Emission Properties of Diblock Copolymers Composed of Poly(ethylene glycol) and Dense 1,2,3-Triazole Blocks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Preparation of Monopropargyl-Terminated Poly(ethylene glycol) (EGm-P)
2.4. Preparation of EGm-b-APn Block Copolymers
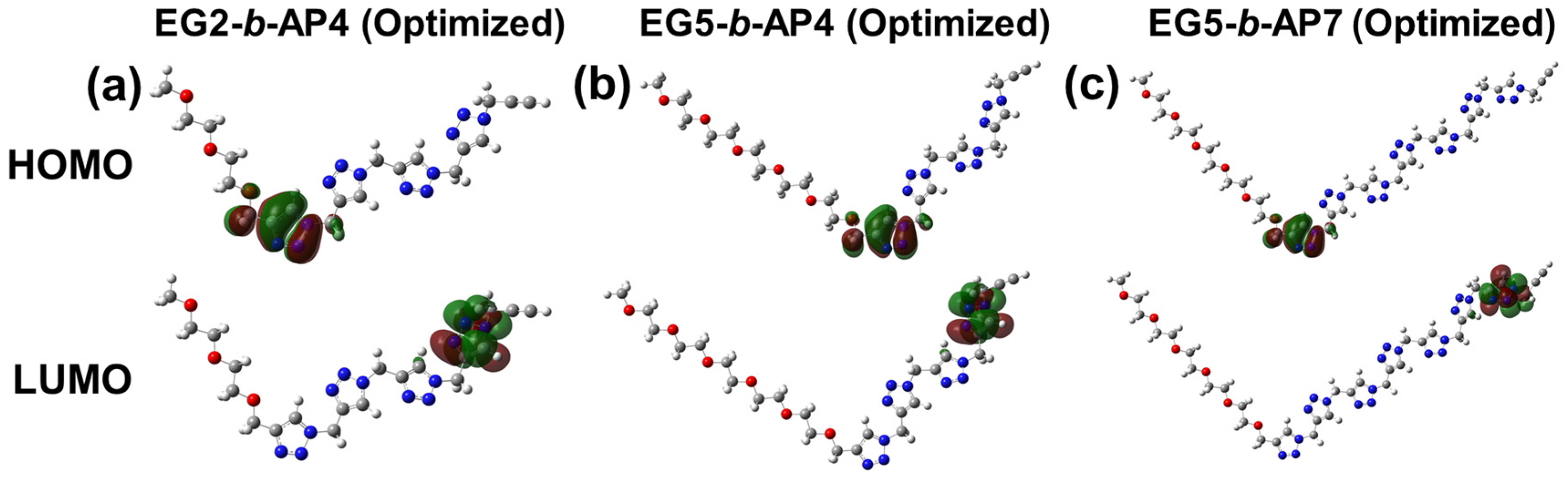
2.5. Density Functional Theory (DFT) Calculations
3. Results and Discussion
3.1. Preparation of EGm-b-APn Block Copolymers
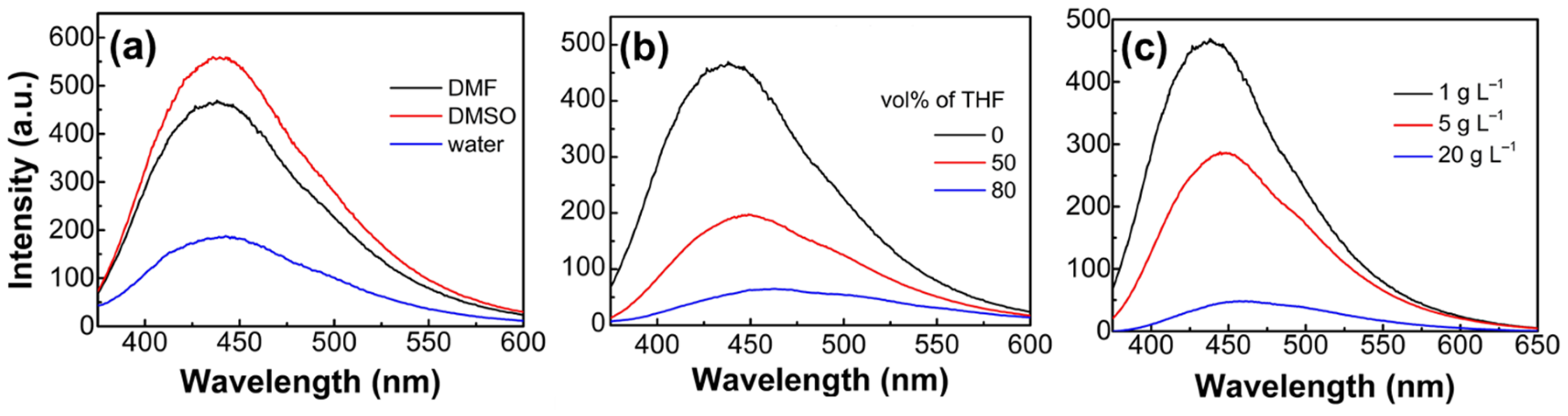
3.2. Emission Properties of EGm-b-APn Block Copolymers
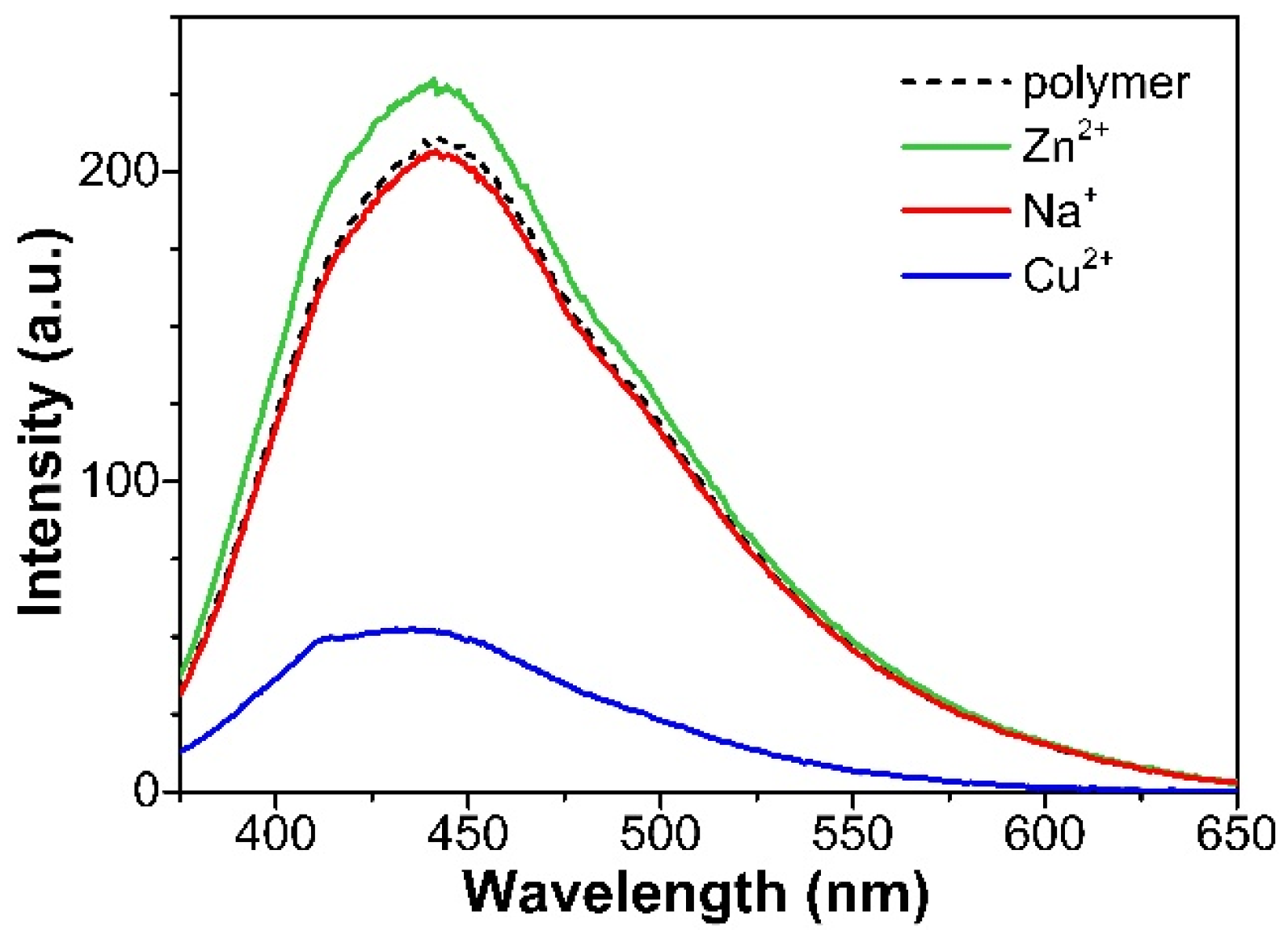
3.3. Metal Ion-Responsive Emission of EGm-b-APn Block Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abboud, J.-L.M.; Foces-Foces, C.; Notario, R.; Trifonov, R.E.; Volovodenko, A.P.; Ostrovskii, V.A.; Alkorta, I.; Elguero, J. Basicity of N-H- and N-methyl-1,2,3-triazoles in the gas phase, in solution, and in the solid state–an experimental and theoretical study. Eur. J. Org. Chem. 2001, 2001, 3013–3024. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discovery Today. 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Dehaen, W.; Bakulev, V.A. Chemistry of 1,2,3-triazoles; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Huisgen, R.; Grashey, R.; Aufderhaar, E.; Kunz, R. 1,3-Dipolar cycloadditions. XIII. Additions of nitrilimines to oximes, azines, and other cn double bonds. Chem. Ber. 1965, 98, 642–649. [Google Scholar] [CrossRef]
- Huisgen, R. The concerted nature of 1,3-dipolar cycloadditions and the question of diradical intermediates. J. Org. Chem. 1976, 41, 403–419. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Fazio, F.; Bryan, M.C.; Blixt, O.; Paulson, J.C.; Wong, C.-H. Synthesis of sugar arrays in microtiter plate. J. Am. Chem. Soc. 2002, 124, 14397–14402. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Lahann, J. Click chemistry for biotechnology and materials science. Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Schulze, B.; Schubert, U.S. Beyond click chemistry–supramolecular interactions of 1,2,3-triazoles. Chem. Soc. Rev. 2014, 43, 2522–2571. [Google Scholar] [CrossRef]
