A Simple Methodology to Estimate the Diffusion Coefficient in Pervaporation-Based Purification Experiments
Abstract
:1. Introduction
2. Theoretical Background
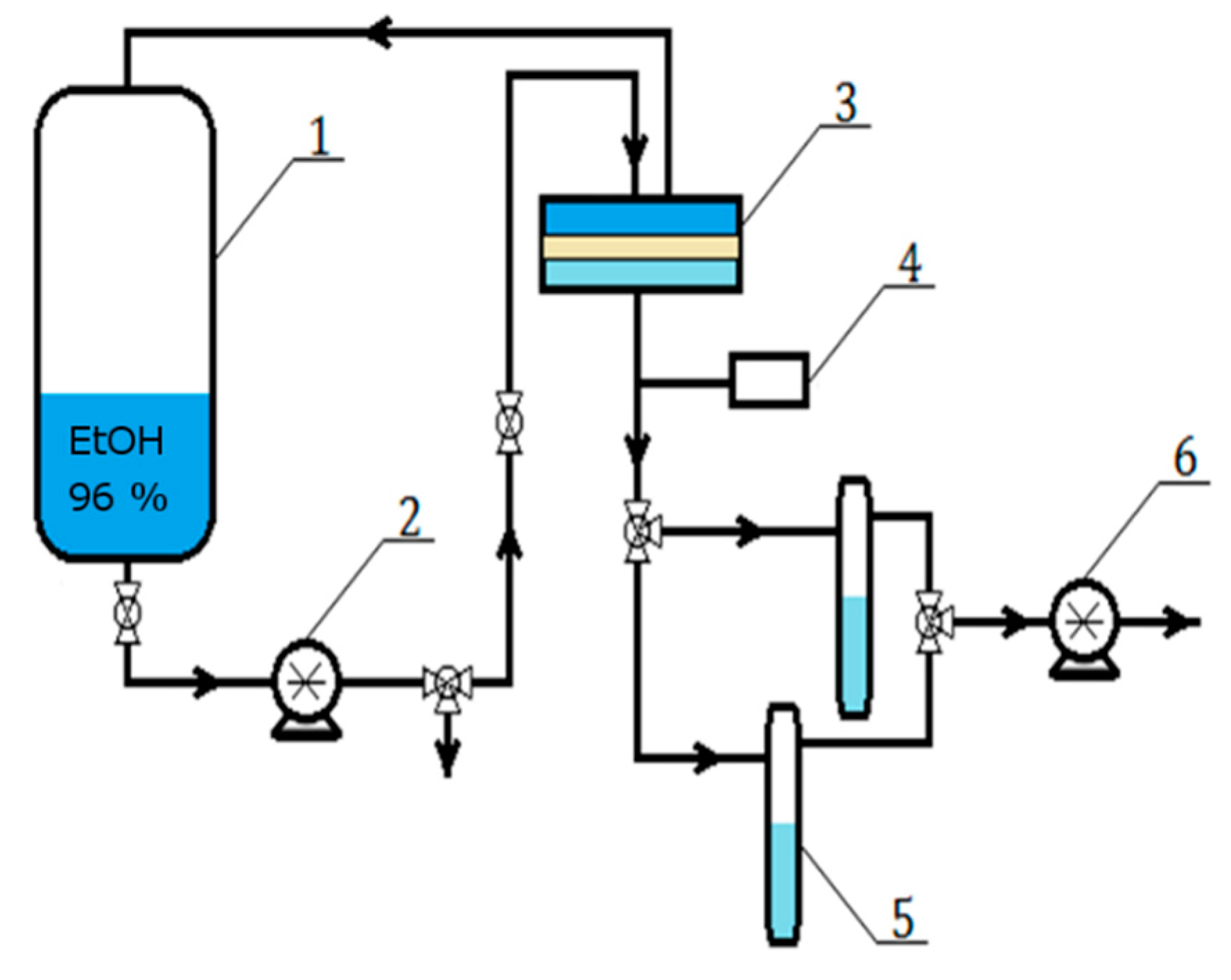
3. Experimental
3.1. Materials
3.2. Membrane Preparation
3.3. Pervaporation Experiments
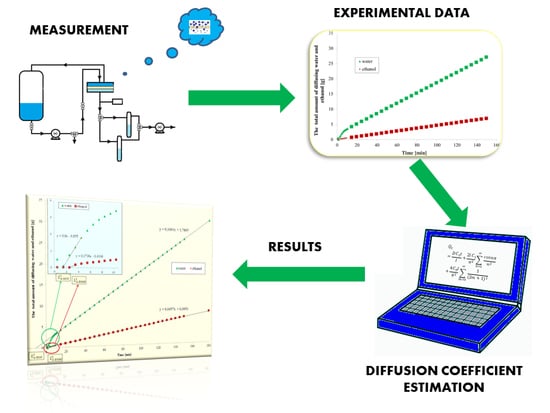
4. Results and Discussion
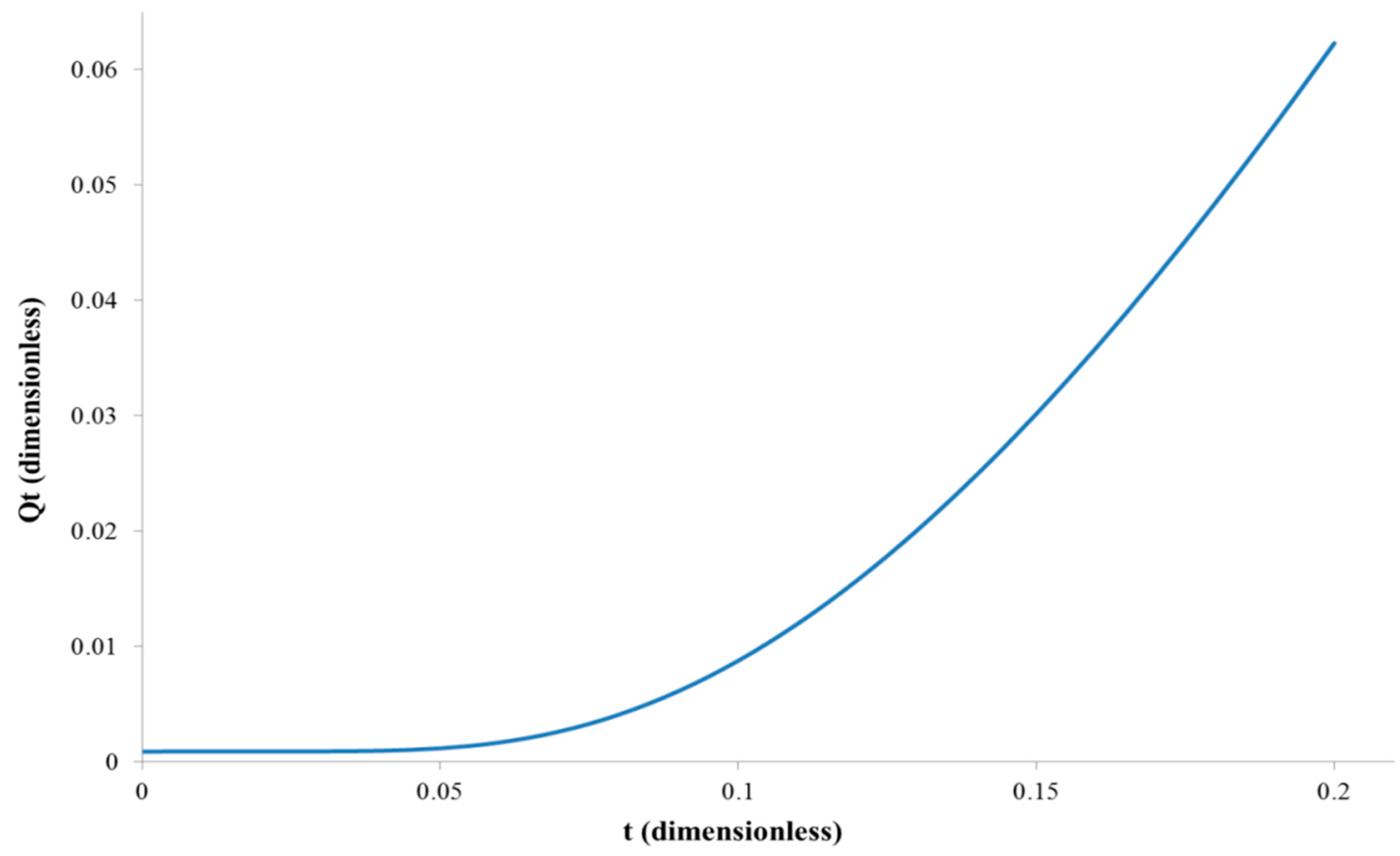
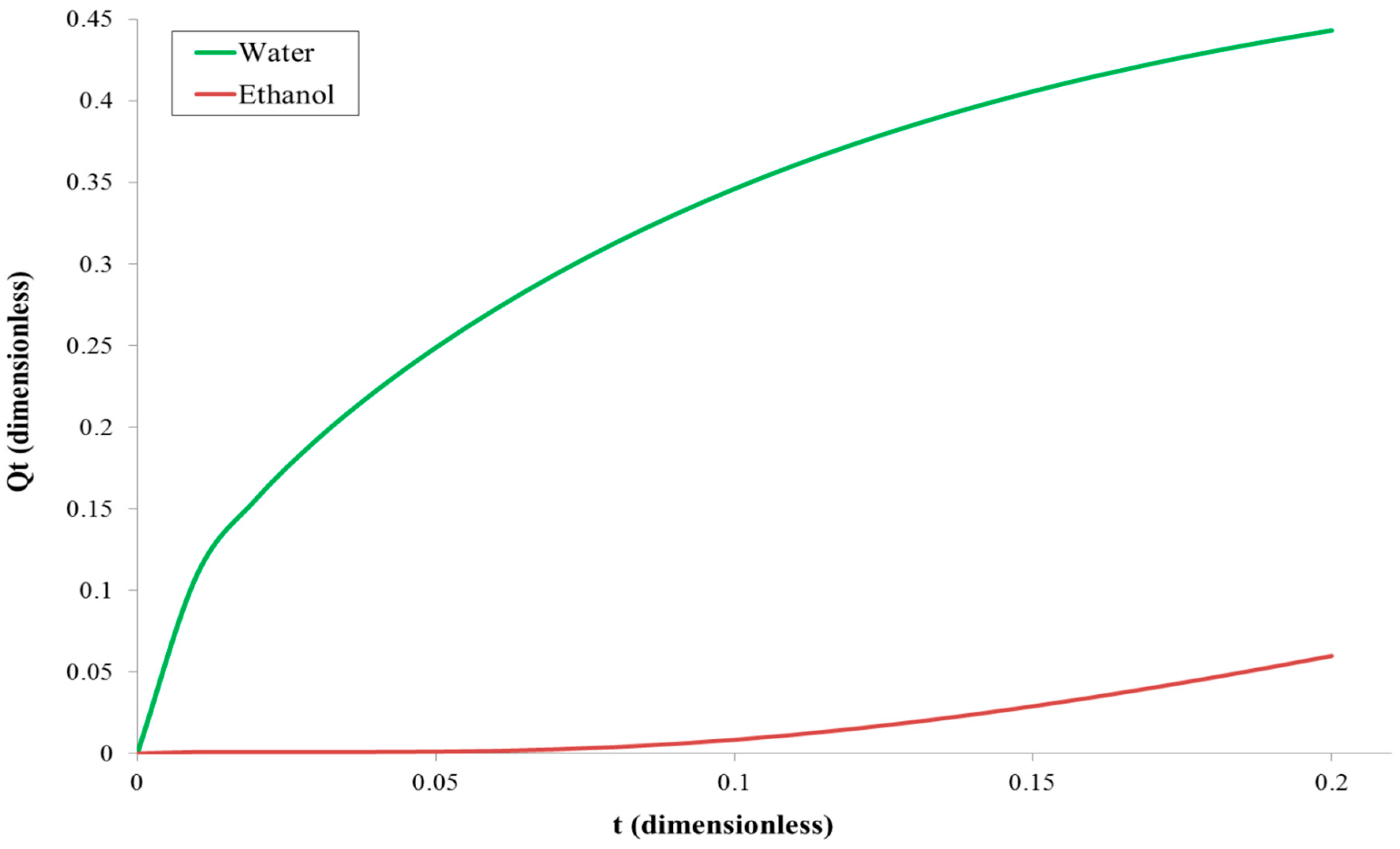
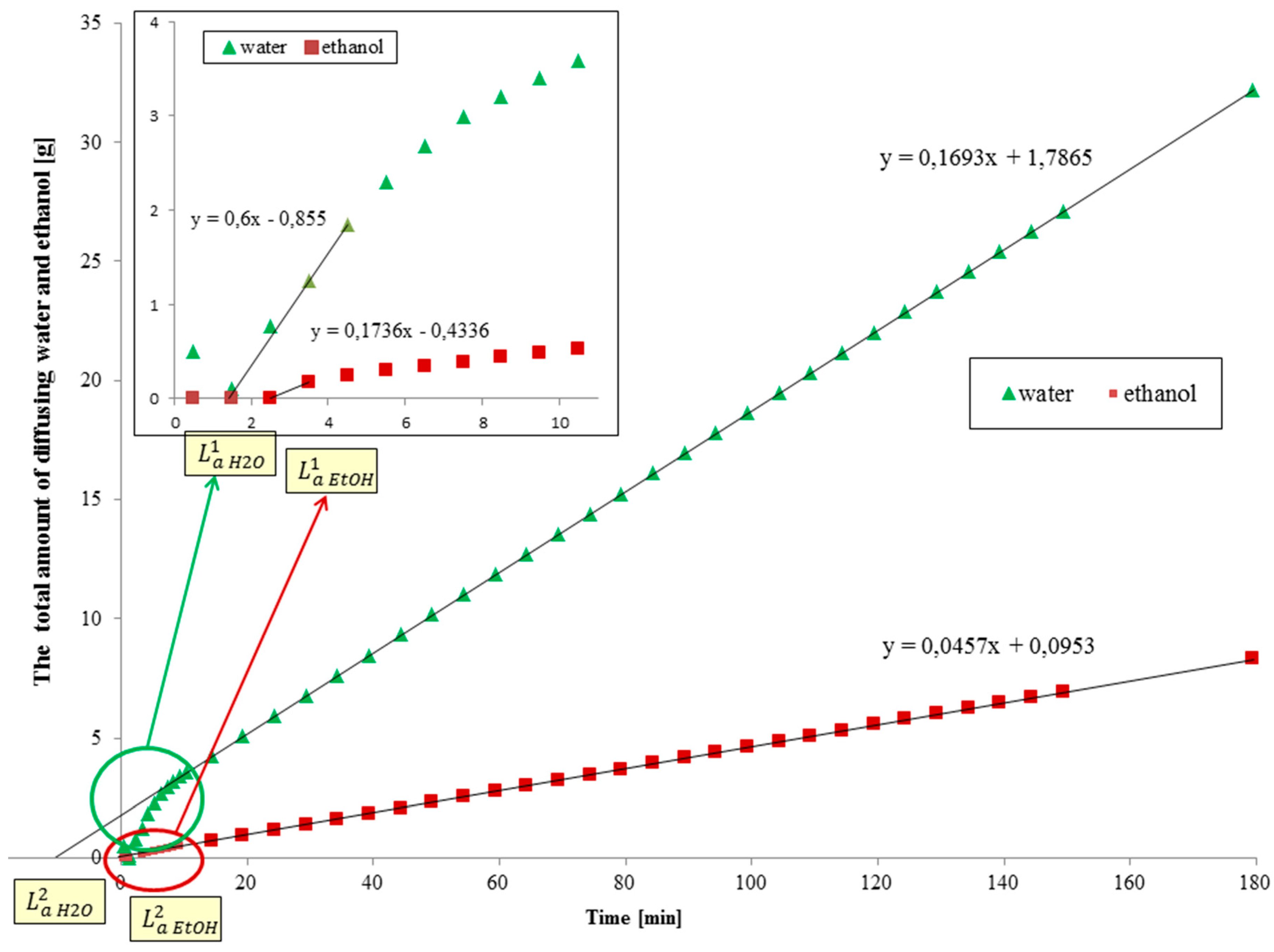
4.1. Diffusion Coefficient Estimation Strategy
4.1.1. Diffusion Coefficient for the Membrane Filled with Feed Solution
4.1.2. Diffusion Coefficient for the Membrane Initially Filled with Water Only
4.1.3. Considering the Effect of Tubing between the Membrane and the Cold Trap
4.1.4. Experimental Evaluation of the Shift in the Asymptote Due to Tubing
4.1.5. Application to Chitosan Pervaporation Membrane
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Basile, A.; Figoli, A.; Khayet, M. Pervaporation, Vapour Permeation and Membrane Distillation, 1st ed.; Woodhead Publishing, Elsevier: Sawston, UK, 2015. [Google Scholar]
- Smitha, B.; Suhanya, D.; Sridhar, S.; Ramakrishna, M. Separation of organic–organic mixtures by pervaporation—A review. J. Membr. Sci. 2004, 241, 1–21. [Google Scholar] [CrossRef]
- Uragami, T. Science and Technology of Separation Membranes; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 413–469. [Google Scholar]
