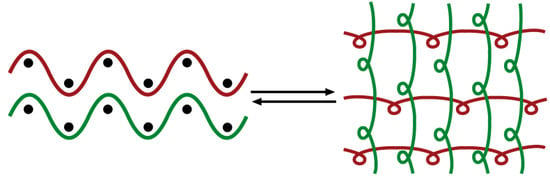
One-Dimensional Mercury Halide Coordination Polymers Based on A Semi-Rigid N-Donor Ligand: Reversible Structural Transformation
Abstract
:1. Introduction
2. Experimental Section
2.1. General Procedures
2.2. Materials
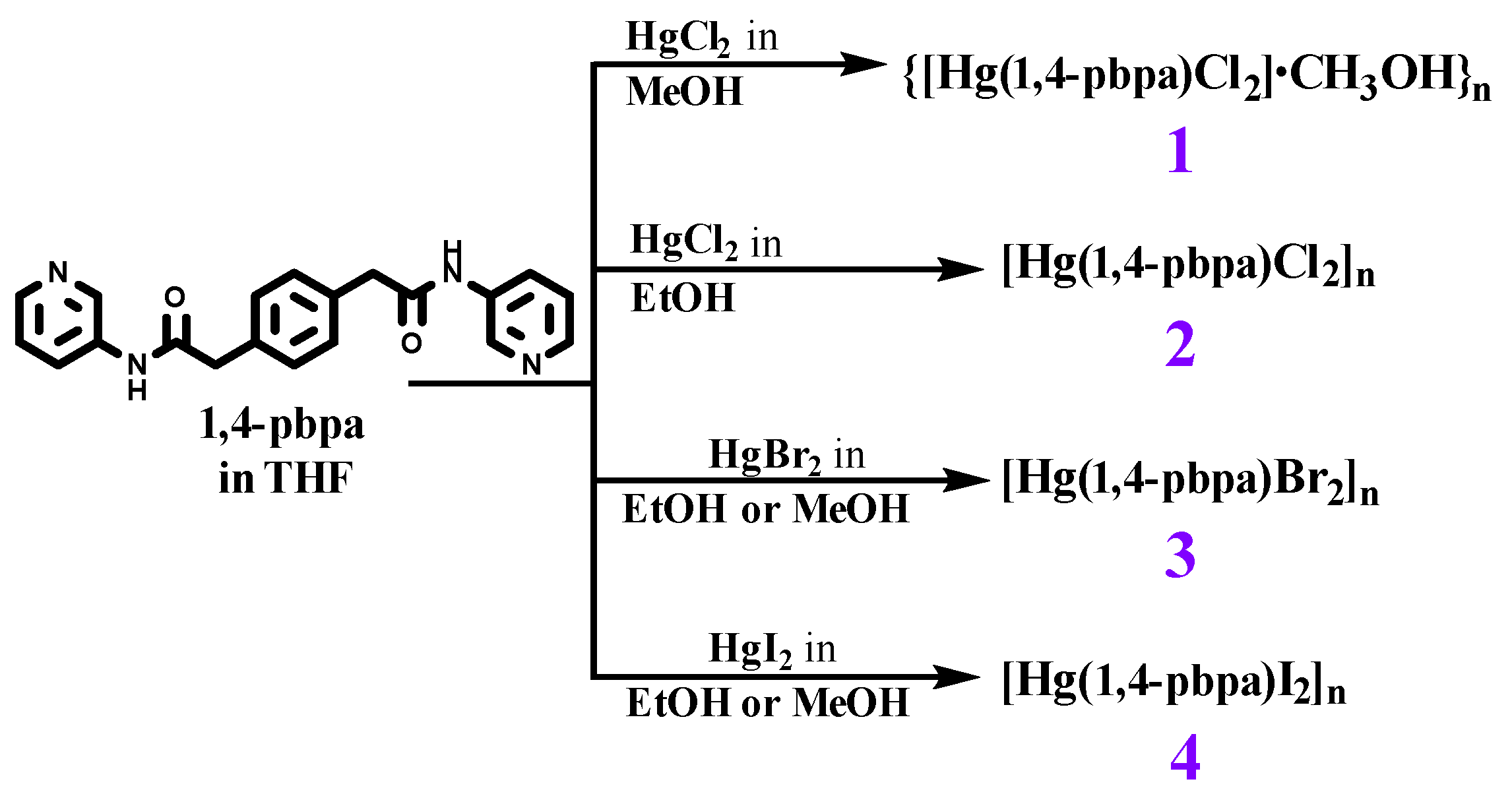
2.3. Preparations
2.3.1. [Hg(1,4-pbpa)Cl2·CH3OH]n, 1
2.3.2. [Hg(1,4-pbpa)Cl2]n, 2
2.3.3. [Hg(1,4-pbpa)Br2]n, 3
2.3.4. [Hg(1,4-pbpa)I2]n, 4
2.4. X-ray Crystallography
3. Results and Discussion
3.1. Synthesis
3.2. Structural Descriptions
3.2.1. Structure of 1
3.2.2. Structures of 2–4
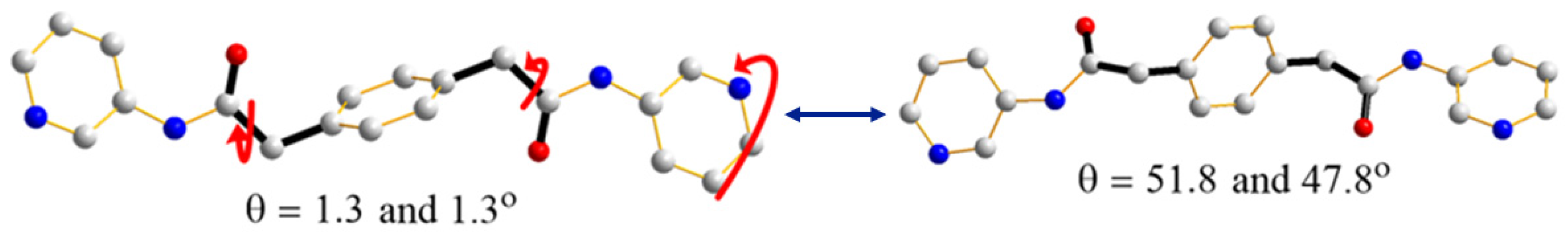
3.2.3. Ligand Conformation
3.2.4. Structural Comparisons
3.2.5. Structural Transformation
3.2.6. Emission Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar]
- Kole, G.K.; Vittal, J.J. Solid-state reactivity and structural transformations involving coordination polymers. Chem. Soc. Rev. 2013, 42, 1755–1775. [Google Scholar] [CrossRef] [PubMed]
- Morsali, A.; Masoomi, M.Y. Structures and properties of mercury(II) coordination polymers. Coord. Chem. Rev. 2009, 253, 1882–1905. [Google Scholar] [CrossRef]
- Mahat Chhetri, P.; Yang, X.-K.; Chen, J.-D. Solvent-mediated reversible structural transformation of mercury iodide coordination polymers: Role of halide anions. Cryst. Growth Des. 2017, 17, 4801–4809. [Google Scholar] [CrossRef]
- Mahat Chhetri, P.; Yang, X.-K.; Chen, J.-D. Mercury halide coordination polymers exhibiting reversible structural transformation. CrystEngComm 2018, 20, 2126–2134. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Zaręba, J.K.; Bauzá, A.; Kubicki, M.; Bartyzel, A.; Keramidas, A.D.; Butusov, L.; Mirosławh, B.; Frontera, A. Recurrent supramolecular motifs in discrete complexes and coordination polymers based on mercury halides: Prevalence of chelate ring stacking and substituent effects. CrystEngComm 2018, 20, 1065–1076. [Google Scholar] [CrossRef]
- Cheng, P.-C.; Yeh, C.-W.; Hsu, W.; Chen, T.-R.; Wang, H.-W.; Chen, J.-D.; Wang, J.-C. Ag(I) complexes containing flexible N,N′-di(3-pyridyl)adipoamide ligands: Syntheses, structures, ligand conformations, and crystal-to-crystal transformations. Cryst. Growth Des. 2012, 12, 943–953. [Google Scholar] [CrossRef]
