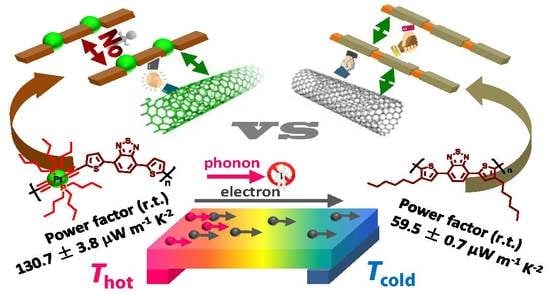
Boosting the Adhesivity of π-Conjugated Polymers by Embedding Platinum Acetylides towards High-Performance Thermoelectric Composites
Abstract
:1. Introduction
2. Experimental
2.1. Instrumentation
2.2. Materials Preparation
2.2.1. Synthesis of the Polymer P(TBT-Pt)
2.2.2. Synthesis of Polymer P(TBT)
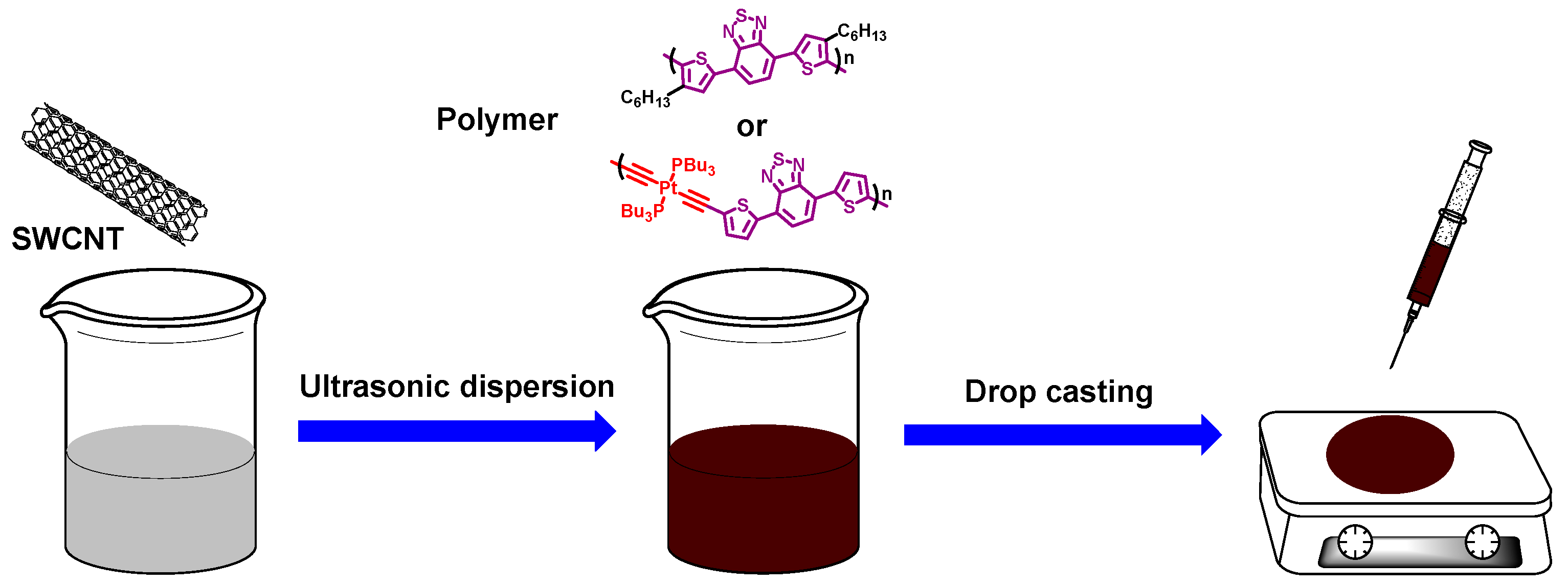
2.3. Preparation of the Composite Films
2.4. Density Functional Theory Simulations
2.5. Thermoelectric Measurements
3. Results and Discussion
3.1. Material Synthesis and Characterization
3.2. DFT Simulation, Electrochemical and Photophysical Properties
3.3. Thermoelectric Properties
3.4. SEM and TEM Image Studies
3.5. Raman Spectroscopy
3.6. X-ray Photoelectron Spectroscopy Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, X.; Pan, C.; Liang, A.; Wang, L.; Wong, W.-Y. Thermoelectric properties of composite films prepared with benzodithiophene derivatives and carbon nanotubes. Compos. Sci. Technol. 2017, 145, 40–45. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Liu, B. Carbon Nanotube-Based Organic Thermoelectric Materials for Energy Harvesting. Polymers 2018, 10, 1196. [Google Scholar] [CrossRef]
- Kroon, R.; Mengistie, D.A.; Kiefer, D.; Hynynen, J.; Ryan, J.D.; Yu, L.; Muller, C. Thermoelectric plastics: From design to synthesis, processing and structure-property relationships. Chem. Soc. Rev. 2016, 45, 6147–6164. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Liang, Z. Solution processed organic thermoelectrics: towards flexible thermoelectric modules. Energy Environ. Sci. 2015, 8, 401–422. [Google Scholar] [CrossRef]
- Lee, M.J.; Ahn, J.H.; Sung, J.H.; Heo, H.; Jeon, S.G.; Lee, W.; Song, J.Y.; Hong, K.H.; Choi, B.; Lee, S.H.; et al. Thermoelectric materials by using two-dimensional materials with negative correlation between electrical and thermal conductivity. Nat. Commun. 2016, 7, 12011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Song, L.; Madsen, G.K.; Fischer, K.F.; Zhang, W.; Shi, X.; Iversen, B.B. Designing high-performance layered thermoelectric materials through orbital engineering. Nat. Commun. 2016, 7, 10892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liao, J.; Tang, Y.; Gu, M.; Ming, C.; Qiu, P.; Bai, S.; Shi, X.; Uher, C.; Chen, L. Realizing a thermoelectric conversion efficiency of 12% in bismuth telluride/skutterudite segmented modules through full-parameter optimization and energy-loss minimized integration. Energy Environ. Sci. 2017, 10, 956–963. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.; Wang, D.; Zhu, G.; Li, J. Preparation and thermoelectric properties of polythiophene/multiwalled carbon nanotube composites. Synth. Met. 2013, 181, 79–85. [Google Scholar] [CrossRef]
