Influence of Combustion Modifiers on the Cure Kinetics of Glycidyl Azide Polymer Based Propellant-Evaluated through Rheo-Kinetic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Experimental Instruments and Conditions
2.3. Test Methods and Curing Model
3. Results and Discussion
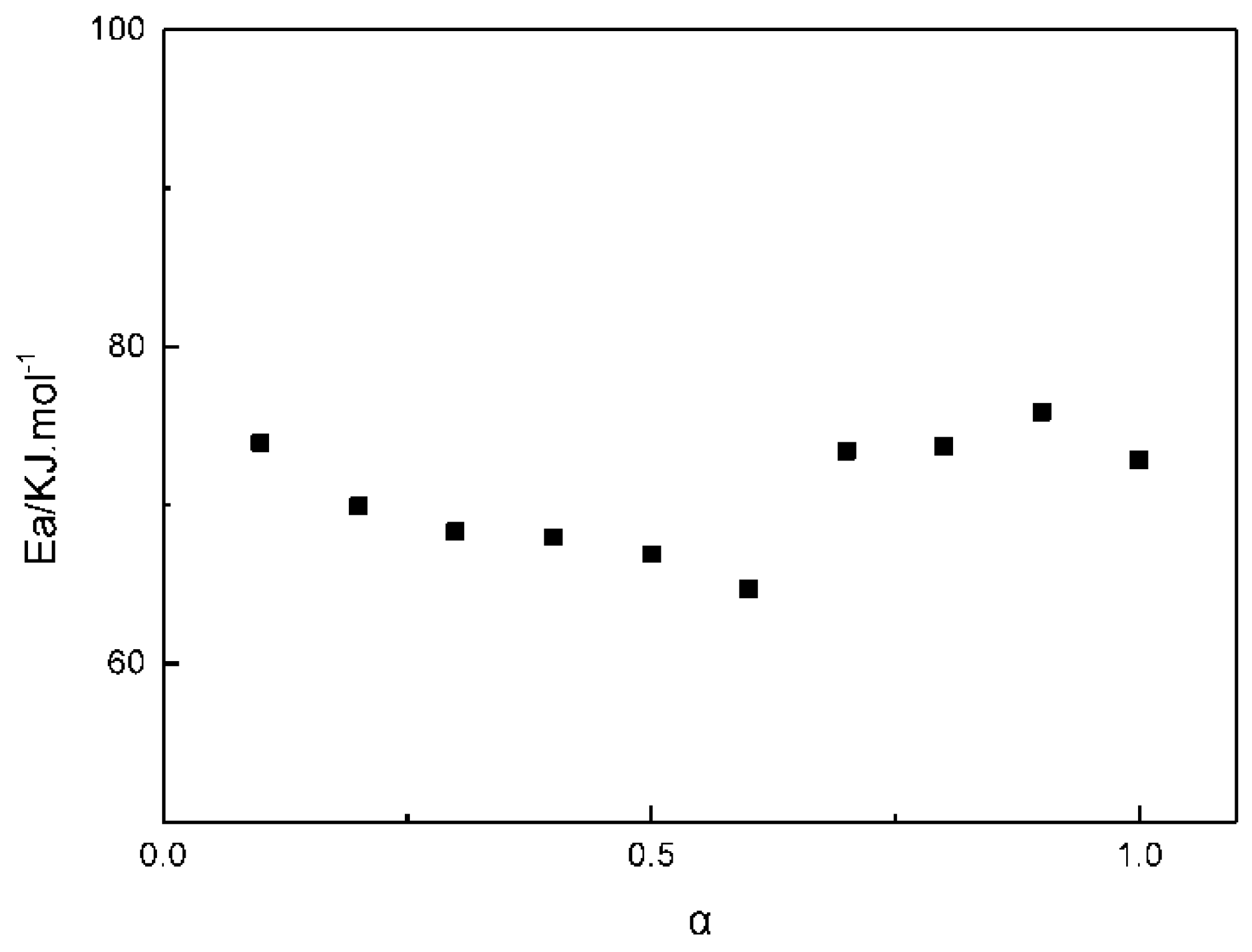
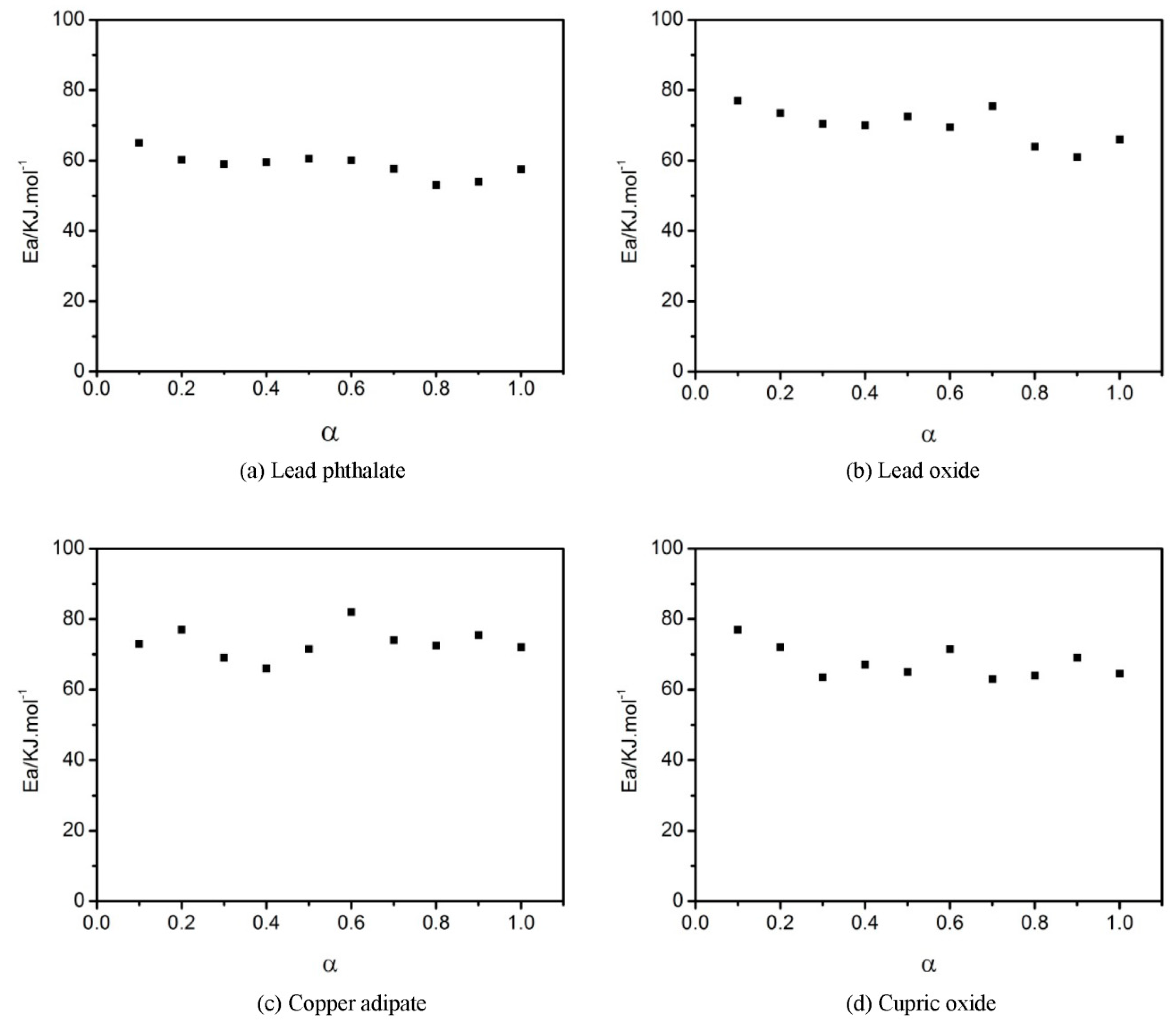
3.1. The Apparent Activation Energy of the GAPSP Curing Reaction
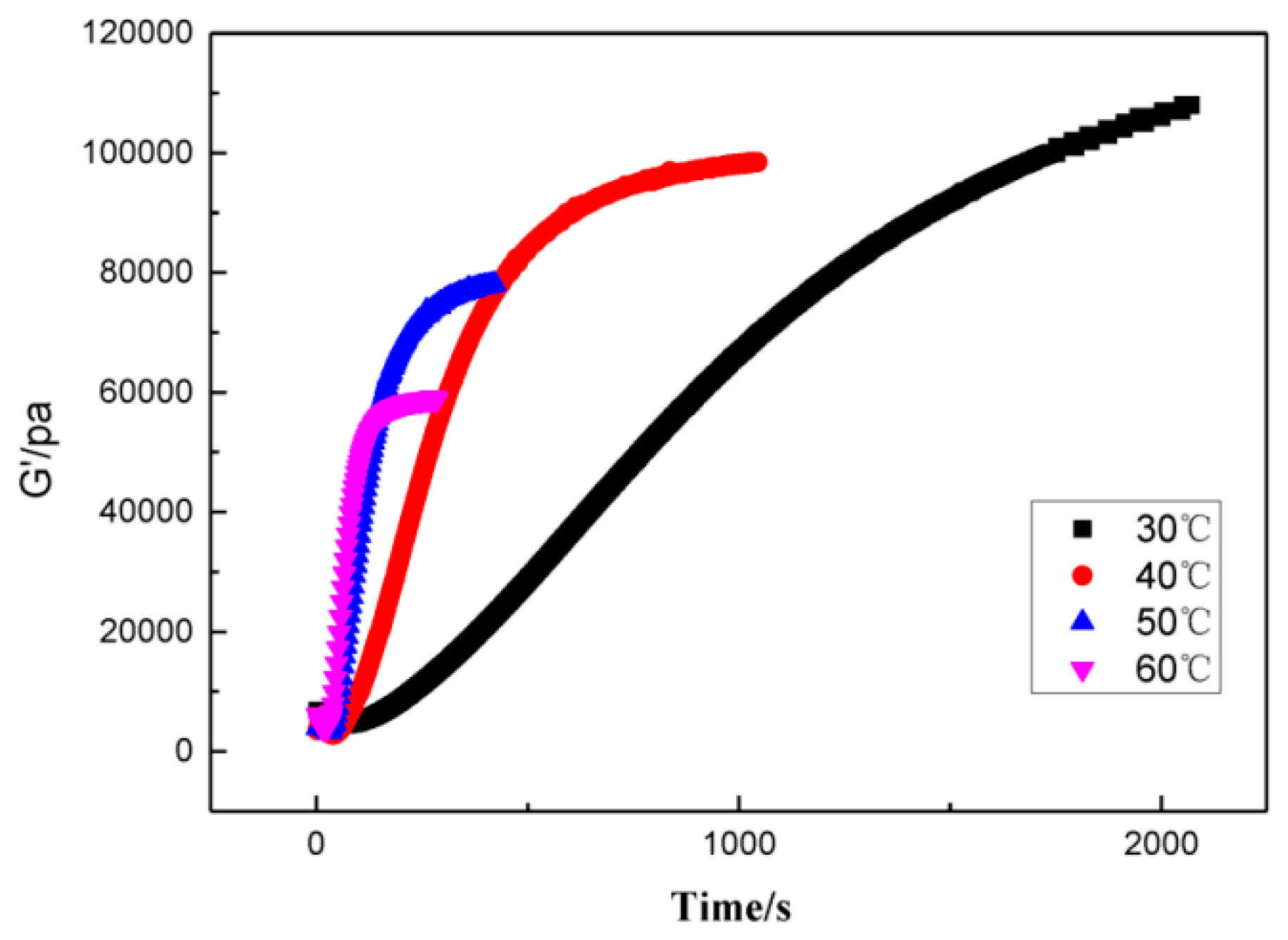
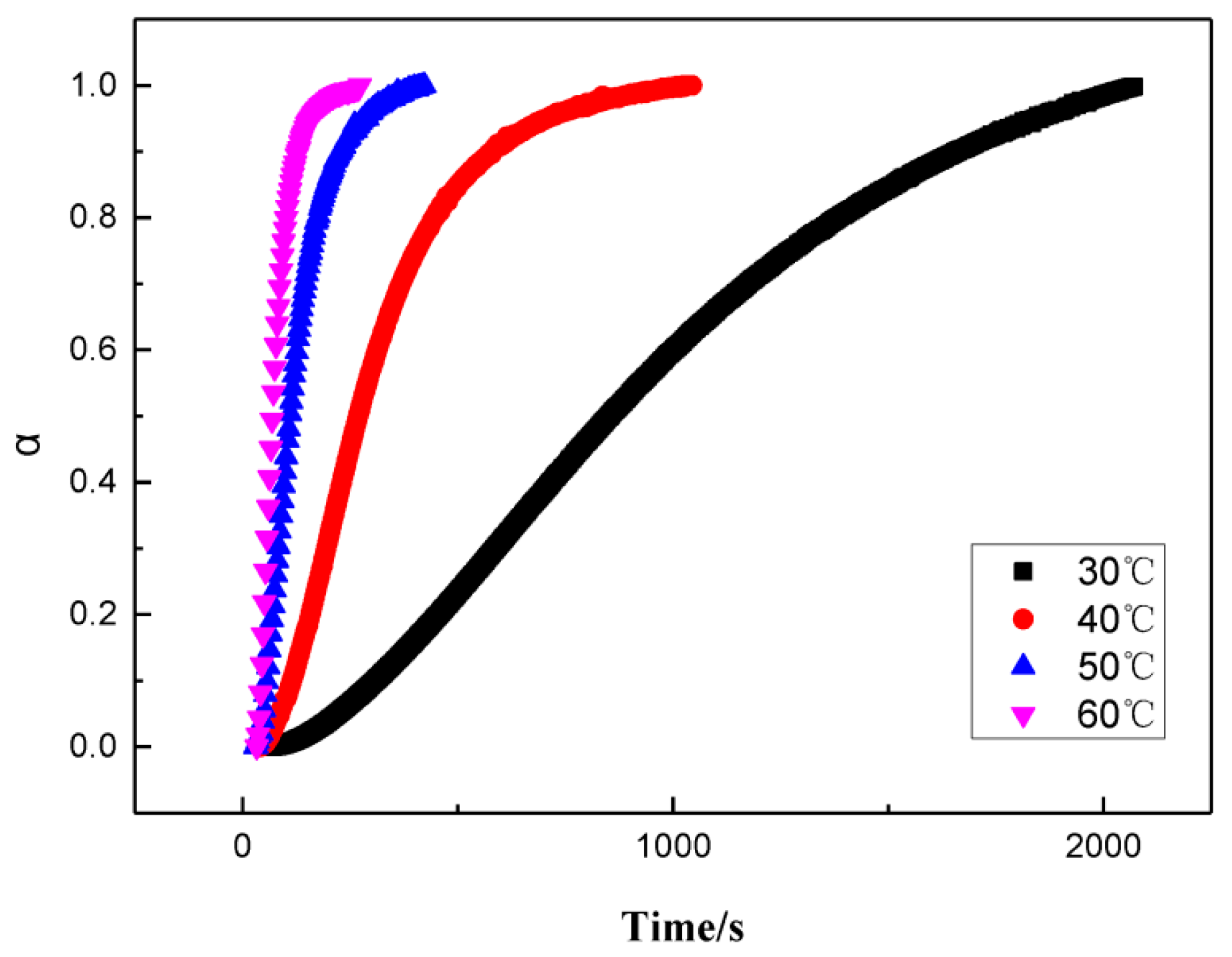
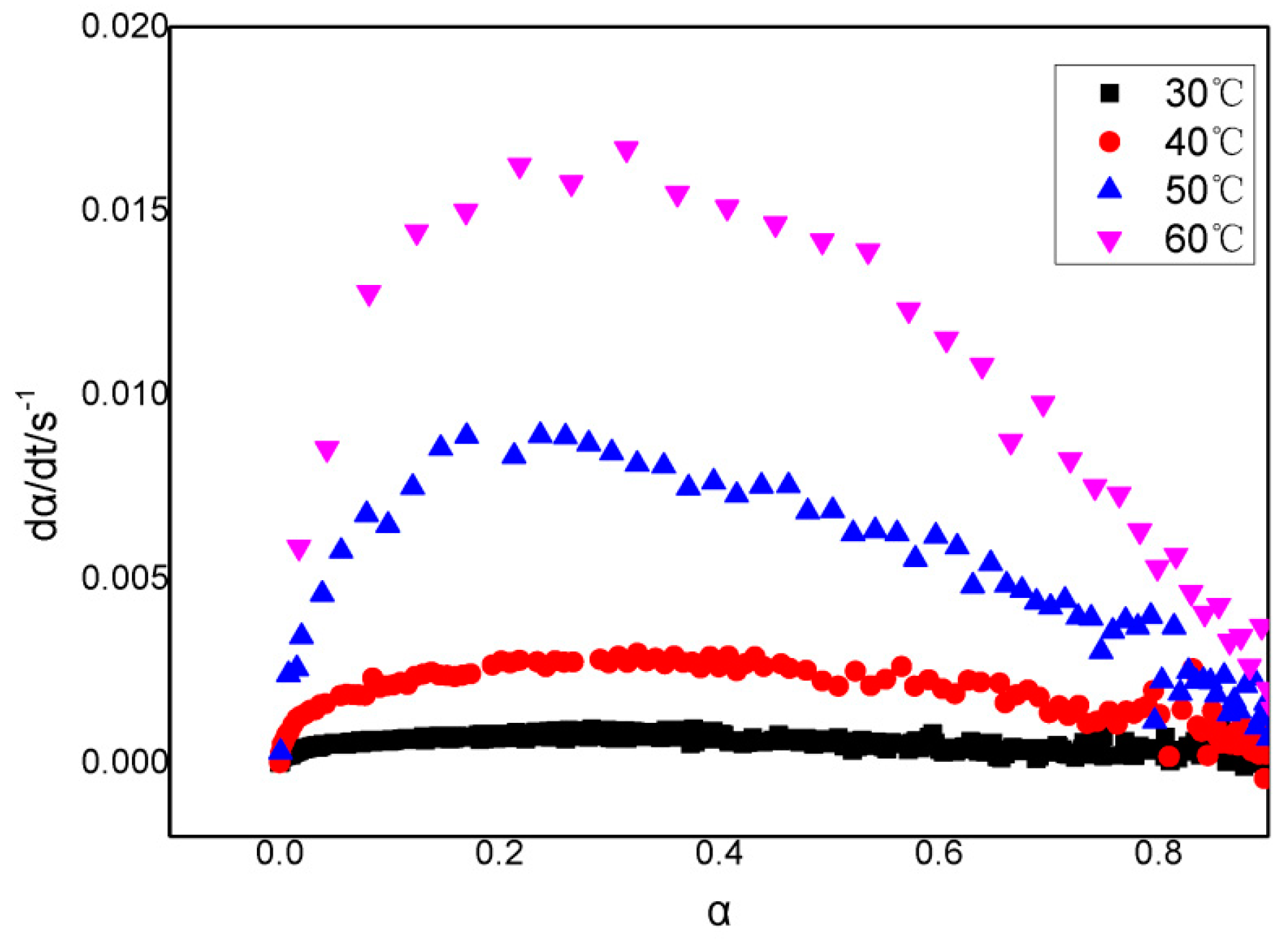
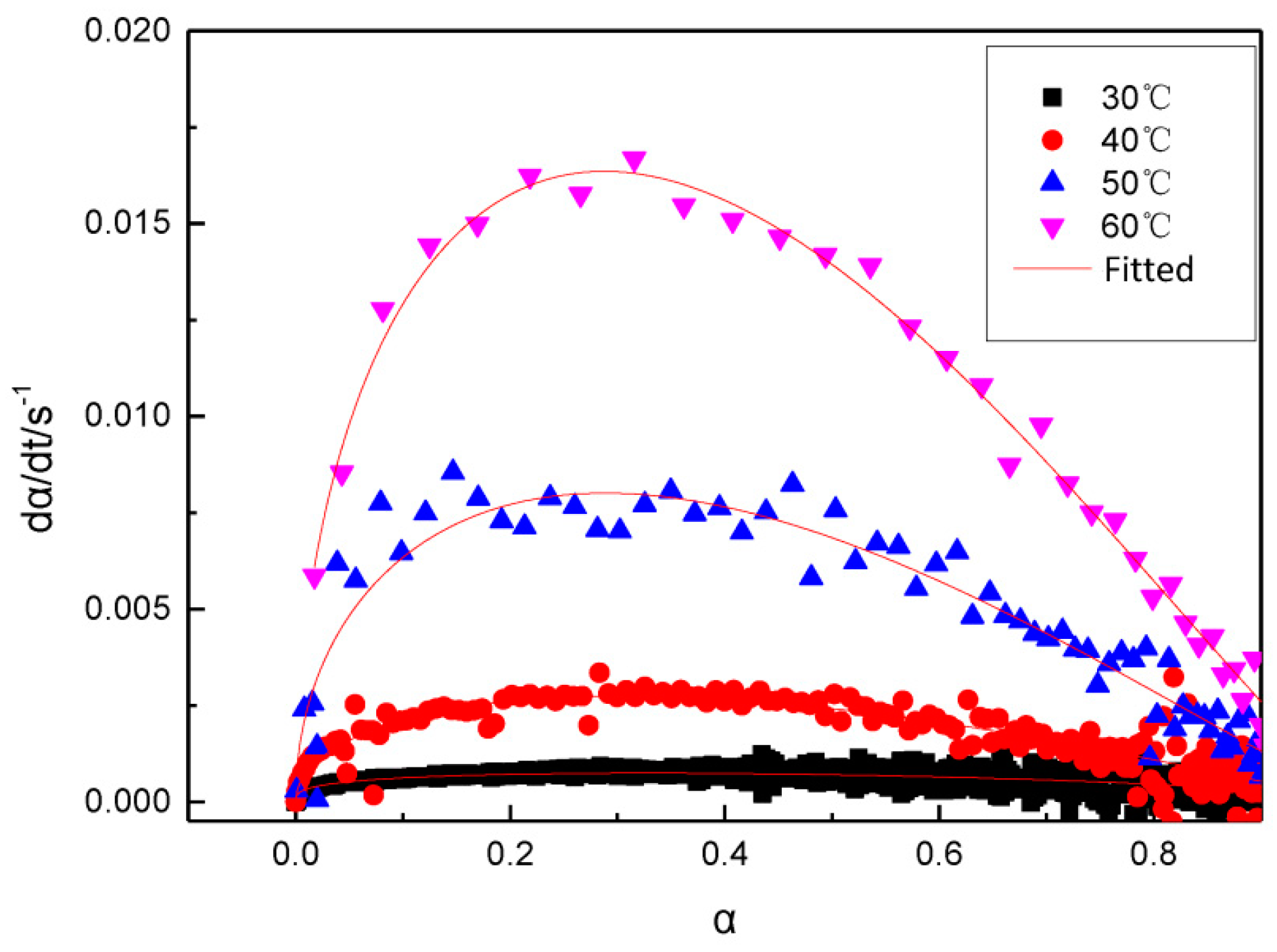
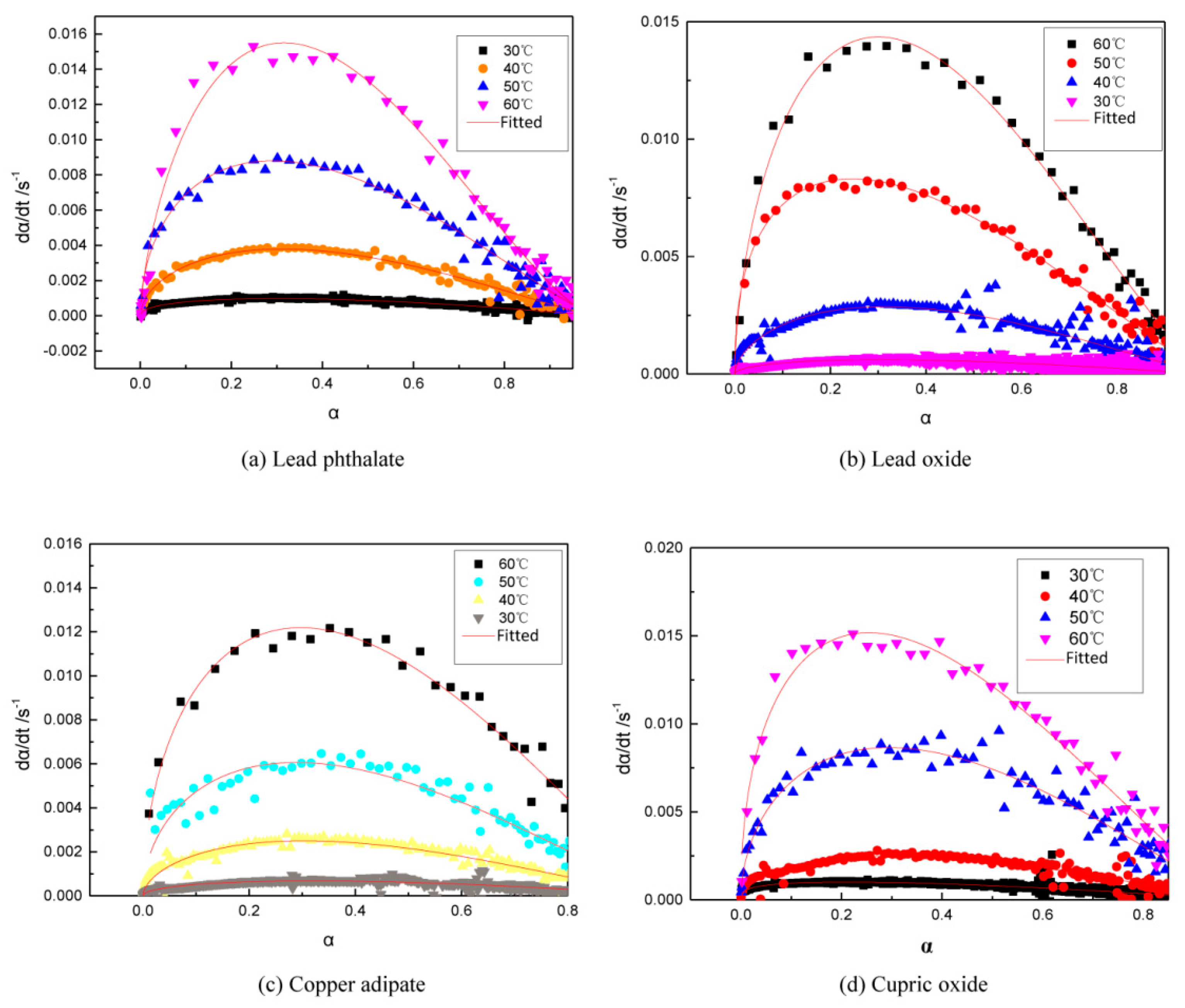
3.2. A model for the Curing Reaction of GAPSPs
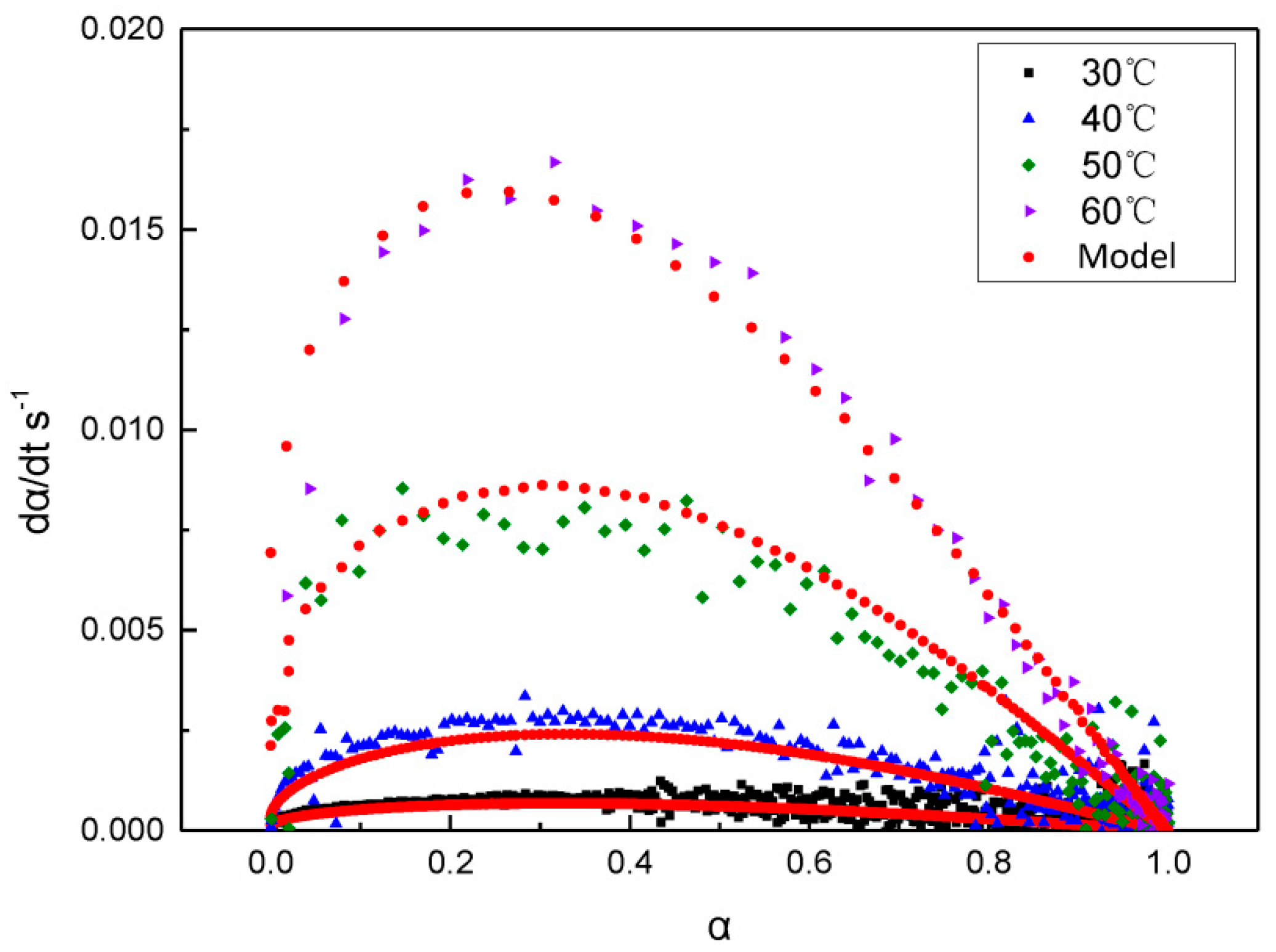
3.3. GAPSPs Curing Kinetic Equation
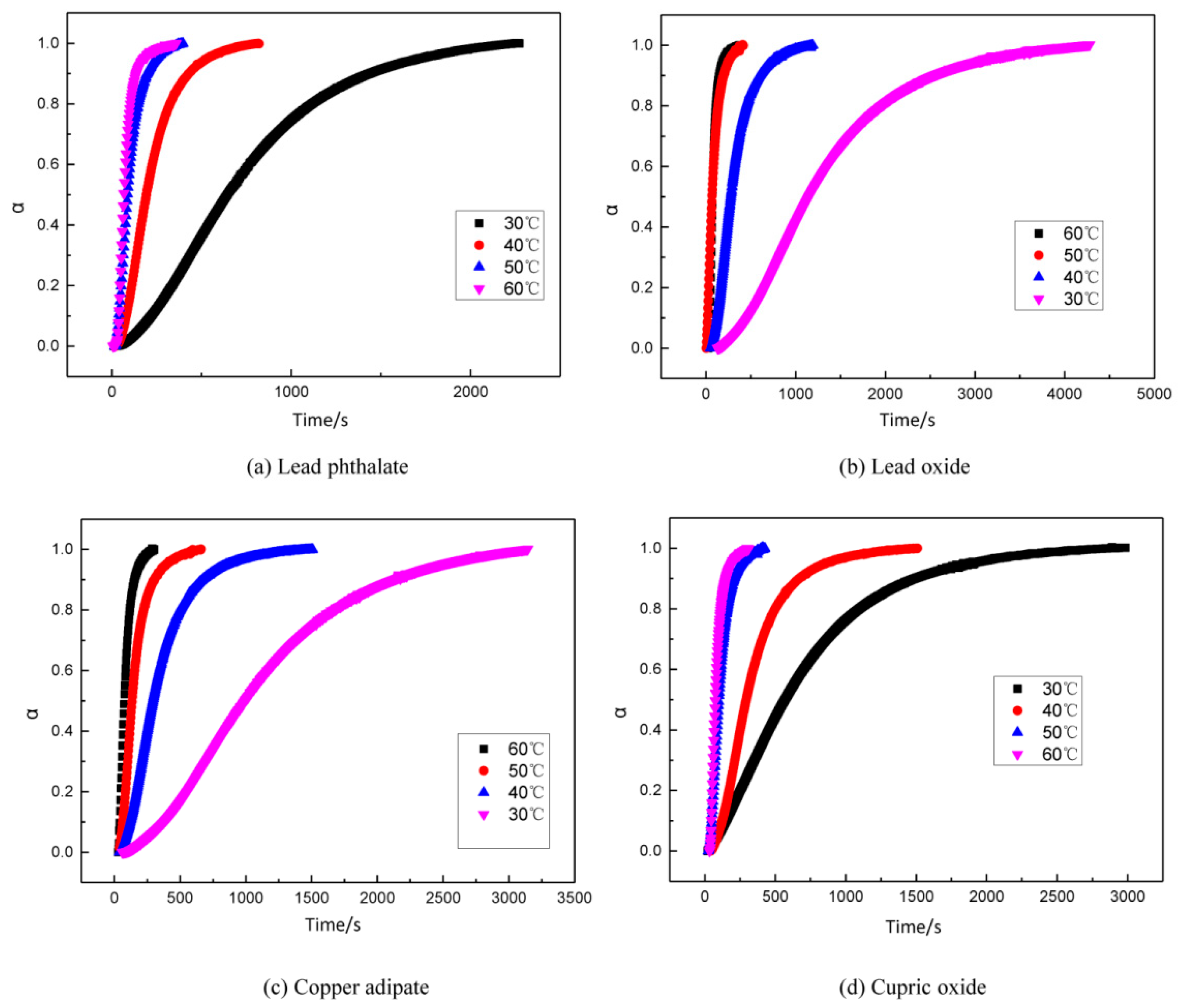
3.4. The Influence of Combustion Modifiers on the Curing of GAPSPs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hussein, A.; Zeman, S.; Elbeih, A. Thermo-analytical study of glycidyl azide polymer and its effect on different cyclic nitramines. Thermochim. Acta 2018, 660, 110–123. [Google Scholar] [CrossRef]
