Development of Polyhydroxyalkanoate-Based Polyurethane with Water-Thermal Response Shape-Memory Behavior as New 3D Elastomers Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
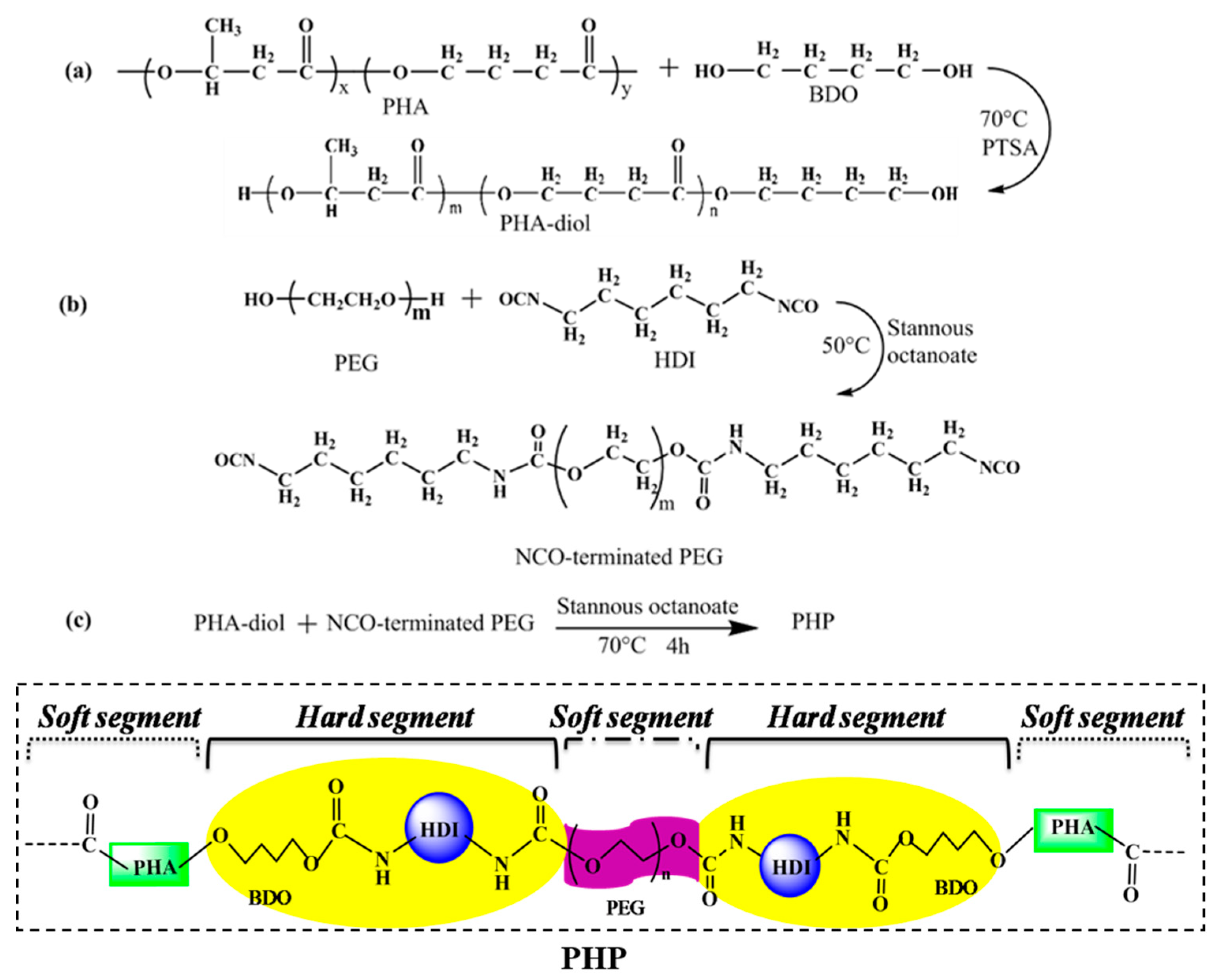
2.2. PEG–HDI–PHA Synthesis
2.3. Characterization
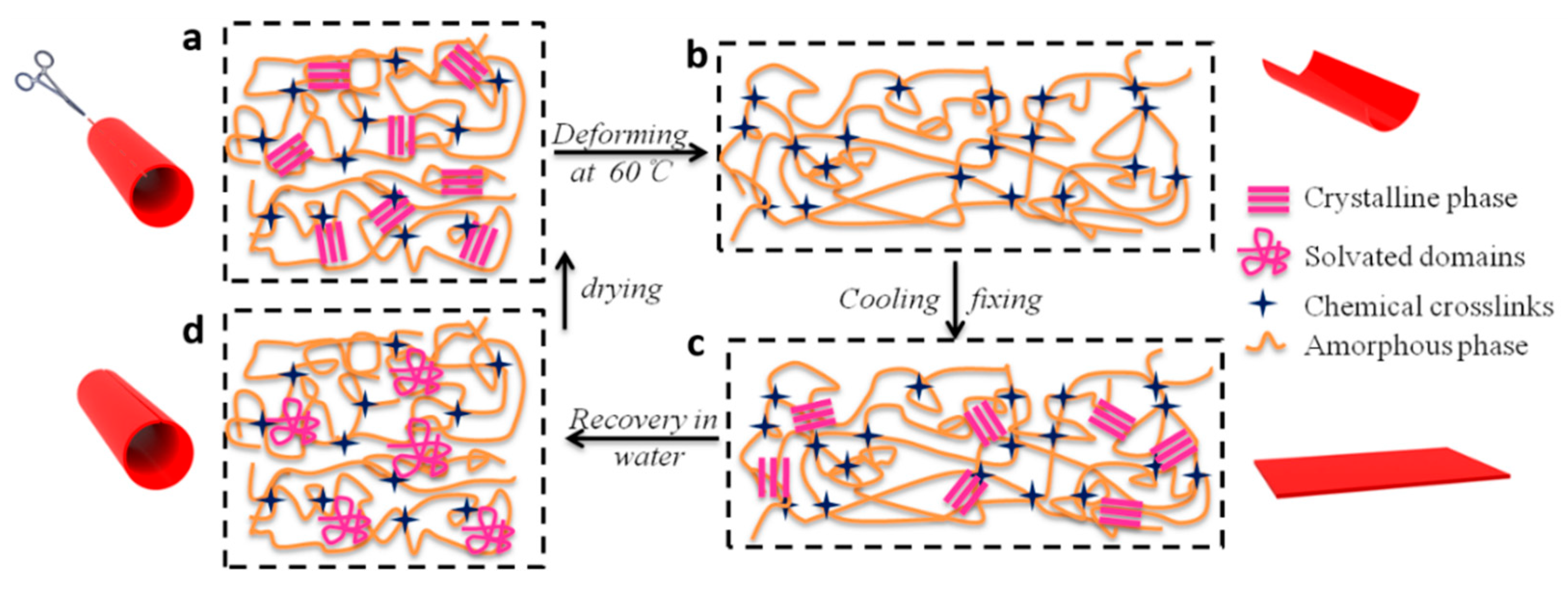
2.4. Shape-Memory Properties
3. Results and Discussion
3.1. Structural Analysis
3.2. Fabrication of PEG–HDI–PHA Elastomers into 3D Scaffolds
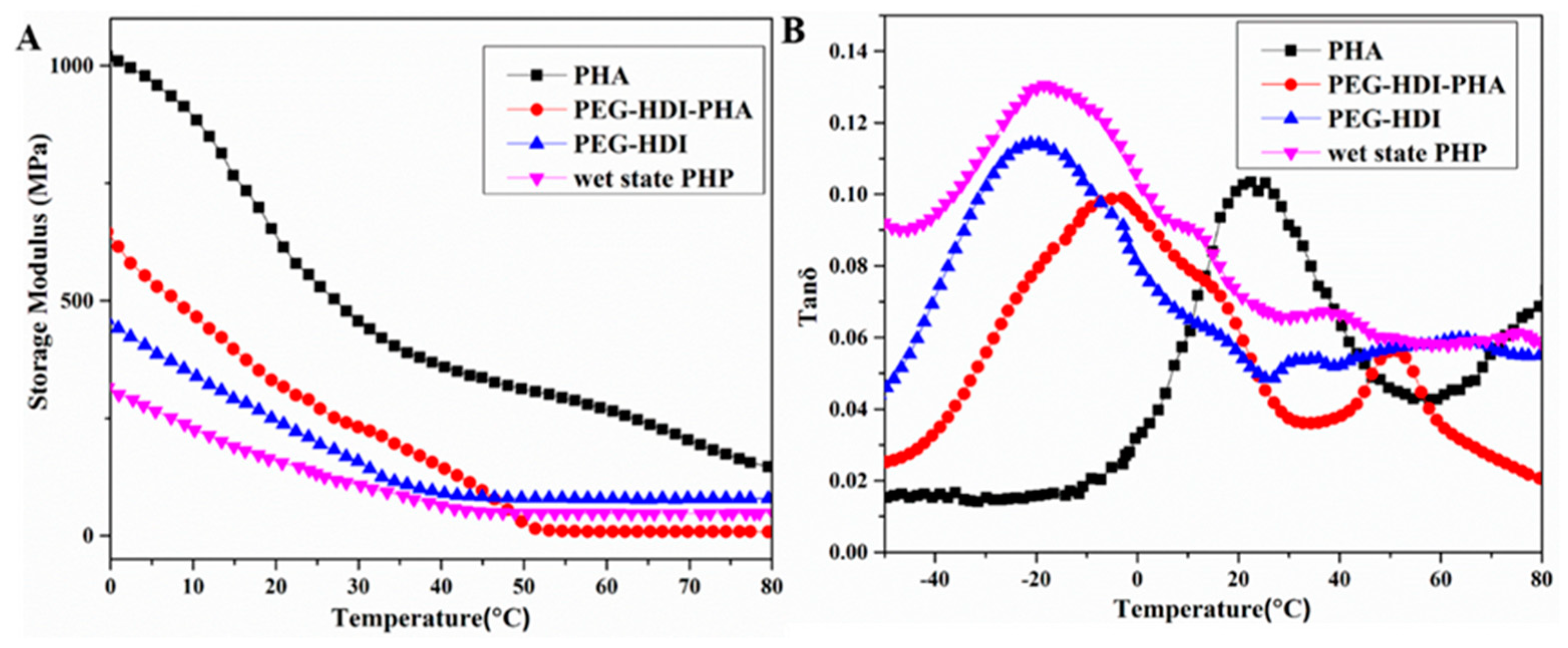
3.3. Mechanical Properties
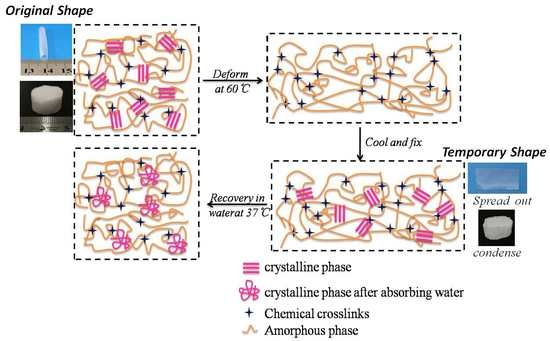
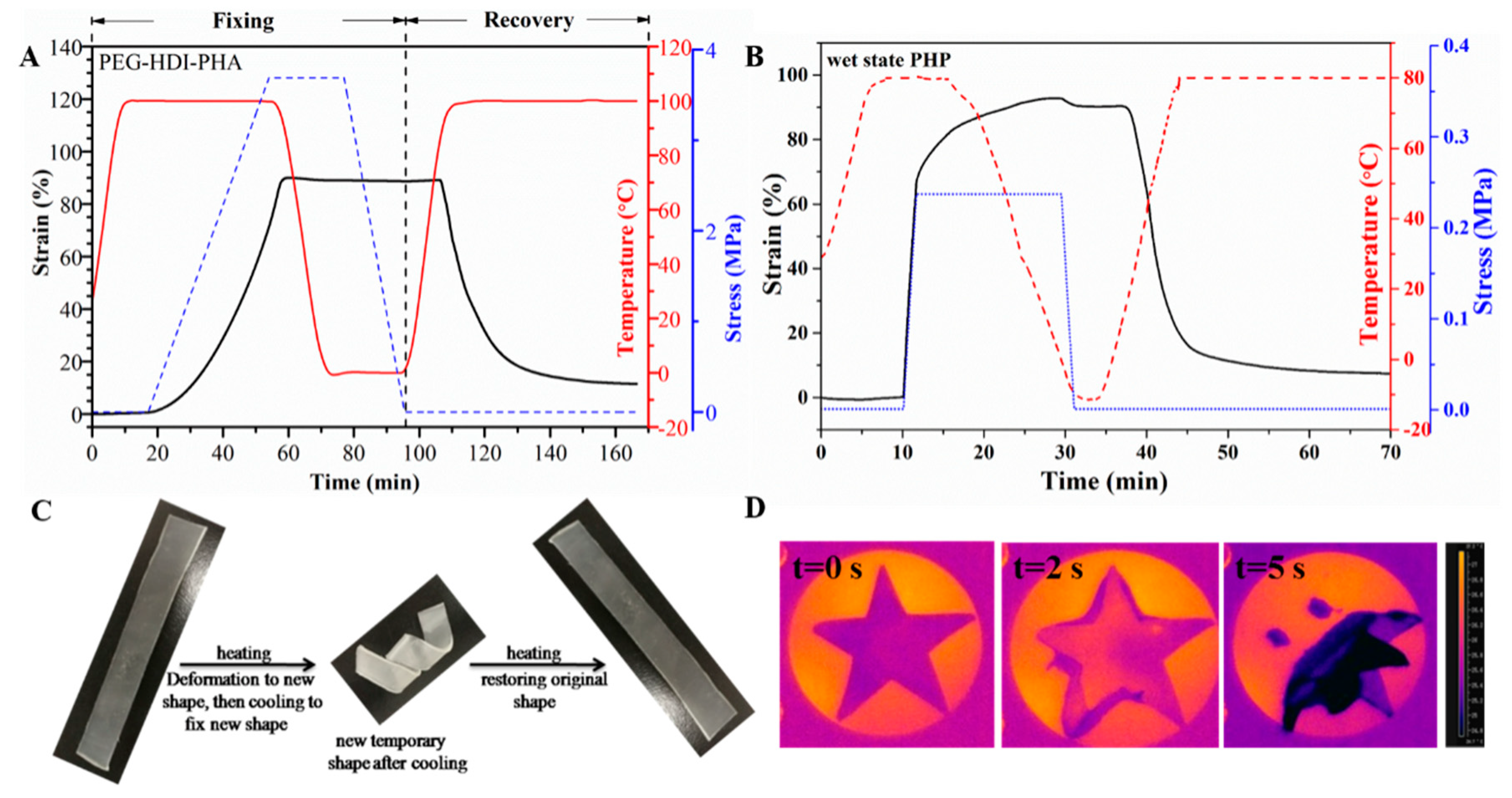
3.4. Water/Thermal Dual-Responsive Shape-Memory Properties
3.5. Mechanism of Water-Thermal Response Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Jian, Y.; Le, X.; Lu, W.; Ma, C.; Zhang, J.; Huang, Y.; Huang, C.F.; Chen, T. Actuating and Memorizing Bilayer Hydrogels for a Self-Deformed Shape Memory Function. Chem. Commun. 2018, 54, 1229. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yuan, H.; Chen, S.; Yang, H.; Ge, Z.; Zhuo, H.; Liu, J. Development of Supramolecular Liquid-Crystalline Polyurethane Complexes Exhibiting Triple-Shape Functionality Using a One-Step Programming Process. J. Mater. Chem. A 2014, 2, 10169–10181. [Google Scholar] [CrossRef]