- Bertram, J.R.; Penn, A.; Nee, M.J.; Rathnayake, H. A Novel n-Type Organosilane-Metal Ion Hybrid of Rhodamine B and Copper Cation for Low-Temperature Thermoelectric Materials. ACS Appl. Mater. Interfaces 2017, 9, 10946–10954. [Google Scholar] [CrossRef]
- Nakashima, Y.; Nakashima, N.; Fujigaya, T. Development of air-stable n-type single-walled carbon nanotubes by doping with 2-(2-methoxyphenyl)-1,3-dimethyl-2,3-dihydro-1H-benzo[d]imidazole and their thermoelectric properties. Synth. Met. 2017, 225, 76–80. [Google Scholar] [CrossRef]
- Zhou, X.; Liang, A.; Pan, C.; Wang, L. Effects of oxidative doping on the thermoelectric performance of polyfluorene derivatives/carbon nanotube composite films. Org. Electron. 2018, 52, 281–287. [Google Scholar] [CrossRef]
- Pan, C.; Wang, L.; Zhou, W.; Cai, L.; Xie, D.; Chen, Z.; Wang, L. Preparation and Thermoelectric Properties Study of Bipyridine-Containing Polyfluorene Derivative/SWCNT Composites. Polymers 2019, 11, 278. [Google Scholar] [CrossRef]
- Dennler, G.; Chmielowski, R.; Jacob, S.; Capet, F.; Roussel, P.; Zastrow, S.; Nielsch, K.; Opahle, I.; Madsen, G.K.H. Are Binary Copper Sulfides/Selenides Really New and Promising Thermoelectric Materials? Adv. Energy Mater. 2014, 4, 1301581. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, H.; Snyder, G.J. Band engineering of thermoelectric materials. Adv. Mater. 2012, 24, 6125–6135. [Google Scholar] [CrossRef]
- Xiao, C.; Li, Z.; Li, K.; Huang, P.; Xie, Y. Decoupling interrelated parameters for designing high performance thermoelectric materials. Acc. Chem. Res. 2014, 47, 1287–1295. [Google Scholar] [CrossRef]
- Du, F.-P.; Qiao, X.; Wu, Y.-G.; Fu, P.; Liu, S.-P.; Zhang, Y.-F.; Wang, Q.-Y. Fabrication of Porous Polyvinylidene Fluoride/Multi-Walled Carbon Nanotube Nanocomposites and Their Enhanced Thermoelectric Performance. Polymers 2018, 10, 797. [Google Scholar] [CrossRef]
- Cho, C.; Wallace, K.L.; Tzeng, P.; Hsu, J.-H.; Yu, C.; Grunlan, J.C. Outstanding Low Temperature Thermoelectric Power Factor from Completely Organic Thin Films Enabled by Multidimensional Conjugated Nanomaterials. Adv. Energy Mater. 2016, 6, 1502168. [Google Scholar] [CrossRef]
- Meng, C.; Liu, C.; Fan, S. A Promising Approach to Enhanced Thermoelectric Properties Using Carbon Nanotube Networks. Adv. Mater. 2010, 22, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Aich, R.B.; Blouin, N.; Bouchard, A.; Leclerc, M. Electrical and Thermoelectric Properties of Poly(2,7-Carbazole) Derivatives. Chem. Mater. 2009, 21, 751–757. [Google Scholar] [CrossRef]