- Rana, L.K.; Sharma, S.; Hundal, G. First report on crystal engineering of Hg(II) halides with fully substituted 3,4-pyridinedicarboxamides: Generation of two-dimensional coordination polymers and linear zig-zag chains of mercury metal ions. Cryst. Growth Des. 2016, 16, 92–107. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Bauzá, A.; Gurbanov, A.V.; Zubkov, F.I.; Maniukiewicz, W.; Rodriguez-Diéguez, A.; López-Torres, E.; Frontera, A. The role of unconventional stacking interactions in the supramolecular assemblies of Hg(II) coordination compounds. CrystEngComm 2016, 18, 9056–9066. [Google Scholar] [CrossRef]
- Thapa, K.B.; Chen, J.-D. Crystal engineering of coordination polymers containing flexible bis-pyridyl-bis-amide ligands. CrystEngComm 2015, 17, 4611–4626. [Google Scholar] [CrossRef]
- Thapa, K.B.; Hsu, Y.-F.; Lin, H.-C.; Chen, J.-D. Hg(II) supramolecular isomers: Structural transformation and photoluminescence change. CrystEngComm 2015, 17, 7574–7582. [Google Scholar] [CrossRef]
- Mobin, S.M.; Srivastava, A.K.; Mathur, P.; Lahiri, G.K. Reversible single-crystal to single-crystal transformations in a Hg(II) derivative. 1D-polymeric chain ⇌ 2D-networking as a function of temperature. Dalton Trans. 2010, 39, 8698–8705. [Google Scholar] [CrossRef] [PubMed]
- Burchell, T.J.; Eisler, D.J.; Puddephatt, R.J. Self-assembly using dynamic coordination chemistry and hydrogen bonding: Mercury(II) macrocycles, polymers and sheets. Inorg. Chem. 2004, 43, 5550–5557. [Google Scholar] [CrossRef] [PubMed]
- Burchell, T.J.; Puddephatt, R.J. Self-assembly of chiral coordination polymers and macrocycles: A metal template effect on the polymer−macrocycle equilibrium. Inorg. Chem. 2005, 44, 3718–3730. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-L.; Hsu, Y.-F.; Wu, C.-J.; Yeh, C.-W.; Chen, J.-D.; Wang, J.-C. Structural diversity in the d10 metal complexes containing N,N′-di(3-pyridyl)oxamide. Polyhedron 2012, 33, 280–288. [Google Scholar] [CrossRef]
- Maity, K.; Kundu, T.; Banerjee, R.; Biradha, K. One-dimensional water cages with repeat units of (H2O)24 resembling pagodane trapped in a 3D coordination polymer: Proton conduction and tunable luminescence emission by adsorption of anionic dyes. CrystEngComm 2015, 17, 4439–4443. [Google Scholar] [CrossRef]
- Bruker AXS, APEX2, V2008.6; SAD ABS V2008/1; SAINT+ V7.60A; SHELXTL V6.14; Bruker AXS Inc.: Madison, WI, USA, 2008.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. Coordination polymers and molecular structures among complexes of mercury(II) halides with selected 1-benzoylthioureas. Polyhedron 2015, 90, 47–57. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford Science Publications: Oxford, UK, 2001. [Google Scholar]
