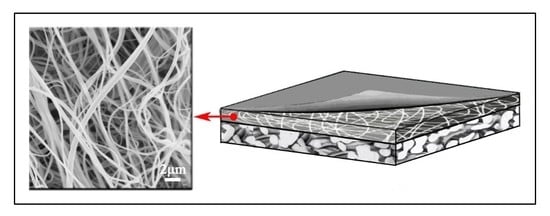
Solution Blown Nylon 6 Nanofibrous Membrane as Scaffold for Nanofiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Nylon 6 Nanofibers by Solution Blowing
2.3. Preparation of HNM by Hot Pressing
2.4. Preparation of PA Active Layer by Interfacial Polymerization
2.5. Characterization
2.5.1. Scanning Electron Microscopy (SEM)
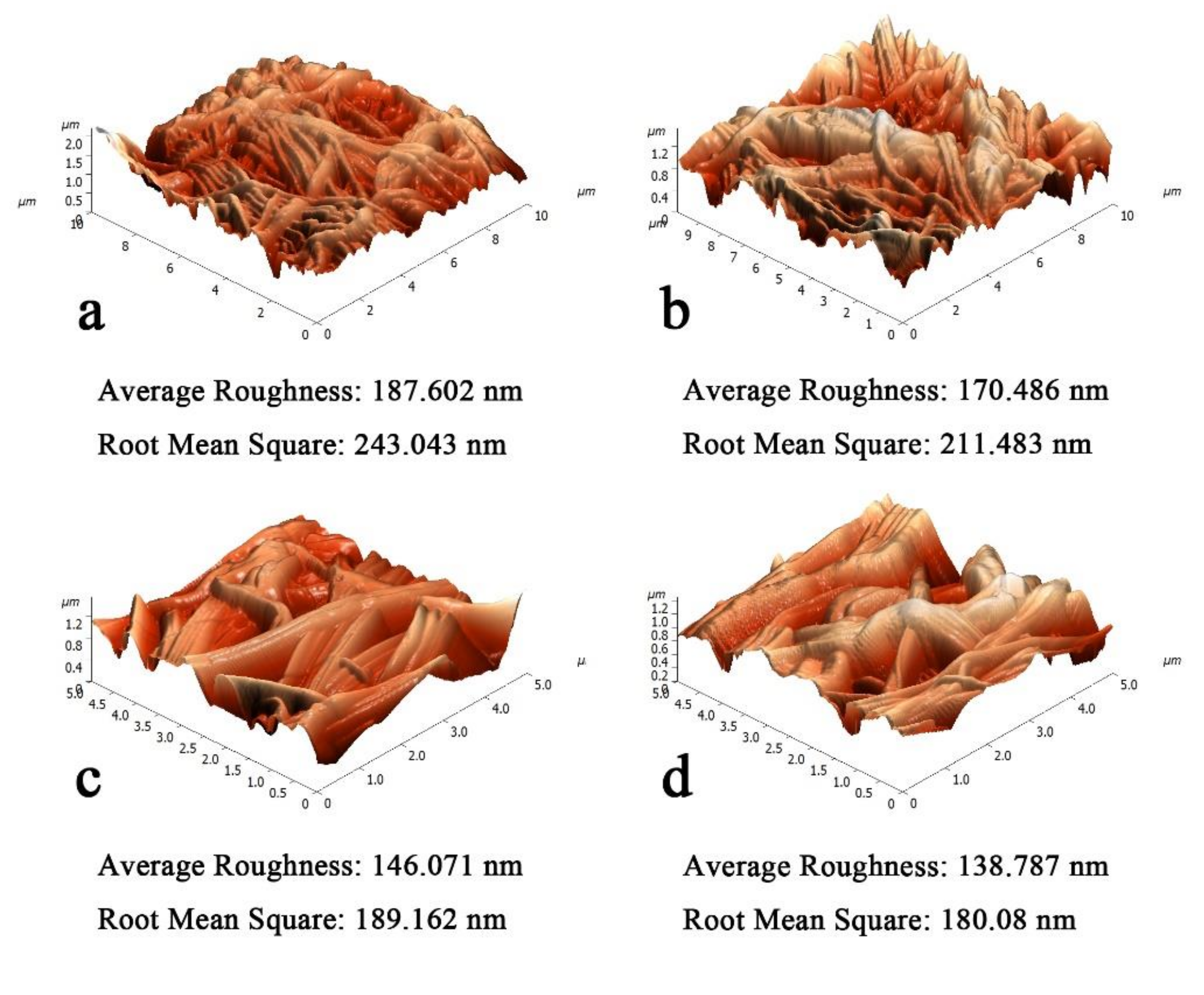
2.5.2. Atomic Force Microscopy (AFM)
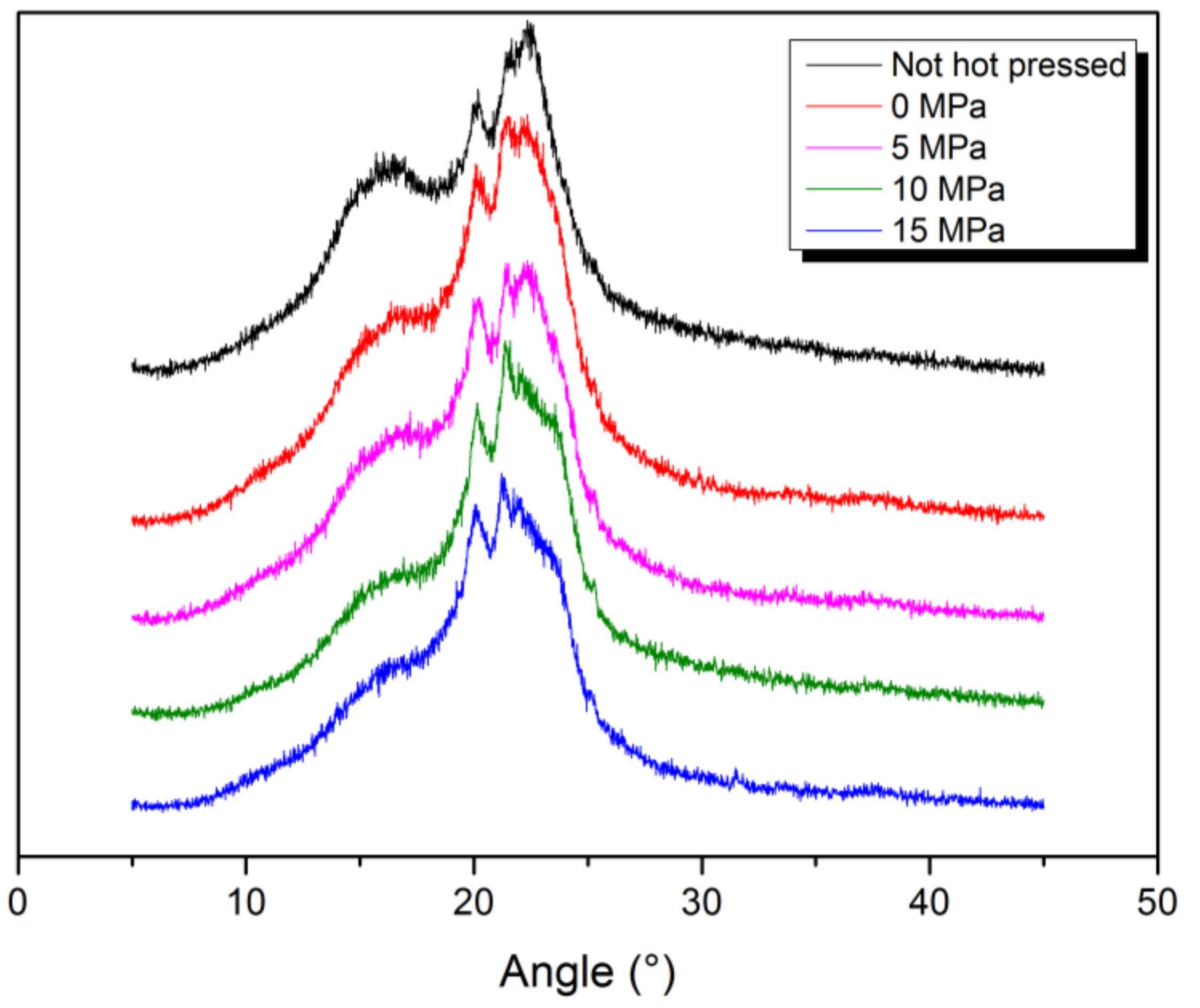
2.5.3. X-ray Diffractometer (XRD)
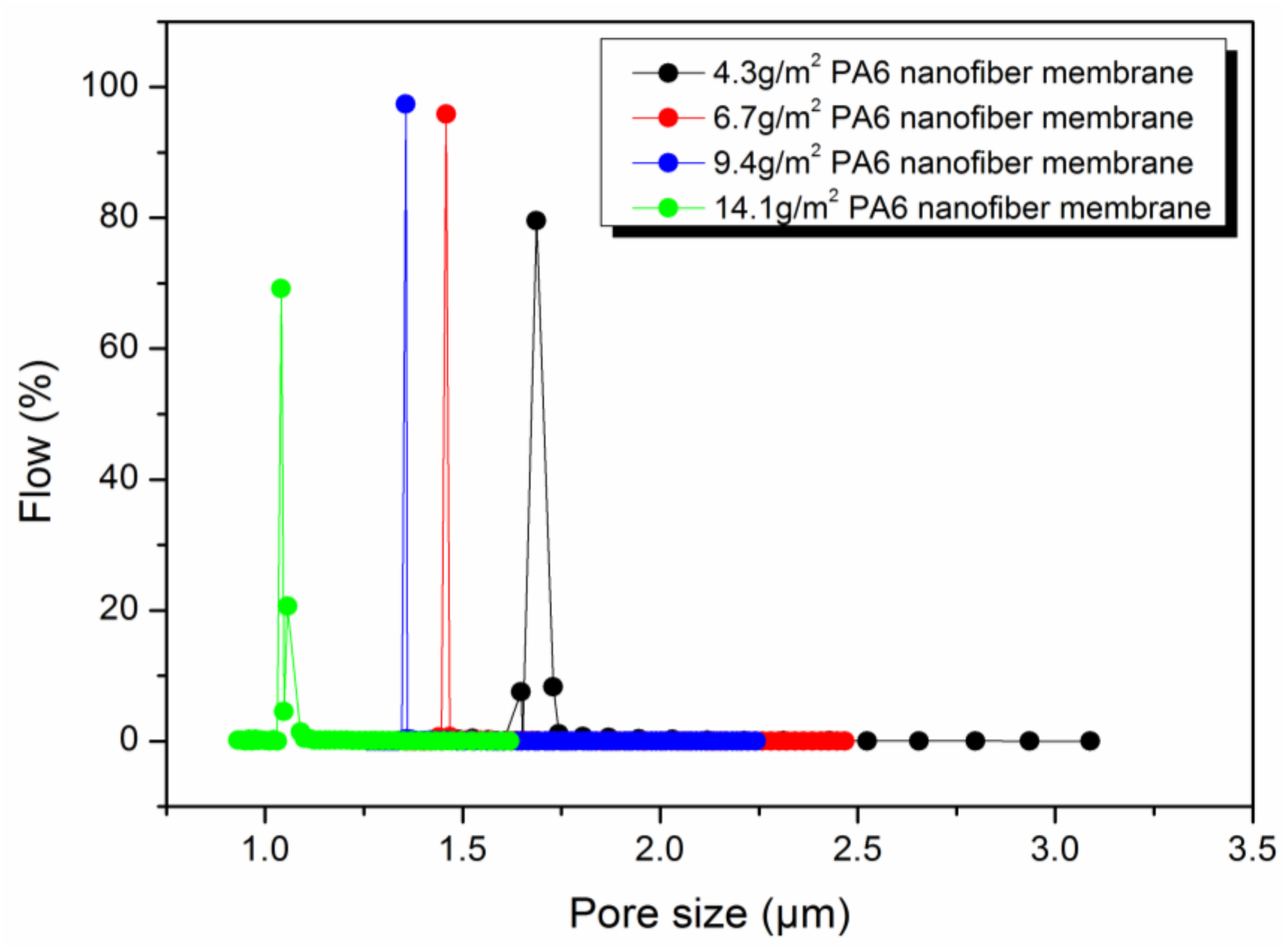
2.5.4. Determination of Porosity (%) and Pore Size
2.5.5. Measure of Contact Angle
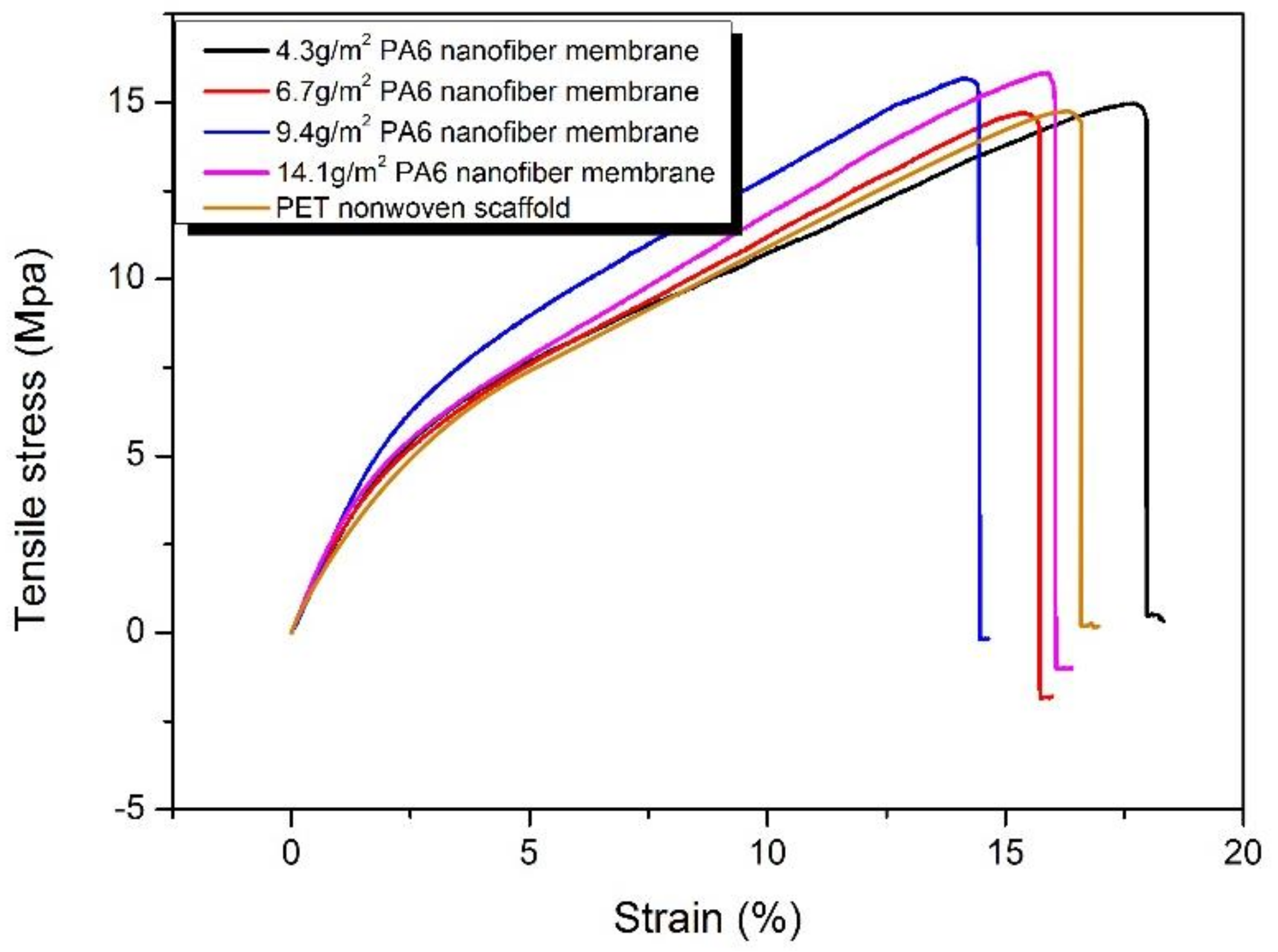
2.5.6. Mechanical Characterization
2.5.7. Characterization of NFNF
3. Results and Discussion
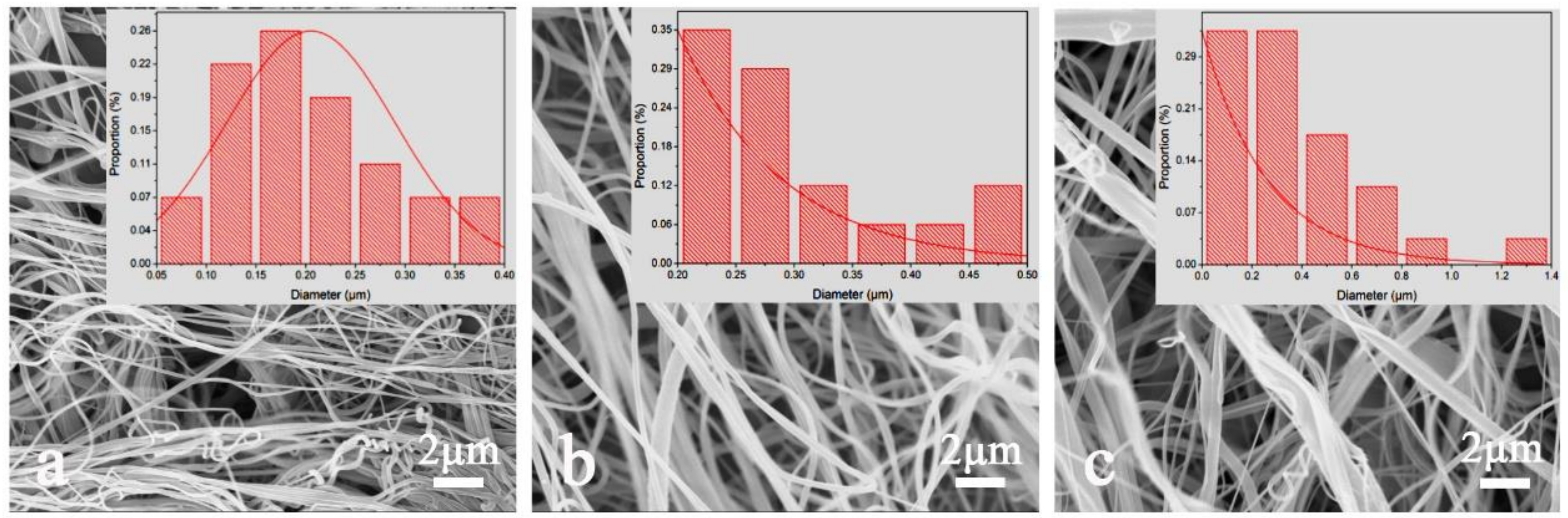
3.1. Effect of Solution Concentration on Nylon 6 Nanofiber Forming
3.2. Effect of Pressure in Hot Pressing on HNM
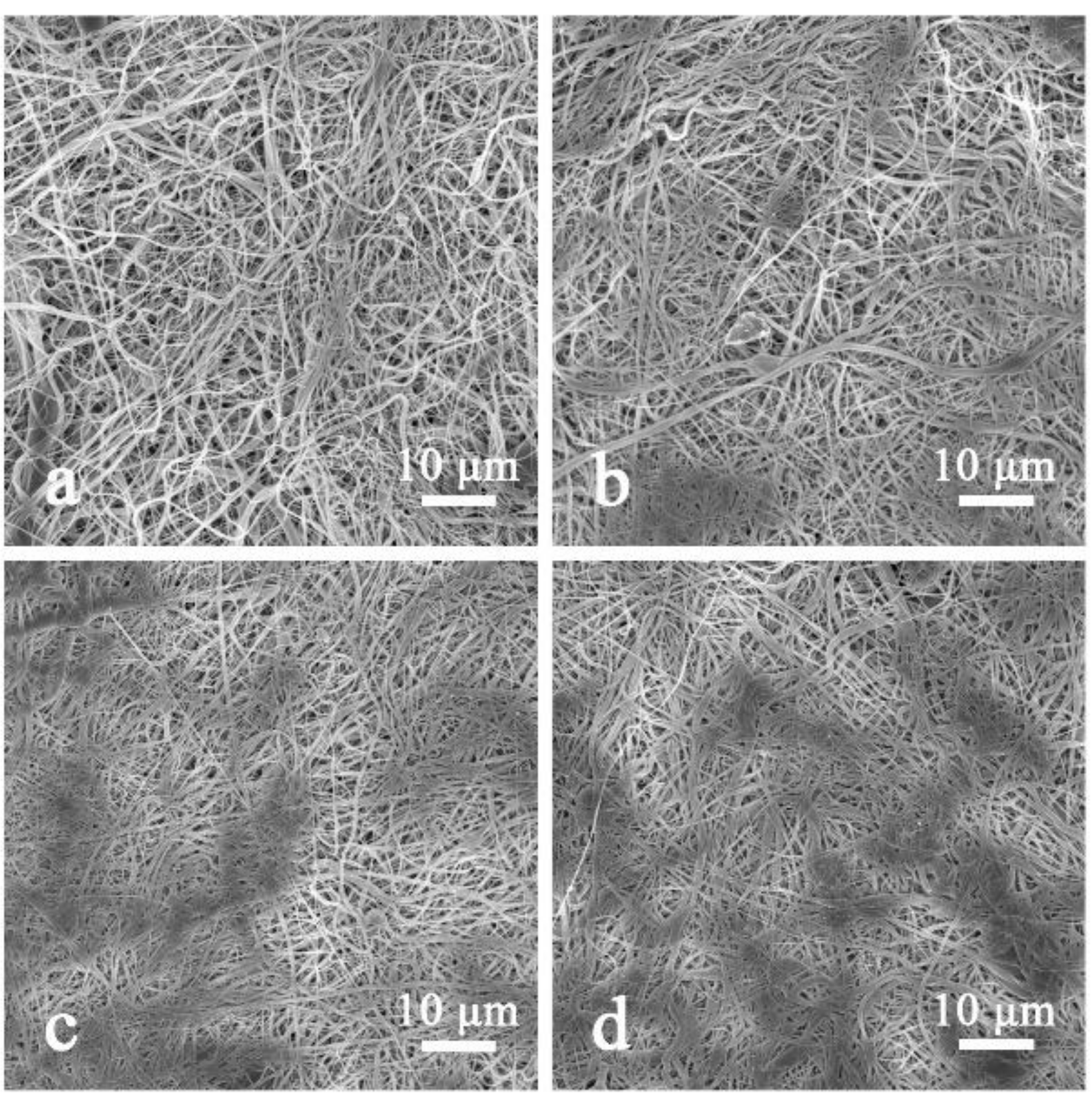
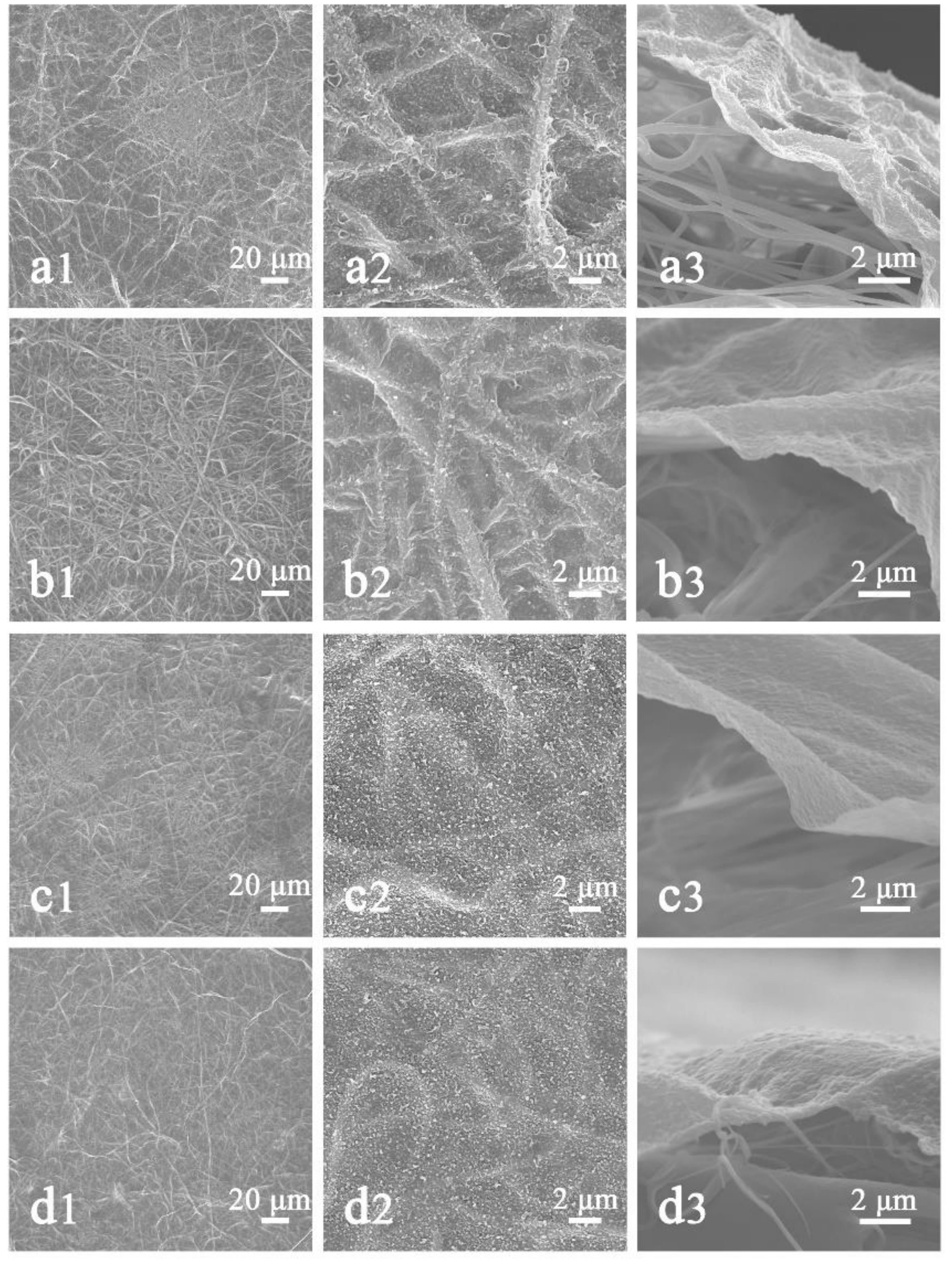
3.3. The Effect of Areal Density on HNM
3.4. Effect of HNM on NFNFM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harrison, I.J.; Green, P.A.; Farrell, T.A.; Juffe-Bignoli, D.; Sáenz, L.; Vörösmarty, C.J. Protected areas and freshwater provisioning: A global assessment of freshwater provision, threats and management strategies to support human water security: Protected areas and freshwater provisioning. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 103–120. [Google Scholar] [CrossRef]
