Hydrodynamic Properties of Polymers Screening the Electrokinetic Flow: Insights from a Computational Study
Abstract
:1. Introduction
2. Methods
2.1. MD Simulation
2.2. Navier-Stokes-Brinkman Model
3. Results and Discussion
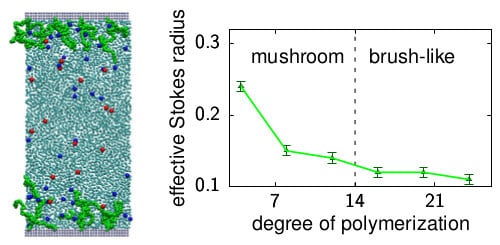
3.1. The Stokes Radius of Beads in Polymeric Coatings
- The hydrodynamic radius decreases at a decreasing slope with increasing the degree of polymerization, N. This is in agreement with the decreasing center-line flow velocity (i.e., the solvent velocity measured in the middle of the slit). This result is a new finding and implies that depends on the configuration of polymers, as suggested by Monteferrante et al. [47].
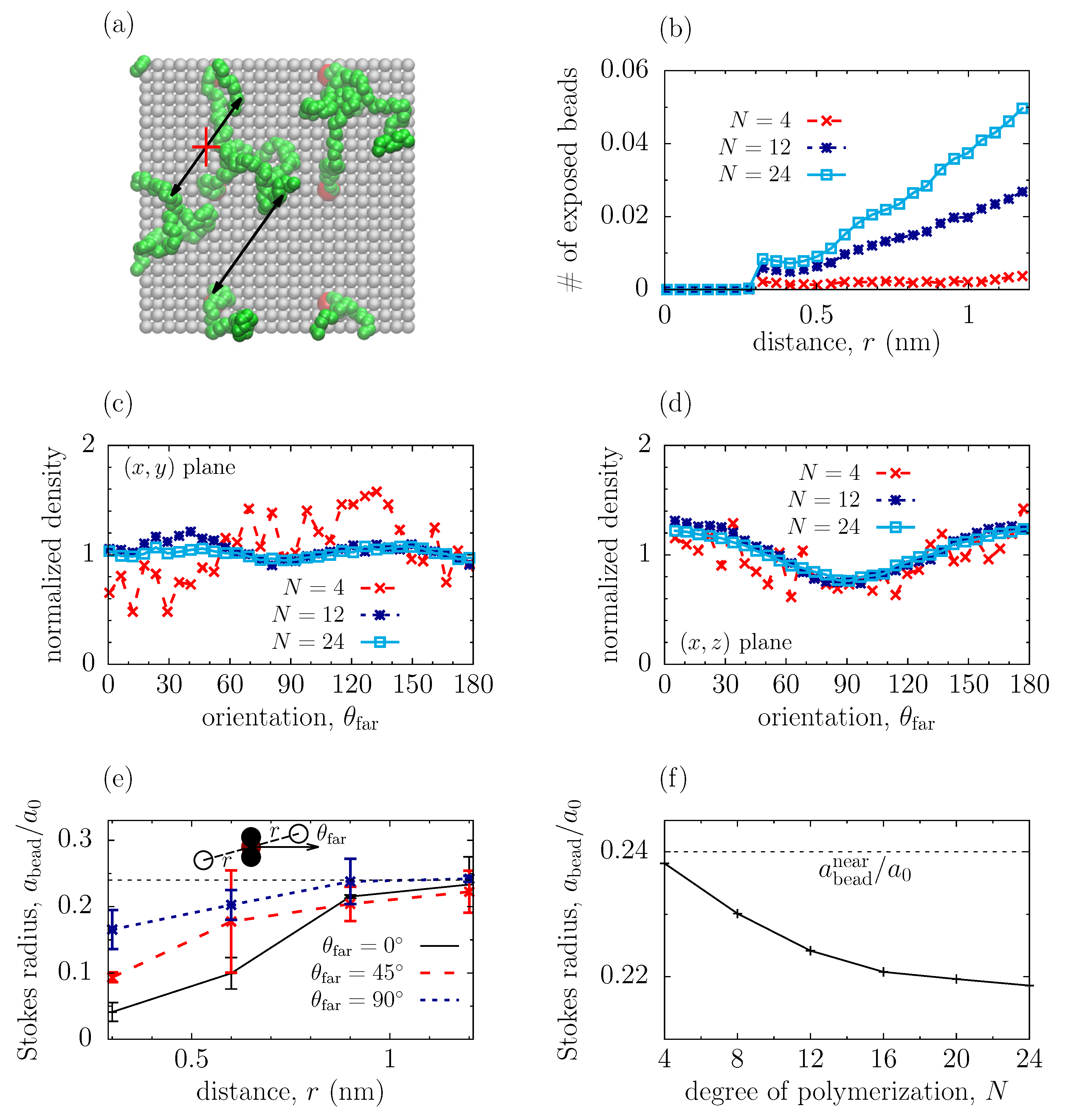
3.2. Structure of Polymeric Coatings
3.3. Hydrodynamic Screening by Near Beads
3.4. Shielding by Far Beads
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hou, X.; Guo, W.; Jiang, L. Biomimetic smart nanopores and nanochannels. Chem. Soc. Rev. 2011, 40, 2385–2401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Zhao, Q.; Yuan, J.Y. Porous Polyelectrolytes: The Interplay of Charge and Pores for New Functionalities. Angew. Chem. Int. Ed. 2018, 57, 6754–6773. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Banik, M.; Chen, G.; Sinha, S.; Mukherjee, R. Polyelectrolyte brushes: Theory, modelling, synthesis and applications. Soft Matter 2015, 11, 8550–8583. [Google Scholar] [CrossRef] [PubMed]
- Ballou, B.; Lagerholm, B.C.; Ernst, L.A.; Bruchez, M.P.; Waggoner, A.S. Noninvasive imaging of quantum dots in mice. Bioconjugate Chem. 2004, 15, 79–86. [Google Scholar] [CrossRef]
- Barenholz, Y. Liposome application: Problems and prospects. Curr. Opin. Colloid Interface Sci. 2001, 6, 66–77. [Google Scholar] [CrossRef]
- Shendruk, T.N.; Hickey, O.A.; Slater, G.W.; Harden, J.L. Electrophoresis: When hydrodynamics matter. Curr. Opin. Colloid Interface Sci. 2012, 17, 74–82. [Google Scholar] [CrossRef]
- Savariar, E.N.; Krishnamoorthy, K.; Thayumanavan, S. Molecular discrimination inside polymer nanotubules. Nat. Nanotechnol. 2008, 3, 112–117. [Google Scholar] [CrossRef]
- Adiga, S.P.; Brenner, D.W. Stimuli-Responsive Polymer Brushes for Flow Control through Nanopores. J. Funct. Biomater. 2012, 3, 239–256. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, R.; Werner, C.; Duval, J.F.L. Recent Progress and Perspectives in the Electrokinetic Characterization of Polyelectrolyte Films. Polymers 2016, 8, 7. [Google Scholar] [CrossRef]
- Cao, Q.Q.; You, H. Electroosmotic Flow in Mixed Polymer Brush-Grafted Nanochannels. Polymers 2016, 8, 438. [Google Scholar] [CrossRef]