- Kroon, R.; Kiefer, D.; Stegerer, D.; Yu, L.; Sommer, M.; Mueller, C. Polar Side Chains Enhance Processability, Electrical Conductivity, and Thermal Stability of a Molecularly p-Doped Polythiophene. Adv. Mater. 2017, 29, 1700930. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Chen, L.; Zhang, W.; Liufu, S.; Chen, X. Enhanced Thermoelectric Performance of Single-Walled Carbon Nanotubes/Polyaniline Hybrid Nanocomposites. ACS Nano 2010, 4, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Chen, G.; Guo, C.-Y. Enhanced thermoelectric performance by self-assembled layered morphology of polypyrrole nanowire/single-walled carbon nanotube composites. Compos. Sci. Technol. 2016, 129, 130–136. [Google Scholar] [CrossRef]
- Du, Y.; Niu, H.; Li, J.; Dou, Y.; Shen, S.Z.; Jia, R.; Xu, J. Morphologies Tuning of Polypyrrole and Thermoelectric Properties of Polypyrrole Nanowire/Graphene Composites. Polymers 2018, 10, 1143. [Google Scholar] [CrossRef]
- Song, H.; Cai, K.; Wang, J.; Shen, S. Influence of polymerization method on the thermoelectric properties of multi-walled carbon nanotubes/polypyrrole composites. Synth. Met. 2016, 211, 58–65. [Google Scholar] [CrossRef]
- Liu, J.; Yu, H.-Q. Thermoelectric Enhancement in Polyaniline Composites with Polypyrrole-Functionalized Multiwall Carbon Nanotubes. J. Electron. Mater. 2014, 43, 1181–1187. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Choi, K.; Grunlan, J.C.; Yu, C. Improved Thermoelectric Behavior of Nanotube-Filled Polymer Composites with Poly(3,4-ethylenedioxythiophene) Poly(styrenesulfonate). ACS Nano 2010, 4, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Yu, C. Highly Doped Carbon Nanotubes with Gold Nanoparticles and Their Influence on Electrical Conductivity and Thermopower of Nanocomposites. PLoS ONE 2012, 7, 16033. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Anoop, G.; Lee, H.J.; Kim, C.; Park, J.-W.; Choi, J.; Kim, H.; Kim, Y.-J.; Lee, E.; Lee, S.-G.; et al. Enhanced thermoelectric performance of PEDOT: PSS/PANI-CSA polymer multilayer structures. Energy Environ. Sci. 2016, 9, 2806–2811. [Google Scholar] [CrossRef]
- Bounioux, C.; Diaz-Chao, P.; Campoy-Quiles, M.; Martin-Gonzalez, M.S.; Goni, A.R.; Yerushalmi-Rozene, R.; Mueller, C. Thermoelectric composites of poly(3-hexylthiophene) and carbon nanotubes with a large power factor. Energy Environ. Sci. 2013, 6, 918–925. [Google Scholar] [CrossRef]
- Du, Y.; Shen, S.Z.; Yang, W.D.; Cai, K.F.; Casey, P.S. Preparation and characterization of multiwalled carbon nanotube/poly(3-hexylthiophene) thermoelectric composite materials. Synth. Met. 2012, 162, 375–380. [Google Scholar] [CrossRef]