- Muntha, S.T.; Kausar, A.; Siddiq, M. Advances in polymeric nanofiltration membrane: A review. J. Macromol. Sci. Part D Rev. Polym. Process. 2016, 56, 841–856. [Google Scholar] [CrossRef]
- Lim, S.K.; Goh, K.; Bae, T.-H.; Wang, R. Polymer-based membranes for solvent-resistant nanofiltration: A review. Chin. J. Chem. Eng. 2017, 25, 1653–1675. [Google Scholar] [CrossRef]
- Pendergast, M.T.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Li, S.; Luo, J.; Wan, Y. Regenerable biocatalytic nanofiltration membrane for aquatic micropollutants removal. J. Membr. Sci. 2018, 549, 120–128. [Google Scholar] [CrossRef]
- Abdellah, M.H.; Pérez-Manríquez, L.; Puspasari, T.; Scholes, C.A.; Kentish, S.E.; Peinemann, K.-V. A catechin/cellulose composite membrane for organic solvent nanofiltration. J. Membr. Sci. 2018, 567, 139–145. [Google Scholar] [CrossRef]
- Li, K.; Ma, W.; Han, H.; Xu, C.; Han, Y.; Wang, D.; Ma, W.; Zhu, H. Selective recovery of salt from coal gasification brine by nanofiltration membranes. J. Environ. Manag. 2018, 223, 306–313. [Google Scholar] [CrossRef]
- Kaur, S.; Sundarrajan, S.; Rana, D.; Matsuura, T.; Ramakrishna, S. Influence of electrospun fiber size on the separation efficiency of thin film nanofiltration composite membrane. J. Membr. Sci. 2012, 392, 101–111. [Google Scholar] [CrossRef]
- Prakash Rao, A.; Joshi, S.V.; Trivedi, J.J.; Devmurari, C.V.; Shah, V.J. Structure–performance correlation of polyamide thin film composite membranes: Effect of coating conditions on film formation. J. Membr. Sci. 2003, 211, 13–24. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Dong, Y.; Zhao, X.; Jiang, Z.; Zhang, R.; Zhao, J. Separation performance of thin-film composite nanofiltration membrane through interfacial polymerization using different amine monomers. Desalination 2014, 333, 59–65. [Google Scholar] [CrossRef]