- Dudek, G.; Turczyn, R. Application of chitosan membranes for permeation and pervaporation. In Chitosan Based Materials and Its Applications; Dotto, G.L., Pinto, L.A.A., Eds.; Frontiers in Biomaterials; Bentham Science Publishers Ltd.: Sharjah, UAE, 2017; Chapter 10; pp. 281–324. [Google Scholar]
- Cheng, X.; Pan, F.; Wang, M.; Li, W.; Song, Y.; Liu, G.; Yang, H.; Gao, B.; Wu, H.; Jiang, Z. Hybrid membranes for pervaporation separations. J. Membr. Sci. 2017, 541, 329–346. [Google Scholar] [CrossRef]
- Graham, T. On the absorption and dialytic separation of gases by colloid septa. Philos. Trans. 1866, 156, 399–439. [Google Scholar]
- Klinov, A.V.; Akberov, R.R.; Fazlyev, A.R.; Farakhov, M.I. Experimental investigation and modelling through using the solution-diffusion concept of pervaporation dehydration of ethanol and isopropanol by ceramic membranes HybSi. J. Membr. Sci. 2017, 524, 321–333. [Google Scholar] [CrossRef]
- Schaetzel, P.; Vauclair, C.; Nguyen, Q.T.; Bouzerar, R. A simplified solution–diffusion theory in pervaporation: The total solvent volume fraction model. J. Membr. Sci. 2004, 244, 117–127. [Google Scholar] [CrossRef]
