Medicine. Polymers. 2019; 11(2):341. https://doi.org/10.3390/polym11020341
Chicago/Turabian StyleLi, Xiaoran, Zhenni Chen, Haimin Zhang, Yan Zhuang, He Shen, Yanyan Chen, Yannan Zhao, Bing Chen, Zhifeng Xiao, and Jianwu Dai. 2019. "Aligned Scaffolds with Biomolecular Gradients for Regenerative Medicine" Polymers 11, no. 2: 341. https://doi.org/10.3390/polym11020341