- Gu, Z.; Cui, S.; Liu, S.; An, Q.; Qin, Z.; Guo, H. Superhydrophilic nanofiltration membrane with antifouling property through in-situ mineralization of Ce2(CO3)3 nanoparticles. J. Taiwan Inst. Chem. Eng. 2018, 88, 70–77. [Google Scholar] [CrossRef]
- Shan, L.; Liang, Y.; Prozorovska, L.; Jennings, G.K.; Ji, S.; Lin, S. Multifold enhancement of loose nanofiltration membrane performance by intercalation of surfactant assemblies. Environ. Sci. Technol. Lett. 2018, 5, 668–674. [Google Scholar] [CrossRef]
- Rajaeian, B.; Rahimpour, A.; Tade, M.O.; Liu, S. Fabrication and characterization of polyamide thin film nanocomposite (tfn) nanofiltration membrane impregnated with tio2 nanoparticles. Desalination 2013, 313, 176–188. [Google Scholar] [CrossRef]
- Yung, L.; Ma, H.; Wang, X.; Yoon, K.; Wang, R.; Hsiao, B.S.; Chu, B. Fabrication of thin-film nanofibrous composite membranes by interfacial polymerization using ionic liquids as additives. J. Membr. Sci. 2010, 365, 52–58. [Google Scholar] [CrossRef]
- Wang, X.; Yeh, T.-M.; Wang, Z.; Yang, R.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. Nanofiltration membranes prepared by interfacial polymerization on thin-film nanofibrous composite scaffold. Polymer 2014, 55, 1358–1366. [Google Scholar] [CrossRef]
- Ramon, G.Z.; Wong, M.C.Y.; Hoek, E.M.V. Transport through composite membrane, part 1: Is there an optimal support membrane? J. Membr. Sci. 2012, 415, 298–305. [Google Scholar] [CrossRef]