- Heintz, A.; Stephan, W. A generalized solution—Diffusion model of the pervaporation process through composite membranes Part I. Prediction of mixture solubilities in the dense active layer using the UNIQUAC model. J. Membr. Sci. 1994, 89, 143–151. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on Pervaporation: Theory, Membrane Performance, and Application to Intensification of Esterification Reaction. J. Eng. 2015, 2015, 1–24. [Google Scholar] [CrossRef]
- Yeom, C.K.; Huang, R.Y.M. A new method for determining the diffusion coefficients of penetrants through polymeric membranes from steady state pervaporation experiments. J. Membr. Sci. 1992, 68, 11–20. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Rhim, J. Theoretical estimations of diffusion coefficients. J. Appl. Polym. Sci. 1990, 41, 535–546. [Google Scholar] [CrossRef]
- Walcher, T.; Gbritz, D. A method for determining intrinsic diffusion coefficients in the pervaporation process. Macromol. Chem. Phys. 1995, 196, 429–439. [Google Scholar] [CrossRef]
- Borys, P.; Grzywna, Z.J. An analytical solution to the idealized batch pervaporation experiment: A way to determine the diffusion and partition coefficients. J. Membr. Sci. 2015, 476, 525–529. [Google Scholar] [CrossRef]
- Taylor, R.; Krishna, R. Multicomponent Mass Transfer, 1st ed.; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Krishna, R.; Wesselingh, J.A. The Maxwell-Stefan approach to mass transfer. Chem. Eng. Sci. 1997, 52, 861–911. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Lin, Y.; Chen, C. Crosslinked poly(vinyl alcohol) membranes for separation of dimethyl carbonate/methanol mixtures by pervaporation. Chem. Eng. J. 2009, 146, 71–78. [Google Scholar] [CrossRef]
- Shameli, A.; Ameri, E. Synthesis of cross-linked PVA membranes embedded with multi-wall carbon nanotubes and their application to esterification of acetic acid with methanol. Chem. Eng. J. 2017, 309, 381–396. [Google Scholar] [CrossRef]
- Dudek, G.; Strzelewicz, A.; Turczyn, R.; Krasowska, M.; Rybak, A. The Study of Ethanol/Water Vapors Permeation through Sulfuric Acid Cross-Linked Chitosan Magnetic Membranes. Sep. Sci. Technol. 2014, 49, 1761. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, M.; Lu, L.; Yin, X.; Chen, J.; Jiang, Z. Intensifying esterification reaction between lactic acid and ethanol by pervaporation dehydration using chitosan–TEOS hybrid membranes. Chem. Eng. J. 2009, 155, 800–809. [Google Scholar] [CrossRef]
- Dudek, G.; Turczyn, R.; Gnus, M.; Konieczny, K. Pervaporative dehydration of ethanol/water mixture through hybrid alginate membranes with ferroferic oxide nanoparticles. Sep. Purif. Technol. 2018, 193, 398–407. [Google Scholar] [CrossRef]
- Adoor, S.G.; Rajineekanth, V.; Nadagouda, M.N.; Rao, K.C.; Dionysiou, D.D.; Aminabhavi, T.M. Exploration of nanocomposite membranes composed of phosphotungstic acid in sodium alginate for separation of aqueous–organic mixtures by pervaporation. Sep. Purif. Technol. 2013, 113, 64–74. [Google Scholar] [CrossRef]