- Cao, Q.Q.; Zuo, C.C.; Li, L.J.; Zhang, Y.H. Modulation of electroosmotic flow by electric field-responsive polyelectrolyte brushes: A molecular dynamics study. Microfluid. Nanofluid. 2012, 12, 649–655. [Google Scholar] [CrossRef]
- Chen, G.; Sachar, H.S.; Das, S. Efficient electrochemomechanical energy conversion in nanochannels grafted with end-charged polyelectrolyte brushes at medium and high salt concentration. Soft Matter 2018, 14, 5246–5255. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Cordero, R.; Tran, H.; Ober, C.K. 50th anniversary perspective: Polymer brushes: Novel surfaces for future materials. Macromolecules 2017, 50, 4089–4113. [Google Scholar] [CrossRef]
- Lyklema, J. Fundamentals of Interface and Colloid Science; Academic: San Diego, CA, USA, 1995. [Google Scholar]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic: London, UK, 1981. [Google Scholar]
- Gomez, F.A. Biological Applications of Microfluidics; Wiley-Interscience: Hoboken, NJ, USA, 2008. [Google Scholar]
- Karniadakis, G.; Beskok, A.; Aluru, N.R. Microflows and Nanoflows; Springer: New York, NY, USA, 2005. [Google Scholar]
- Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal Dispersions; Cambridge University Press: Cambridge, MA, USA, 1989. [Google Scholar]
- Wiersema, P.H.; Loeb, A.L.; Overbeek, J.T.G. Calculation of the electrophoretic mobility of a spherical colloid particle. J. Colloid Interface Sci. 1966, 22, 78–99. [Google Scholar] [CrossRef] [Green Version]
- Duval, J.F.L.; Gaboriaud, F. Progress in electrohydrodynamics of soft microbial particle interphases. Curr. Opin. Colloid Interface Sci. 2010, 15, 184–195. [Google Scholar] [CrossRef]
- Debye, P.; Bueche, A.M. Intrinsic Viscosity, Diffusion, and Sedimentation Rate of Polymers in Solution. J. Chem. Phys. 1948, 16, 573–579. [Google Scholar] [CrossRef]
- Saville, D.A. Electrokinetic properties of fuzzy colloidal particles. J. Colloid Interface Sci. 2000, 222, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H. On the general expression for the electrophoretic mobility of a soft particle. J. Colloid Interface Sci. 2000, 228, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J.; Saville, D.A. ‘Exact’ solutions of the full electrokinetic model for soft spherical colloids: Electrophoretic mobility. Colloids Surf. A Physicochem. Eng. Asp. 2005, 267, 31–49. [Google Scholar] [CrossRef]