- Misdan, N.; Lau, W.J.; Ismail, A.F.; Matsuura, T. Formation of thin film composite nanofiltration membrane: Effect of polysulfone substrate characteristics. Desalination 2013, 329, 9–18. [Google Scholar] [CrossRef]
- Malmali, M.; Wickramasinghe, S.R.; Tang, J.; Cong, H. Sugar fractionation using surface-modified nanofiltration membranes. Sep. Purif. Technol. 2016, 166, 187–195. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Xu, Z.-L.; Xue, S.-M.; Wei, Y.-M.; Yang, H. A chlorine-tolerant nanofiltration membrane prepared by the mixed diamine monomers of pip and bhttm. J. Membr. Sci. 2016, 498, 374–384. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hoek, E.M.V. Impacts of support membrane structure and chemistry on polyamide–polysulfone interfacial composite membranes. J. Membr. Sci. 2009, 336, 140–148. [Google Scholar] [CrossRef]
- Misdan, N.; Lau, W.J.; Ismail, A.F.; Matsuura, T.; Rana, D. Study on the thin film composite poly(piperazine-amide) nanofiltration membrane: Impacts of physicochemical properties of substrate on interfacial polymerization formation. Desalination 2014, 344, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Chong, C.; Shuang, L.; Zhao, W.; Qiang, W.; Nie, S.; Sun, S.; Zhao, C. The hydrodynamic permeability and surface property of polyethersulfone ultrafiltration membranes with mussel-inspired polydopamine coatings. J. Membr. Sci. 2012, 417, 228–236. [Google Scholar] [CrossRef]