- Xu, S.; Liu, L.; Wang, Y. Network cross-linking of polyimide membranes for pervaporation dehydration. Sep. Purif. Technol. 2017, 185, 215–226. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y. Novel thermally cross-linked polyimide membranes for ethanol dehydration via pervaporation. J. Membr. Sci. 2015, 496, 142–155. [Google Scholar] [CrossRef]
- Moulik, S.; Nazia, S.; Vani, B.; Sridhar, S. Pervaporation separation of acetic acid/water mixtures through sodium alginate/polyaniline polyion complex membrane. Sep. Purif. Technol. 2016, 170, 30–39. [Google Scholar] [CrossRef]
- Chapman, P.; Loh, X.X.; Livingston, A.G.; Li, K.; Oliveira, T.A.C. Polyaniline membranes for the dehydration of tetrahydrofuran by pervaporation. J. Membr. Sci. 2008, 309, 102–111. [Google Scholar] [CrossRef]
- Villegas, M.; Vidaurre, E.F.C.; Gottifredi, J.C. Sorption and pervaporation of methanol/water mixtures with poly(3-hydroxybutyrate) membranes. Chem. Eng. Res. Des. 2015, 94, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; Bourgeois, D.; Belfort, G. Sorption, diffusion, and pervaporation of organics in polymer membranes. J. Membr. Sci. 1989, 44, 161–181. [Google Scholar] [CrossRef]