- Hafner, S.; Keicher, T.; Klapotke, T. Copolymers based on GAP and 1,2-Epoxyhexane as Promising Prepolymers for Energetic Binder Systems. Propell. Explos. Pyrot. 2018, 43, 126–135. [Google Scholar] [CrossRef]
- Kim, H.; Jang, Y.; Noh, S.; Jeong, J.; Kim, D.; Kang, B.; Kang, T.; Choi, H.; Rhee, H. Ecofriendly synthesis and characterization of carboxylated GAP copolymers. RSC Adv. 2018, 8, 20032–20038. [Google Scholar] [CrossRef]
- Agawane, N.; Soman, R.; Wagh, R.; Athar, J.; Talawar, M. Optimization of Curing Agents for Linear Difunctional Glycidyl Azide Polymer (GAP), with and without Isocyanate, for Binder Applications. Cent. Eur. J. Energ. Mater. 2018, 15, 206–222. [Google Scholar]
- Huang, T.; Jin, B.; Peng, R. Synthesis and Characterization of Fullerene-Glycidyl Azide Polymer and Its Thermal Decomposition. Polymers 2015, 7, 896–908. [Google Scholar] [CrossRef]
- Sun, Q.; Sang, C.; Wang, Z.; Luo, Y. The study of mechanical and creep properties of glycidyl azide polyol energetic thermoplastic elastomer binder with bonding group with RDX and its interface reinforcement mechanism. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- Tang, C.; Lee, Y.; Litzinger, T. Simultaneous temperature and species measurements of the glycidyl azide polymer (GAP) propellant during laser-induced decomposition. Combust. Flame 1999, 117, 244–256. [Google Scholar] [CrossRef]