- Lee, W.; Hong, C.T.; Kwon, O.H.; Yoo, Y.; Kang, Y.H.; Lee, J.Y.; Cho, S.Y.; Jang, K.S. Enhanced thermoelectric performance of bar-coated SWCNT/P3HT thin films. ACS Appl. Mater. Interfaces 2015, 7, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, W.; Xu, H.; Wang, W. Pulse Electropolymerization and Thermoelectrical Performances of Carbon Nanotubes/Polyaniline Composite Film. ECS J. Solid State Sci. Technol. 2016, 5, M27–M30. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, J.; Zhao, L.; Wu, J.; Li, Q. Thermoelectric performance of conducting aerogels based on carbon nanotube/silver nanocomposites with ultralow thermal conductivity. RSC Adv. 2016, 6, 109878–109884. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Q.; Qu, S.; Shi, W.; Chen, L. Influence of electronic type of SWNTs on the thermoelectric properties of SWNTs/PANI composite films. Org. Electron. 2016, 39, 146–152. [Google Scholar] [CrossRef]
- Li, J.; Lai, C.; Xiang, X.; Wang, L. Synthesis and characterization of poly-Schiff bases with a donor-acceptor structure containing thiophene units as thermoelectric materials. J. Mater. Chem. C 2015, 3, 2693–2701. [Google Scholar] [CrossRef]
- Li, J.; Lai, C.; Jia, X.; Wang, L.; Xiang, X.; Ho, C.-L.; Li, H.; Wong, W.-Y. Effect of electron donor/acceptor substituents on the Seebeck coefficient and thermoelectric properties of poly(3-methylthiophene methine)s/graphite composites. Composites Part B 2015, 77, 248–256. [Google Scholar] [CrossRef]
- Wang, L.; Pan, C.; Liang, A.; Zhou, X.; Zhou, W.; Wan, T.; Wang, L. The effect of the backbone structure on the thermoelectric properties of donor–acceptor conjugated polymers. Polym. Chem. 2017, 8, 4644–4650. [Google Scholar] [CrossRef]
- Wang, X.Z.; Wang, Q.; Yan, L.; Wong, W.Y.; Cheung, K.Y.; Ng, A.; Djurisic, A.B.; Chan, W.K. Very-low-bandgap metallopolyynes of platinum with a cyclopentadithiophenone ring for organic solar cells absorbing down to the near-infrared spectral region. Macromol. Rapid Commun. 2010, 31, 861–867. [Google Scholar] [CrossRef]
- Cekli, S.; Winkel, R.W.; Schanze, K.S. Effect of Oligomer Length on Photophysical Properties of Platinum Acetylide Donor-Acceptor-Donor Oligomers. J. Phys. Chem. A 2016, 120, 5512–5521. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.Y.; Wang, X.Z.; He, Z.; Djurisic, A.B.; Yip, C.T.; Cheung, K.Y.; Wang, H.; Mak, C.S.; Chan, W.K. Metallated conjugated polymers as a new avenue towards high-efficiency polymer solar cells. Nat. Mater. 2007, 6, 521–527. [Google Scholar] [CrossRef]
- Motaghiani, S.; Mirabbaszadeh, K. Density functional study of platinum polyyne monomer, oligomer, and polymer: Ground state geometrical and electronic structures. Int. J. Q. Chem. 2013, 113, 1650–1659. [Google Scholar] [CrossRef]
- Yin, X.; Peng, Y.; Luo, J.; Zhou, X.; Gao, C.; Wang, L.; Yang, C. Tailoring the framework of organic small molecule semiconductors towards high-performance thermoelectric composites via conglutinated carbon nanotube webs. J. Mater. Chem. A 2018, 6, 8323–8330. [Google Scholar] [CrossRef]