- McDonald, K.P.; Hua, Y.; Flood, A.H. 1,2,3-Triazoles and the expanding utility of charge neutral CH⋯anion interactions. In Anion Recognition in Supramolecular Chemistry; Gale, P.A., Dehaen, W., Eds.; Springer: Berlin, Germany, 2010; pp. 341–366. [Google Scholar]
- Ghosh, K.; Panja, A.; Panja, S. Cholesterol appended bis-1,2,3-triazoles as simple supramolecular gelators for the naked eye detection of Ag+, Cu2+ and Hg2+ ions. New J. Chem. 2016, 40, 3476–3483. [Google Scholar] [CrossRef]
- Jung, S.-H.; Ryu, J.; Sohn, D.; Lee, H.-i. Time-dependent increase in aqueous solubility caused by the gradual disruption of hydrophobic aggregation. Polym. Chem. 2012, 3, 1002–1006. [Google Scholar] [CrossRef]
- Hua, Y.; Flood, A.H. Click chemistry generates privileged ch hydrogen-bonding triazoles: The latest addition to anion supramolecular chemistry. Chem. Soc. Rev. 2010, 39, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Meudtner, R.M.; Hecht, S. Responsive backbones based on alternating triazole-pyridine/benzene copolymers: From helically folding polymers to metallosupramolecularly crosslinked gels. Macromol. Rapid Commun. 2008, 29, 347–351. [Google Scholar] [CrossRef]
- Pourghaz, Y.; Dongare, P.; Thompson, D.W.; Zhao, Y. Click functionalized poly(p-phenylene ethynylene)s as highly selective and sensitive fluorescence turn-on chemosensors for Zn2+ and Cd2+ ions. Chem. Commun. 2011, 47, 11014–11016. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, W.; Han, H.; Sun, R.; Xie, M.; Liao, X. Hyperbranched poly(triazole) with thermal and metal ion dual stimuli-responsiveness. Polym. Chem. 2015, 6, 4801–4808. [Google Scholar] [CrossRef]