- Michael, N. Compounding of glycidyl azide polymer with nitrocellulose and its influence on the properties of propellants. Propell. Explos. Pyrot. 2000, 25, 236–240. [Google Scholar]
- Wu, Y.; Luo, Y.; Ge, Z. Properties and Application of a Novel Type of Glycidyl Azide Polymer (GAP)-Modified Nitrocellulose Powders. Propell. Explos. Pyrot. 2015, 40, 67–73. [Google Scholar] [CrossRef]
- Wu, Y.; Ge, Z.; Luo, Y. Properties and application of a novel type of glycidyl azide polymer modified double-base spherical powders. J. Therm. Anal. Calorim. 2016, 124, 107–115. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, Z.; Luo, Y. Fabrication and properties of glycidyl azide polymer-modified nitrocellulose spherical powders. J. Therm. Anal. Calorim. 2017, 129, 1555–1562. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, J.; Shi, L.; Lin, X.; Zhang, J. Study on Curing Kinetics of Diallyl-Bearing Epoxy Resin Using Sulfur as Curing Agent. Thermochim. Acta 2012, 538, 36–42. [Google Scholar] [CrossRef]
- Li, X.; Yu, B.; Zhang, D. Cure behavior and thermomechanical properties of phthalonitrile-polyhedral oligomeric silsesquioxane copolymers. Polymers 2017, 9, 334. [Google Scholar] [CrossRef]
- Garschke, C.; Parlevliet, P.; Weimer, C.; Fox, B. Cure kinetics and viscosity modelling of a high-performance epoxy resin film. Polym. Test. 2013, 32, 150–157. [Google Scholar] [CrossRef]
- Mc, C.; Andrews, G.P.; Laverty, T.P. Rheological evaluation of the isothermal cure characteristics of medical grade silicone elastomers. J. Appl. Polym. Sci. 2010, 116, 2320–2327. [Google Scholar]
- Trottier, E.; Affrossman, S.; Pethrick, R.A. Rheological studies of the cure of epoxy/polyester powder coatings containing titanium dioxide. J. Coat. Technol. Res. 2012, 9, 725–733. [Google Scholar] [CrossRef]
- Jaruchattada, J.; Fuongfuchat, A.; Pattamaprom, C. Rheological investigation of cure kinetics and adhesive strength of polyurethane acrylate adhesive. J. Appl. Polym. Sci. 2012, 123, 2344–2350. [Google Scholar] [CrossRef]
- He, L.; He, W.; Ma, Z. Effect of components on the curing of glycidyl azide polymer spherical propellant through rheological method. R. Soc. Open Sci. 2018, 5, 181282. [Google Scholar] [CrossRef]