- Suja, P.S.; Reshmi, C.R.; Sagitha, P.; Sujith, A. Electrospun nanofibrous membranes for water purification. Polym. Rev. 2017, 57, 467–504. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, X.; Li, Y.; Zhou, G.; Wang, J. Porous nanofibrous composite membrane for unparalleled proton conduction. J. Membr. Sci. 2018, 550, 136–144. [Google Scholar] [CrossRef]
- Kang, N.; Lin, F.; Zhao, W.; Lombardi, J.P.; Almihdhar, M.; Liu, K.; Yan, S.; Kim, J.; Luo, J.; Hsiao, B.S.; et al. Nanoparticle–nanofibrous membranes as scaffolds for flexible sweat sensors. ACS Sens. 2016, 1, 1060–1069. [Google Scholar] [CrossRef]
- Feroz, H.; Bai, M.; Kwon, H.; Brezovec, J.; Peng, J.; Kumar, M. Can fibrous mats outperform current ultrafiltration and microfiltration membranes? Ind. Eng. Chem. Res. 2017, 56, 10438–10447. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Jacobs, N.V.; Luyt, A.S. Nanofibrous alginate membrane coated with cellulose nanowhiskers for water purification. Cellulose 2017, 1–11. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. High flux nanofiltration membranes based on interfacially polymerized polyamide barrier layer on polyacrylonitrile nanofibrous scaffolds. J. Membr. Sci. 2009, 326, 484–492. [Google Scholar] [CrossRef]
- Xu, G.-R.; Liu, X.-Y.; Xu, J.-M.; Li, L.; Su, H.-C.; Zhao, H.-L.; Feng, H.-J. High flux nanofiltration membranes based on layer-by-layer assembly modified electrospun nanofibrous substrate. Appl. Surf. Sci. 2018, 434, 573–581. [Google Scholar] [CrossRef]
- Wang, X.; Fang, D.; Hsiao, B.S.; Chu, B. Nanofiltration membranes based on thin-film nanofibrous composites. J. Membr. Sci. 2014, 469, 188–197. [Google Scholar] [CrossRef]