- Ban, J.; Mu, L.; Yang, J.; Chen, S.; Zhuo, H. New Stimulus-Responsive Shape-Memory Polyurethanes Capable of Uv Light-Triggered Deformation, Hydrogen Bond-Mediated Fixation, and Thermal-Induced Recovery. J. Mater. Chem. A 2017, 5. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Chen, H.; Gong, T.; Li, W.; Yang, G.; Zhou, S. Design of Triple-Shape Memory Polyurethane with Photo-Cross-Linking of Cinnamon Groups. Appl. Mate. Interfaces 2013, 5, 10520–10528. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, G.; Xu, S.; Ma, T.; Nie, J. Biodegradable Magnetic-Sensitive Shape Memory Poly(ε-Caprolactone)/Fe3o4 Nanocomposites. J. Appl. Polym. Sci. 2018, 135, 45652. [Google Scholar]
- Yu, J.; Xia, H.; Teramoto, A.; Ni, Q.Q. Fabrication and Characterization of Shape Memory Polyurethane Porous Scaffold for Bone Tissue Engineering. J. Biomed. Mater. Res. Part A 2017, 105, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Hsieh, C.T.; Sun, Y.M. Synthesis and Characterization of Waterborne Polyurethane Containing Poly(3-Hydroxybutyrate) as New Biodegradable Elastomers. J. Mater. Chem. B 2015, 3, 9089–9097. [Google Scholar] [CrossRef]
- Bao, M.; Zhou, Q.; Dong, W.; Lou, X.; Zhang, Y. Ultrasound-Modulated Shape Memory and Payload Release Effects in a Biodegradable Cylindrical Rod Made of Chitosan-Functionalized Plga Microspheres. Biomacromolecules 2013, 14, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Julich-Gruner, K.K.; Löwenberg, C.; Neffe, A.T.; Behl, M.; Lendlein, A. Recent Trends in the Chemistry of Shape-Memory Polymers. Macromol. Chem. Phys. 2013, 214, 527–536. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, H.; Chang, R.; Shan, G.; Bao, Y.; Pan, P. Stereocomplexed and Homochiral Polyurethane Elastomers with Tunable Crystallizability and Multishape Memory Effects. ACS Macro Lett. 2018, 7, 233–238. [Google Scholar] [CrossRef]
- Xie, T. Tunable Polymer Multi-Shape Memory Effect. Nature 2010, 464, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Jing, M.; Liu, Z.; Peng, D.; Liu, T.; Qiang, F. Microfibrillated Cellulose Reinforced Bio-Based Poly(Propylene Carbonate) with Dual-Responsive Shape Memory Properties. Rsc Adv. 2016, 6, 7560–7567. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-Responsive Bio-Based Polymeric Systems and Their Applications. J.Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Wong, Y.S.; Salvekar, A.V.; Zhuang, K.D.; Liu, H.; Birch, W.R.; Tay, K.H.; Huang, W.M.; Venkatraman, S.S. Bioabsorbable Radiopaque Water-Responsive Shape Memory Embolization Plug for Temporary Vascular Occlusion. Biomaterials 2016, 102, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.M.; Yang, B.; An, L.; Li, C.; Chan, Y.S. Water-Driven Programmable Polyurethane Shape Memory Polymer: Demonstration and Mechanism. Appl. Phys. Lett. 2005, 86, 109. [Google Scholar] [CrossRef]
- Chan, B.Q.Y.; Heng, S.J.W.; Liow, S.S.; Zhang, K.; Xian, J.L. Dual-Responsive Hybrid Thermoplastic Shape Memory Polyurethane. Mater. Chem. Front. 2016, 1, 767–779. [Google Scholar] [CrossRef]