- Pulyalina, A.; Polotskaya, G.; Goikhman, M.; Podeshvo, I.; Chernitsa, B.; Kocherbitov, V.; Toikka, A. Novel approach to determination of sorption in pervaporation process: A case study of isopropanol dehydration by polyamidoimideurea membranes. Sci. Rep. 2017, 7, 8415–8427. [Google Scholar] [CrossRef] [PubMed]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Ball, I.J.; Huang, S.; Wolf, R.A.; Shimano, J.Y.; Kaner, R.B. Pervaporation studies with polyaniline membranes and blends. J. Membr. Sci. 2000, 174, 161–176. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Miyazaki, Y.; Nakao, S.; Tsuru, T.; Kimura, S. Membrane Design for Pervaporation or Vapor Permeation Separation Using a Filling-Type Membrane Concept. Ind. Eng. Chem. Res. 1998, 37, 177–184. [Google Scholar] [CrossRef]
- Felder, R.M.; Huvard, G.S. Permeation, diffusion, and sorption of gases and vapors. In Methods of Experimental Physics: Polymers; Fava, R.A., Ed.; Academic Press: New York, NY, USA, 1980; pp. 315–377. [Google Scholar]
- Borys, P.; Rybak, A.; Grzywna, Z.J. On the Analysis of Concentration-Dependent Diffusion Using Transient Sorption and Permeation Measurements by the D1–D8 System. Ind. Eng. Chem. Res. 2013, 52, 8887–8896. [Google Scholar] [CrossRef]
- Zauderer, E. Partial Differential Equations of Applied Mathematics, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Metzler, R.; Klafter, J. The random walk’s guide to anomalous diffusion: A fractional dynamics approach. Phys. Rep. 2000, 339, 1–77. [Google Scholar] [CrossRef]