- He, W.; He, L.; Ma, Z. The kinetic and viscosity analysis of glycidyl azide polymer spherical propellant. J. Therm. Anal. Calorim. 2016, 124, 943–950. [Google Scholar]
- He, W.; He, L.; Ma, Z. Using rheometry to study the curing kinetics of glycidyl azide polymer spherical propellant by non-isothermal method. Rheol. Acta 2016, 55, 365–371. [Google Scholar]
- He, W.; He, L.; Ma, Z.; Xiao, Z.; Guo, Y. Research on effect of Al on curing property of GAP spherical propellant by rheological isothemal measuring method. Acta Armamentarii 2016, 37, 1023–1029. [Google Scholar]
- Raghavan, S.; Chen, L.; McDowell, C. Rheological study of crosslinking and gelation in chlorobutyl elastomer systems. Polymer 1996, 37, 5869–5875. [Google Scholar] [CrossRef]
- Lucio, B.; Fuente, J.L. Rheological cure characterization of an advanced functional polyurethane. Thermochim. Acta 2014, 596, 6–13. [Google Scholar] [CrossRef]
- Xu, W.; Bao, S.; Shen, S. Differential Scanning Calori-Metric Study on The Curing Behavior of Epoxy Resin/Diethylenetriamine/Organic-montmorillonite Nanocomposite. J. Polym. Sci. Pol. Phys. 2003, 41, 378–386. [Google Scholar] [CrossRef]