- Zhao, T.; Mei, T.; Cui, Y.; Chao, D.; He, H.; Guo, M. Reactive Macromolecular Micelle Crosslinked Highly Elastic Hydrogel with Water-Triggered Shape-Memory Behaviour. Polym. Chem. 2014, 5, 4965–4973. [Google Scholar] [CrossRef]
- Agarwal, S.; Jiang, S.; Chen, Y. Progress in the Field of Water and/or TemperatureTriggered Polymer Actuators. Macromol. Mater. Eng. 2018, 304, 1800548. [Google Scholar] [CrossRef]
- Cabanlit, M.; Maitland, D.; Wilson, T.; Simon, S.; Wun, T.; Gershwin, M.E.; Van de Water, J. Polyurethane Shape-Memory Polymers Demonstrate Functional Biocompatibility in Vitro. Macromol. Biosci. 2010, 7, 48–55. [Google Scholar] [CrossRef]
- Gong, T.; Zhao, K.; Liu, X.; Lu, L.; Liu, D.; Zhou, S. A Dynamically Tunable, Bioinspired Micropatterned Surface Regulates Vascular Endothelial and Smooth Muscle Cells Growth at Vascularization. Small 2016, 12, 5769–5778. [Google Scholar] [CrossRef]
- Cai, W.; Zheng, Y.; Yi, S.; Fan, J.; Qin, Q.; Zhao, Z. A Novel Biodegradable Polyurethane Based on Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) and Poly(Ethylene Glycol) as Promising Biomaterials with the Improvement of Mechanical Properties and Hemocompatibility. Polym. Chem. 2016, 7, 6120–6132. [Google Scholar]
- Pan, J.; Li, G.; Chen, Z.; Chen, X.; Zhu, W.; Xu, K. Alternative Block Polyurethanes Based on Poly(3-Hydroxybutyrate--4-Hydroxybutyrate) and Poly(Ethylene Glycol). Biomaterials 2009, 30, 2975–2984. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Liu, T.; Qin, Z.; Cong, S.; Bai, Q.W.; Wang, H. Surface Patterning of Hydrogels for Programmable and Complex Shape Deformations by Ion Inkjet Printing. Adv. Funct. Mater. 2017, 27, 1701962. [Google Scholar]