- Chapman, S.; Cowling, T.G. The Mathematical Theory of Non-Uniform Gases; Cambridge University Press: London, UK, 1970. [Google Scholar]
- Qian, X.; Li, N.; Wang, Q.; Jia, S. Chitosan/graphene oxide mixed matrix membrane with enhanced water permeability for high-salinity water desalination by pervaporation. Desalination 2018, 438, 83–96. [Google Scholar] [CrossRef]
- Mulder, M.H.V.; Smolders, C.A. On the mechanism of separation of ethanol/water mixtures by pervaporation I. Calculations of concentration profiles. J. Membr. Sci. 1984, 17, 289–307. [Google Scholar] [CrossRef] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, G.; Borys, P. A Simple Methodology to Estimate the Diffusion Coefficient in Pervaporation-Based Purification Experiments. Polymers 2019, 11, 343. https://doi.org/10.3390/polym11020343
Dudek G, Borys P. A Simple Methodology to Estimate the Diffusion Coefficient in Pervaporation-Based Purification Experiments. Polymers. 2019; 11(2):343. https://doi.org/10.3390/polym11020343
Chicago/Turabian StyleDudek, Gabriela, and Przemysław Borys. 2019. "A Simple Methodology to Estimate the Diffusion Coefficient in Pervaporation-Based Purification Experiments" Polymers 11, no. 2: 343. https://doi.org/10.3390/polym11020343