- Li, B.; Liu, W.; Jiang, Z.; Dong, X.; Wang, B.; Zhong, Y. Ultrathin and stable active layer of dense composite membrane enabled by poly(dopamine). Langmuir 2009, 25, 7368–7374. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Yang, X.; Shi, L.; Cheng, B.; Guan, K.; Kang, W. Solution blowing of submicron-scale cellulose fibers. Carbohydr. Polym. 2012, 90, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Daristotle, J.L.; Behrens, A.M.; Sandler, A.D.; Kofinas, P. A review of the fundamental principles and applications of solution blow spinning. Acs Appl. Mater. Interfaces 2016, 8, 34951–34963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, X.; Lei, S.; Jia, K.; Cheng, B.; Kang, W. Solution blown nanofibrous membrane for microfiltration. J. Membr. Sci. 2013, 429, 66–70. [Google Scholar] [CrossRef]
- Yalcinkaya, B.; Yalcinkaya, F.; Chaloupek, J. Optimisation of thin film composite nanofiltration membranes based on laminated nanofibrous and nonwoven supporting material. Desalin. Water Treat. 2016, 59, 19–30. [Google Scholar] [CrossRef]
- Shi, L.; Tao, X.; Weimin, K.; Xupin, Z.; Bowen, C. Solution blowing nylon 6 nanofiber mats for air filtration. Fibers Polym. 2013, 14, 1485–1490. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Jung, S.G.; Yoon, Y.S.; Ihm, D.W. Details of surface features in aromatic polyamide reverse osmosis membranes characterized by scanning electron and atomic force microscopy. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1429–1440. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, S.; Zhang, X.; Zheng, G. Polyamide thin film composite membranes prepared from 3,4′,5-biphenyl triacyl chloride, 3,3′,5,5′-biphenyl tetraacyl chloride and m -phenylenediamine. J. Membr. Sci. 2007, 289, 258–267. [Google Scholar] [CrossRef]