- Oyefusi, A.; Chen, J. Reprogrammable Chemical 3d Shaping for Origami, Kirigami, and Reconfigurable Molding. Angew. Chem. 2017, 56, 8250. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.M.; Skrovanek, D.J.; Hu, J.; Painter, P.C. Hydrogen Bonding in Polymer Blends. 1. Ftir Studies of Urethane-Ether Blends. Macromolecules 1988, 21, 59–65. [Google Scholar] [CrossRef]
- Datta, J.; Głowińska, E. Effect of Hydroxylated Soybean Oil and Bio-Based Propanediol on the Structure and Thermal Properties of Synthesized Bio-Polyurethanes. Ind. Crops Prod. 2014, 61, 84–91. [Google Scholar] [CrossRef]
- Chan, C.H.; Kummerlöwe, C.; Kammer, H.W. Crystallization and Melting Behavior of Poly(3-Hydroxybutyrate)-Based Blends. Macromol. Chem. Phys. 2004, 205, 664–675. [Google Scholar] [CrossRef]
- Xue, L.; Dai, S.; Li, Z. Biodegradable Shape-Memory Block Co-Polymers for Fast Self-Expandable Stents. Biomaterials 2010, 31, 8132–8140. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, R.; Fujimoto, K.L.; Hong, Y.; Guan, J.; Toma, C.; Tobita, K.; Wagner, W.R. Biodegradable Elastic Patch Plasty Ameliorates Left Ventricular Adverse Remodeling after Ischemia–Reperfusion Injury: A Preclinical Study of a Porous Polyurethane Material in a Porcine Model. J. Thorac. Cardiovasc. Surg. 2013, 146, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Cheng, C.; He, Y.S.; Lyu, C.; Wang, Y.; Yu, J.; Qiu, L.; Zou, D.; Li, D. Multilayered Graphene Hydrogel Membranes for Guided Bone Regeneration. Adv. Mater. 