- Chang, M.-N.; Yang, X.-K.; Mahat Chhetri, P.; Chen, J.-D. Metal and ligand effects on the construction of divalent coordination polymers based on bis-pyridyl-bis-amide and polycarboxylate ligands. Polymers 2017, 9, 691. [Google Scholar] [CrossRef]
- Thapa, K.B.; Yang, X.-K.; Chen, J.-D. Mg(II) coordination polymers based on flexible isomeric tetracarboxylate ligands: Syntheses, structures, structural transformation and luminescent properties. Polymers 2018, 10, 371. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-S.; Lees, A.J. Transition metal based supramolecular systems: Synthesis, photophysics, photochemistry and their potential applications as luminescent anion chemosensors. Coord. Chem. Rev. 2002, 230, 171–192. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-lived emissive probes for time-resolved photoluminescence bioimaging and biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Ni, J.; Wang, Q.; Ding, Y.; Ng, S.W.; Zhu, W.; Xie, Y. Synthesis, structures, and photoluminescence of zinc(II), cadmium(II), and mercury(II) coordination polymers constructed from two novel tetrapyridyl ligands. Cryst. Growth Des. 2010, 10, 1611–1622. [Google Scholar] [CrossRef]







| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Formula | C21H22Cl2HgN4O3 | C20H18Cl2HgN4O2 | C20H18Br2HgN4O2 | C20H18HgI2N4O2 |
| Fw | 649.91 | 617.87 | 706.79 | 800.77 |
| Crystal System | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space Group | P21/m | P21/c | P21/c | P21/c |
| a, Å | 5.0705(1) | 9.3764(5) | 9.3217(1) | 9.3679(2) |
| b, Å | 20.6636(5) | 13.3232(7) | 13.4660(1) | 13.6718(3) |
| c, Å | 11.4320(3) | 17.1229(10) | 17.2458(2) | 17.3825(5) |
| α,° | 90 | 90 | 90 | 90 |
| β,° | 92.3088(18) | 97.490(2) | 96.741(1) | 95.555(1) |
| ϒ,° | 90 | 90 | 90 | 90 |
| V, Å3 | 1196.81(5) | 2120.8(2) | 2149.83(4) | 2215.83(9) |
| Temperature, K | 296 (2) | 299 (2) | 296 (2) | 296 (2) |
| Dcal, g cm−3 | 1.803 | 1.935 | 2.184 | 2.400 |
| F(000) | 628 | 1184 | 1328 | 1472 |
| Z | 2 | 4 | 4 | 4 |
| µ(Mo Kα), mm−1 | 6.682 | 7.533 | 10.9 | 9.756 |
| Range(2θ) for data collection, ° | 4.07 to 56.74 | 3.88 to 56.63 | 3.84 to 56.60 | 3.79 to 56.68 |
| Reflections collected | 11011 | 19495 | 21154 | 20206 |
| Independent reflections | 3057 [R(int) = 0.0491] | 5275 [R(int) = 0.0260] | 5335 [R(int) = 0.0353] | 5516 [R(int) = 0.0323] |