- Mishra, V.; Jung, S.-H.; Park, J.M.; Jeong, H.M.; Lee, H.-i. Triazole-containing hydrogels for time-dependent sustained drug release. Macromol. Rapid Commun. 2014, 35, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Song, H.B.; Baranek, A.; Worrell, B.T.; Cook, W.D.; Bowman, C.N. Photopolymerized triazole-based glassy polymer networks with superior tensile toughness. Adv. Funct. Mater. 2018, 28, 1801095. [Google Scholar] [CrossRef] [PubMed]
- Hashidzume, A.; Nakamura, T.; Sato, T. Copper-catalyzed azide-alkyne cycloaddition oligomerization of 3-azido-1-propyne derivatives. Polymer 2013, 54, 3448–3451. [Google Scholar] [CrossRef]
- Nakano, S.; Hashidzume, A.; Sato, T. Quarternization of 3-azido-1-propyne oligomers obtained by copper(I)-catalyzed azide–alkyne cycloaddition polymerization. Beilstein J. Org. Chem. 2015, 11, 1037–1042. [Google Scholar] [CrossRef]
- Jourdain, A.; Serghei, A.; Drockenmuller, E. Enhanced ionic conductivity of a 1,2,3-triazolium-based poly(siloxane ionic liquid) homopolymer. ACS Macro Lett. 2016, 5, 1283–1286. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Obadia, M.M.; Serghei, A.; Livi, S.; Duchet-Rumeau, J.; Drockenmuller, E. 1,2,3-Triazolium-based epoxy-amine networks: Ion-conducting polymer electrolytes. Macromol. Rapid Commun. 2016, 37, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Obadia, M.M.; Drockenmuller, E. Poly(1,2,3-triazolium)s: A new class of functional polymer electrolytes. Chem. Commun. 2016, 52, 2433–2450. [Google Scholar] [CrossRef] [PubMed]
- Toal, S.J.; Trogler, W.C. Polymer sensors for nitroaromatic explosives detection. J. Mater. Chem. 2006, 16, 2871–2883. [Google Scholar] [CrossRef]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lam, J.W.Y.; Tang, B.Z. Acetylenic polymers: Syntheses, structures, and functions. Chem. Rev. 2009, 109, 5799–5867. [Google Scholar] [CrossRef]
- Willets, K.A.; Nishimura, S.Y.; Schuck, P.J.; Twieg, R.J.; Moerner, W.E. Nonlinear optical chromophores as nanoscale emitters for single-molecule spectroscopy. Acc. Chem. Res. 2005, 38, 549–556. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Zhou, G. Recent advances of the emitters for high performance deep-blue organic light-emitting diodes. J. Mater. Chem. C 2015, 3, 913–944. [Google Scholar] [CrossRef]