2016, 28, 4025. [Google Scholar] [CrossRef]
- Fujimoto, K.L.; Tobita, K.; Guan, J.; Hashizume, R.; Takanari, K.; Alfieri, C.M.; Yutzey, K.E.; Wagner, W.R. Placement of an Elastic, Biodegradable Cardiac Patch on a Sub-Acute Infarcted Heart Leads to Cellularization with Early Developmental Cardiomyocyte Characteristics. J. Card. Fail. 2012, 18, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, D.; Yang, J.; Lui, K.Y.; Webb, A.R.; Ameer, G.A. Hemocompatibility Evaluation of Poly(Glycerol-Sebacate) in Vitro for Vascular Tissue Engineering. Biomaterials 2010, 82A, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ensley, A.E.; Nerem, R.M.; Wang, Y. Poly(Glycerol Sebacate) Supports the Proliferation and Phenotypic Protein Expression of Primary Baboon Vascular Cells. J. Biomed. Mater. Res. Part A 2010, 83A, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Sundback, C.A.; Shyu, J.Y.; Wang, Y.; Faquin, W.C.; Langer, R.S.; Vacanti, J.P.; Hadlock, T.A. Biocompatibility Analysis of Poly(Glycerol Sebacate) as a Nerve Guide Material. Biomaterials 2005, 26, 5454–5464. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wei, Y.; Hung, C.; Vunjak-Novakovic, G. Chondrogenic Properties of Collagen Type Xi, a Component of Cartilage Extracellular Matrix. Biomaterials 2018, 173, 47. [Google Scholar] [CrossRef] [PubMed]