- Hill, R.J. Hydrodynamics and electrokinetics of spherical liposomes with coatings of terminally anchored poly(ethylene glycol): Numerically exact electrokinetics with self-consistent mean-field polymer. Phys. Rev. E 2004, 70, 051406. [Google Scholar] [CrossRef] [PubMed]
- Harden, J.L.; Long, D.; Ajdari, A. Influence of end-grafted polyelectrolytes on electro-osmosis along charged surfaces. Langmuir 2001, 17, 705–715. [Google Scholar] [CrossRef]
- Sobera, M.P.; Kleijn, C.R. Hydraulic permeability of ordered and disordered single-layer arrays of cylinders. Phys. Rev. E 2006, 74, 036301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczyk, Z.; Sadlej, K.; Wajnryb, E.; Ekiel-Jezewska, M.L.; Warszynski, P. Hydrodynamic radii and diffusion coefficients of particle aggregates derived from the bead model. J. Colloid Interface Sci. 2010, 347, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Moeendarbary, E.; Ng, T.Y.; Zangeneh, M. Dissipative particle dynamics in soft matter and polymeric applications—A review. Int. J. Appl. Mech. 2010, 2, 161–190. [Google Scholar] [CrossRef]
- Grest, G.S. Interfacial sliding of polymer brushes: A molecular dynamics simulation. Phys. Rev. Lett. 1996, 76, 4979–4982. [Google Scholar] [CrossRef]
- Gama Goicochea, A.; Lopez-Esparza, R.; Altamirano, M.A.B.; Rivera-Paz, E.; Waldo-Mendoza, M.A.; Perez, E. Friction coefficient and viscosity of polymer brushes with and without free polymers as slip agents. J. Mol. Liq. 2016, 219, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Eckmann, D.M.; Ayyaswamy, P.S.; Radhakrishnan, R. Hydrodynamic interactions of deformable polymeric nanocarriers and the effect of crosslinking. Soft Matter 2015, 11, 5955–5969. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.A.; Khorosheva, V.A. Electrokinetic measurement of hydrodynamic properties of grafted polymer layers on liposome surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2001, 195, 113–127. [Google Scholar] [CrossRef]
- Raafatnia, S.; Hickey, O.A.; Holm, C. Electrophoresis of a spherical polyelectrolyte-grafted colloid in monovalent salt solutions: Comparison of molecular dynamics simulations with theory and numerical calculations. Macromolecules 2015, 48, 775–787. [Google Scholar] [CrossRef]
- Qiao, R.; Aluru, N.R. Ion concentrations and velocity profiles in nanochannel electroosmotic flows. J. Chem. Phys. 2003, 118, 4692–4701. [Google Scholar] [CrossRef]
- Qiao, R.; Aluru, N.R. Atomistic simulation of KCl transport in charged silicon nanochannels: Interfacial effects. Colloids Surf. A Physicochem. Eng. Asp. 2005, 267, 103–109. [Google Scholar] [CrossRef]