- Xu, W.; Bao, S.; Shen, S. Curing Kinetics of Epoxy Resin-Imidazole-Organic Montmorillonite Nanocomposites Determined by Differential Scanning Calorimetry. J. Appl. Polym. Sci. 2003, 88, 2932–2941. [Google Scholar] [CrossRef]
- Kamal, M.R.; Sourour, S. Kinetics and thermal characterization of thermoset cure. Polym. Eng. Sci. 1973, 13, 59–64. [Google Scholar] [CrossRef]
- Lu, M.; Shim, M.; Kim, S. The macrokinetic model of thermosetting polymers by phase-change theory. Mater. Chem. Phys. 1998, 56, 193–197. [Google Scholar] [CrossRef]
- Lu, M.; Shim, M.; Kim, S. Effect of filler on cure behavior of an epoxy system: Cure modeling. Polym. Eng. Sci. 1999, 39, 274–277. [Google Scholar] [CrossRef]
- Huang, F.; Wang, X.; Li, S. Thermal Stability of Polyetherimide. Polym. Degrad. Stabil. 1987, 18, 247–259. [Google Scholar]
- Liang, Y.; Cheng, B.; Si, Y. Thermal Decomposition Kinetics and Characteristics of Spartina Alterniflora via Thermogravimetric Analysis. Renew. Energy 2014, 68, 111–117. [Google Scholar] [CrossRef]
- Sava, I.; Burescu, A. Study of Thermal Behavior of Polyimides Containing Pendent-Substituted Azobenzene Units. Polym. Bull. 2014, 21, 385. [Google Scholar] [CrossRef]