- Wang, L.; Pan, C.; Chen, Z.; Zhou, X.; Gao, C.; Wang, L. A study of the thermoelectric properties of benzo[1,2-b:4,5-b′] dithiophene-based donor-acceptor conjugated polymers. Polym. Chem. 2018, 9, 4440–4447. [Google Scholar] [CrossRef]
- Zhao, W.; Fan, S.; Xiao, N.; Liu, D.; Tay, Y.Y.; Yu, C.; Sim, D.; Hng, H.H.; Zhang, Q.; Boey, F.; et al. Flexible carbon nanotube papers with improved thermoelectric properties. Energy Environ. Sci. 2012, 5, 5364–5369. [Google Scholar] [CrossRef]
- Van den Berg, M.; Back, J.; Horneber, A.; Meixner, M.; Swider, K.; Ludwigs, S.; Zhang, D. Determination of the Local Morphology within Individual Polymer Domains. Macromolecules 2016, 49, 8219–8227. [Google Scholar] [CrossRef]
- Wood, S.; Kim, J.-H.; Hwang, D.-H.; Kim, J.-S. Effects of Fluorination and Side Chain Branching on Molecular Conformation and Photovoltaic Performance of Donor–Acceptor Copolymers. Chem. Mater. 2015, 27, 4196–4204. [Google Scholar] [CrossRef]
- Wang, L.; Pan, C.; Chen, Z.; Zhou, W.; Gao, C.; Wang, L. Enhanced Thermoelectric Performance of Conjugated Polymer/Single-Walled Carbon Nanotube Composites with Strong Stacking. ACS Appl. Energy Mater. 2018, 1, 5075–5082. [Google Scholar] [CrossRef]
- Kim, G.H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef]








| SWCNT (wt%) (a) | P(TBT-Pt)/SWCNT | P(TBT)/SWCNT | ||||
|---|---|---|---|---|---|---|
| σ [S·cm−1] | S [μV·K−1] | PF [μW·m−1·K−1] | σ [S·cm−1] | S [μV·K−1] | PF [μW·m−1·K−1] | |
| 10% | 17.2 ± 5.6 | 53.5 ± 1.3 | 4.9 ± 1.0 | 1.2 ± 0.6 | 72.4 ± 1.0 | 0.6 ± 0.3 |
| 30% | 199.0 ± 7.8 | 48.1 ± 1.2 | 46.1 ± 2.3 | 28.4 ± 7.8 | 62.9 ± 0.8 | 11.3 ± 2.1 |
| 50% | 328.8 ± 9.3 | 46.8 ± 0.9 | 71.9 ± 3.5 | 360.0 ± 8.1 | 31.8 ± 0.7 | 36.4 ± 0.9 |
| 70% | 493.1 ± 9.5 | 45.9 ± 1.1 | 103.8 ± 4.1 | 548.9 ± 9.4 | 28.6 ± 0.6 | 45.0 ± 1.1 |
| 90% | 665.8 ± 8.9 | 44.3 ± 0.9 | 130.7 ± 3.8 | 864.4 ± 8.8 | 26.2 ± 0.4 | 59.5 ± 0.7 |
| 100% | 934.7 ± 9.9 | 24.9 ± 1.4 | 58.0 ± 1.5 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, T.; Yin, X.; Pan, C.; Liu, D.; Zhou, X.; Gao, C.; Wong, W.-Y.; Wang, L. Boosting the Adhesivity of π-Conjugated Polymers by Embedding Platinum Acetylides towards High-Performance Thermoelectric Composites. Polymers 2019, 11, 593. https://doi.org/10.3390/polym11040593
Wan T, Yin X, Pan C, Liu D, Zhou X, Gao C, Wong W-Y, Wang L. Boosting the Adhesivity of π-Conjugated Polymers by Embedding Platinum Acetylides towards High-Performance Thermoelectric Composites. Polymers. 2019; 11(4):593. https://doi.org/10.3390/polym11040593
Chicago/Turabian StyleWan, Tao, Xiaojun Yin, Chengjun Pan, Danqing Liu, Xiaoyan Zhou, Chunmei Gao, Wai-Yeung Wong, and Lei Wang. 2019. "Boosting the Adhesivity of π-Conjugated Polymers by Embedding Platinum Acetylides towards High-Performance Thermoelectric Composites" Polymers 11, no. 4: 593. https://doi.org/10.3390/polym11040593