- Singh, P.S.; Joshi, S.V.; Trivedi, J.J.; Devmurari, C.V.; Rao, A.P.; Ghosh, P.K. Probing the structural variations of thin film composite ro membranes obtained by coating polyamide over polysulfone membranes of different pore dimensions. J. Membr. Sci. 2006, 278, 19–25. [Google Scholar] [CrossRef]
- Wei, Q.; Haag, R. Universal polymer coatings and their representative biomedical applications. Mater. Horiz. 2015, 2, 567–577. [Google Scholar] [CrossRef]
- Kong, C.; Kanezashi, M.; Yamomoto, T.; Shintani, T.; Tsuru, T. Controlled synthesis of high performance polyamide membrane with thin dense layer for water desalination. J. Membr. Sci. 2010, 362, 76–80. [Google Scholar] [CrossRef]
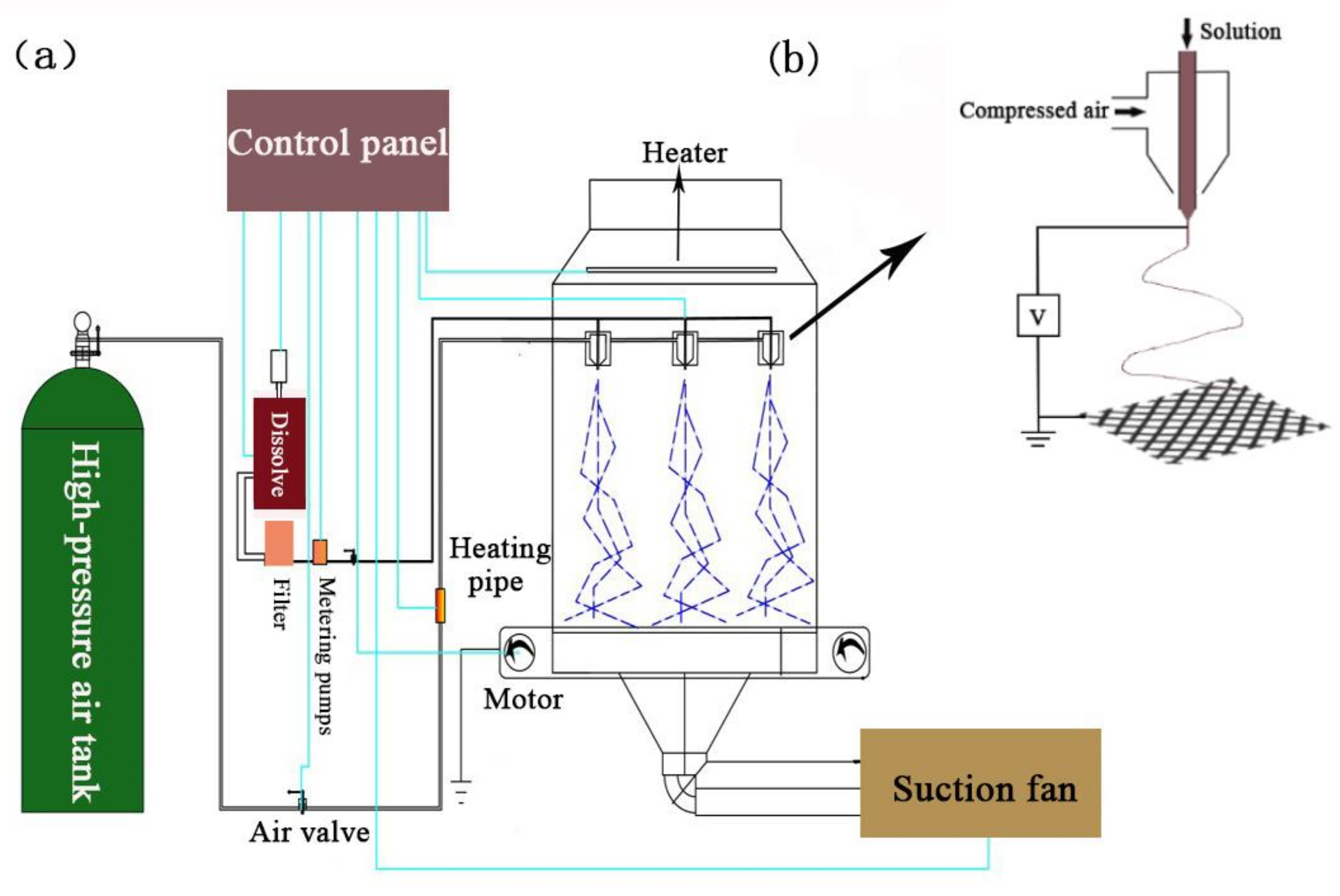

| Process Parameters | Numerical Value |
|---|---|
| Metering pumps | 17.5 r/min |
| Step size | 0.6 ml/r |
| Cabinet temperature | 45 °C |
| Inlet air temperature | 60 °C |
| Metering pump temperature | 30 °C |
| Dissolving kettle temperature | 40 °C |
| Drafting wind pressure | 2 bar |
| Auxiliary voltage | 4 kV |
| Receiving distance | 70 cm |
| Pressures | Maximum Pore Size (μm) | Mean Pore Size (μm) | Minimum Pore Size (μm) |
|---|---|---|---|
| 0 MPa | 2.5243 | 1.7437 | 1.6511 |
| 5 MPa | 2.3154 | 1.6236 | 1.5043 |
| 10 MPa | 2.2208 | 1.5947 | 1.3953 |
| 15 MPa | 2.1147 | 1.5363 | 1.4392 |
| Areal Density (g/m2) | Maximum Pore Size (μm) | Mean Pore Size (μm) | Minimum Pore Size (μm) | Porosity (%) | Contact Angle (°) |