- Pitchaimari, G.; Vijayakumar, C. Functionalized N-(4-Hydroxy Phenyl) Maleimide Monomers: Kinetics of Thermal Polymerization and Degradation Using Model-Free Approach. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Hagen, T.H.; Jensen, T.L.; Unneberg, E.; Stenstrøm, Y.H.; Kristensen, T.E. Curing of glycidyl azide polymer (gap) diol using isocyanate, isocyanate-free, synchronous dual, and sequential dual curing systems. Propell. Explos. Pyrotech. 2015, 40, 275–284. [Google Scholar] [CrossRef]
- Baker, J.; Gaunt, J. The mechanism of the reaction of aryl isocyanates with alcohols and amines. J. Chem. Soc. 1949, 89, 9–18. [Google Scholar] [CrossRef]
- Manu, S.K.; Sekkar, V.; Scariah, K.J.; Varghese, T.L.; Mathew, S. Kinetics of glycidyl azide polymer-based urethane network formation. J. Appl. Polym. Sci. 2008, 110, 908–914. [Google Scholar] [CrossRef]
- Bina, C.K.; Kannan, K.; Ninan, K. DSC study on the effect of isocyanates and catalysts on the HTPB cure reaction. J. Therm. Anal. Calorim. 2004, 78, 753–760. [Google Scholar] [CrossRef]