- Qiao, R.; Aluru, N.R. Scaling of electrokinetic transport in nanometer channels. Langmuir 2005, 21, 8972–8977. [Google Scholar] [CrossRef] [PubMed]
- Tessier, F.; Slater, G.W. Modulation of electroosmotic flow strength with end-grafted polymer chains. Macromolecules 2006, 39, 1250–1260. [Google Scholar] [CrossRef]
- Wu, P.; Qiao, R. Physical origins of apparently enhanced viscosity of interfacial fluids in electrokinetic transport. Phys. Fluids 2011, 23, 072005. [Google Scholar] [CrossRef] [Green Version]
- Siu, S.W.I.; Pluhackova, K.; Boeckmann, R.A. Optimization of the OPLS-AA force field for long hydrocarbons. J. Chem. Theory Comput. 2012, 8, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Schüttelkopf, A.W.; van Aalten, D.M.F. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, I. Polymer Solutions: An Introduction to Physical Properties; John Wiley & Sons Inc.: New York, NY, USA, 2002. [Google Scholar]
- Lindahl, E.; Hess, B.; van der Spoel, D. GROMACS 3.0: A package for molecular simulation and trajectory analysis. J. Mol. Model. 2001, 7, 306–317. [Google Scholar] [CrossRef]
- Koch, D.L.; Sangani, A.S. Particle pressure and marginal stability limits for a homogeneous monodisperse gas-fluidized bed: Kinetic theory and numerical simulations. J. Fluid Mech. 1999, 400, 229–263. [Google Scholar] [CrossRef]
- Batchelor, G.K.; Green, J.T. The determination of the bulk stress in a suspension of spherical particles to order c2. J. Fluid Mech. 1972, 56, 401–427. [Google Scholar] [CrossRef]
- Todd, B.D.; Evans, D.J.; Daivis, P.J. Pressure tensor for inhomogeneous fluids. Phys. Rev. E 1995, 52, 1627–1638. [Google Scholar] [CrossRef]
- Monteferrante, M.; Melchionna, S.; Marconi, U.M.B.; Cretich, M.; Chiari, M.; Sola, L. Electroosmotic flow in polymer-coated slits: A joint experimental/simulation study. Microfluid. Nanofluid. 2015, 18, 475–482. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Sun, T.; Jiang, X.; Kondrat, S. Hydrodynamic Properties of Polymers Screening the Electrokinetic Flow: Insights from a Computational Study. Polymers 2019, 11, 1038. https://doi.org/10.3390/polym11061038
Wu P, Sun T, Jiang X, Kondrat S. Hydrodynamic Properties of Polymers Screening the Electrokinetic Flow: Insights from a Computational Study. Polymers. 2019; 11(6):1038. https://doi.org/10.3390/polym11061038
Chicago/Turabian StyleWu, Peng, Tao Sun, Xikai Jiang, and Svyatoslav Kondrat. 2019. "Hydrodynamic Properties of Polymers Screening the Electrokinetic Flow: Insights from a Computational Study" Polymers 11, no. 6: 1038. https://doi.org/10.3390/polym11061038