- Baroncelli, M.; Van, B.D.E.; Chatterji, S.; Rull, E.T.; Kan, Y.Y.; Koedam, M.; Van, I.H.; Alves, R.; Fratilaapachitei, L.E.; Demmers, J. Human Osteoblast-Derived Extracellular Matrix with High Homology to Bone Proteome Is Osteopromotive. Tissue Eng. Part A 2018, 24, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, J.; Wu, J.; Carlson, A.L.; Silberstein, L.; Putheti, P.; Larocca, R.; Gao, W.; Saito, T.I.; Lo, C.C.; Tsuyuzaki, H. In Vivo Imaging of Treg Cells Providing Immune Privilege to the Haematopoietic Stem-Cell Niche. Nature 2011, 474, 216–219. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, H.; Zou, F.; Chen, S.; Wang, Y. Development of Polyhydroxyalkanoate-Based Polyurethane with Water-Thermal Response Shape-Memory Behavior as New 3D Elastomers Scaffolds. Polymers 2019, 11, 1030. https://doi.org/10.3390/polym11061030
Wang C, Wang H, Zou F, Chen S, Wang Y. Development of Polyhydroxyalkanoate-Based Polyurethane with Water-Thermal Response Shape-Memory Behavior as New 3D Elastomers Scaffolds. Polymers. 2019; 11(6):1030. https://doi.org/10.3390/polym11061030
Chicago/Turabian StyleWang, Cai, Han Wang, Faxing Zou, Shaojun Chen, and Yiping Wang. 2019. "Development of Polyhydroxyalkanoate-Based Polyurethane with Water-Thermal Response Shape-Memory Behavior as New 3D Elastomers Scaffolds" Polymers 11, no. 6: 1030. https://doi.org/10.3390/polym11061030