- Luo, S.; Tan, H.; Chen, F. hydroxyl, Isocyanate and Catalysts in the polyurethane formation. J. Beijing Inst. Technol. 1997, 17, 28–34. [Google Scholar]
| T (°C) | k1/s−1 | k2/s−1 | p | n | Correlation Coefficients |
|---|---|---|---|---|---|
| 30 | 0.000245 | 0.00109 | 0.462 | 0.655 | 0.950 |
| 40 | 0.000102 | 0.0086 | 0.834 | 1.272 | 0.959 |
| 50 | 0.00163 | 0.0238 | 0.671 | 1.276 | 0.970 |
| 60 | 0.00214 | 0.0458 | 0.442 | 1.203 | 0.993 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Zhou, J.; Dai, S.; Ma, Z. Influence of Combustion Modifiers on the Cure Kinetics of Glycidyl Azide Polymer Based Propellant-Evaluated through Rheo-Kinetic Approach. Polymers 2019, 11, 637. https://doi.org/10.3390/polym11040637
He L, Zhou J, Dai S, Ma Z. Influence of Combustion Modifiers on the Cure Kinetics of Glycidyl Azide Polymer Based Propellant-Evaluated through Rheo-Kinetic Approach. Polymers. 2019; 11(4):637. https://doi.org/10.3390/polym11040637
Chicago/Turabian StyleHe, Liming, Jun Zhou, Sulan Dai, and Zhongliang Ma. 2019. "Influence of Combustion Modifiers on the Cure Kinetics of Glycidyl Azide Polymer Based Propellant-Evaluated through Rheo-Kinetic Approach" Polymers 11, no. 4: 637. https://doi.org/10.3390/polym11040637