| Data/restraints/parameters | 3057/1/143 | 5275/0/262 | 5335/0/262 | 5516/0/262 |
| Quality-of-fit Indicator c | 1.009 | 1.032 | 1.022 | 1.022 |
| Final R indices [I>2σ(I)] a,b | R1 = 0.0364, wR2 = 0.0711 | R1 = 0.0255, wR2 = 0.0492 | R1 = 0.0312, wR2 = 0.0556 | R1 = 0.0337, wR2 = 0.0840 |
| R indices (all data) | R1 = 0.0558, wR2 = 0.0783 | R1 = 0.0379, wR2 = 0.0524 | R1 = 0.0537, wR2 = 0.0608 | R1 = 0.0421, wR2 = 0.0882 |
| Complex | Dihedral Angle/° | Conformation | Structure | |||
|---|---|---|---|---|---|---|
| Py-Py | Ph-Py | Py-NCO | NCO–NCO | |||
| 1 | 0 | 69.4 | 28.4 | 0 | trans anti-anti | sinusoidal |
| 2 | 48.4 | 57.0, 81.6 | 32.6, 22.7 | 8.6 | trans syn-anti | helical |
| Complex | Structure | Reference |
|---|---|---|
| [Hg(1,2-pbpa)Cl2]n | Zigzag chain | [4] |
| [Hg(1,2-pbpa)Br2]n | Zigzag chain | [4] |
| [Hg(1,2-pbpa)I2]n | Zigzag chain | [4] |
| {[Hg(1,2-pbpa)I2]·MeCN}n | Helical chain | [4] |
| {[Hg(1,2-pbpa)I2]·MeOH}n | Helical chain | [4] |
| [Hg(1,3-pbpa)Cl2]n | Helical chain | [5] |
| [Hg(1,3-pbpa)Br2]n | Helical chain | [5] |
| [Hg(1,3-pbpa)I2]n | Helical chain | [5] |
| {[Hg(1,3-pbpa)Br2]·MeCN}n | Mesohelical chain | [5] |
| {[Hg(1,3-pbpa)I2]·MeCN}n | Mesohelical chain | [5] |
| {[Hg(1,4-pbpa)Cl2]·MeOH}n, 1 | Sinusoidal chain | This work |
| [Hg(1,4-pbpa)Cl2]n, 2 | Helical chain | This work |
| [Hg(1,4-pbpa)Br2]n, 3 | Helical chain | This work |
| [Hg(1,4-pbpa)I2]n, 4 | Helical chain | This work |
| Compound | Excitation λmax | Emission λmax |
|---|---|---|
| 1 | 372 | 448 |
| 2 | 367 | 438 |
| 3 | 376 | 455 |
| 4 | 396 | 461 |
| 1,4-pbpa | 273 | 330 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahat Chhetri, P.; Yang, X.-K.; Yang, C.-T.; Chen, J.-D. One-Dimensional Mercury Halide Coordination Polymers Based on A Semi-Rigid N-Donor Ligand: Reversible Structural Transformation. Polymers 2019, 11, 436. https://doi.org/10.3390/polym11030436
Mahat Chhetri P, Yang X-K, Yang C-T, Chen J-D. One-Dimensional Mercury Halide Coordination Polymers Based on A Semi-Rigid N-Donor Ligand: Reversible Structural Transformation. Polymers. 2019; 11(3):436. https://doi.org/10.3390/polym11030436
Chicago/Turabian StyleMahat Chhetri, Pradhumna, Xiang-Kai Yang, Chih-Tung Yang, and Jhy-Der Chen. 2019. "One-Dimensional Mercury Halide Coordination Polymers Based on A Semi-Rigid N-Donor Ligand: Reversible Structural Transformation" Polymers 11, no. 3: 436. https://doi.org/10.3390/polym11030436