|---|---|---|---|---|---|
| 4.3 | 2.7978 | 1.7102 | 1.4891 | 78.2 ± 2.6 | 55 ± 0.5 |
| 6.7 | 2.1438 | 1.4632 | 1.4392 | 79.5 ± 1 | 54 ± 0.5 |
| 9.4 | 1.9906 | 1.3580 | 1.2794 | 83.4 ± 1.5 | 51 ± 0.5 |
| 14.1 | 1.4531 | 1.0624 | 0.9316 | 85.0 ± 3 | 52 ± 0.5 |
| Areal Density (g/m2) | Contact Angle (°) | PA Layer Thick-ness (nm) | Average Roughness (nm) | RMS Roughness (nm) |
|---|---|---|---|---|
| 4.3 | 54 ± 0.5 | 69.73 ± 5.4 | 37.5 | 42.0 |
| 6.7 | 53 ± 0.5 | 72.52 ± 3.2 | 32.9 | 39.6 |
| 9.4 | 54 ± 0.5 | 79.85 ± 2.1 | 24.2 | 30.0 |
| 14.1 | 54 ± 0.5 | 86.21 ± 2.9 | 23.7 | 28.1 |
| Areal Density (g/m2) | Flux (L m−2 h−1) | Retention (%) | |||
|---|---|---|---|---|---|
| Water | NaCl | Na2SO4 | NaCl | Na2SO4 | |
| 4.3 | 14.9 | 9.3 | 9.7 | 73.2 | 79.6 |
| 6.7 | 14.7 | 8.7 | 9.4 | 76.5 | 81.3 |
| 9.4 | 13.2 | 8.2 | 8.5 | 80.4 | 84.7 |
| 14.1 | 13.1 | 8.1 | 8.2 | 81.3 | 85.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, G.; Zhuang, X.; Li, S.; Shi, L.; Kang, W.; Cheng, B.; Xu, X. Solution Blown Nylon 6 Nanofibrous Membrane as Scaffold for Nanofiltration. Polymers 2019, 11, 364. https://doi.org/10.3390/polym11020364
Liu Y, Zhang G, Zhuang X, Li S, Shi L, Kang W, Cheng B, Xu X. Solution Blown Nylon 6 Nanofibrous Membrane as Scaffold for Nanofiltration. Polymers. 2019; 11(2):364. https://doi.org/10.3390/polym11020364
Chicago/Turabian StyleLiu, Ya, Gaokai Zhang, Xupin Zhuang, Sisi Li, Lei Shi, Weimin Kang, Bowen Cheng, and Xianlin Xu. 2019. "Solution Blown Nylon 6 Nanofibrous Membrane as Scaffold for Nanofiltration" Polymers 11, no. 2: 364. https://doi.org/10.3390/polym11020364