- Stejskal, E.O.; Tanner, J.E. Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
- Tanner, J.E.; Stejskal, E.O. Restricted self-diffusion of proteins in colloidal systems by the pulse-gradient, spin-echo method. J. Chem. Phys. 1968, 49, 1768–1777. [Google Scholar] [CrossRef]
- Stilbs, P. Fourier transform pulsed-gradient spin-echo studies of molecular diffusion. Prog. Nucl. Magn. Reson. Spectrosc. 1987, 19, 1–45. [Google Scholar] [CrossRef]
- Dimonie, M.; Teodorescu, M. Phase transfer catalysis synthesis of α,ω-diallylpoly(ethylene oxide). Makromol. Chem. Rapid Commun. 1993, 14, 303–307. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Shao, J.; Zhao, X.; Yang, B. Non-conjugated polymer dots with crosslink-enhanced emission in the absence of fluorophore units. Angew. Chem. Int. Ed. 2015, 54, 14626–14637. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.-A.; Huo, Q.; Chi, M.; Veith, G.M.; Binder, A.J.; Dai, S. A “ship-in-a-bottle” approach to synthesis of polymer dots@silica or polymer dots@carbon core-shell nanospheres. Adv. Mater. 2012, 24, 6017–6021. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, L.; Zhou, N.; Zhao, X.; Song, Y.; Maharjan, S.; Zhang, J.; Lu, L.; Wang, H.; Yang, B. The crosslink enhanced emission (CEE) in non-conjugated polymer dots: From the photoluminescence mechanism to the cellular uptake mechanism and internalization. Chem. Commun. 2014, 50, 13845–13848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Xu, L.; Feng, L.; Ji, Y.; Tao, L.; Li, S.; Wei, Y. Biocompatible polydopamine fluorescent organic nanoparticles: Facile preparation and cell imaging. Nanoscale 2012, 4, 5581–5584. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, B.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Price, W.S.; Ide, H.; Arata, Y. Self-diffusion of supercooled water to 238 k using pgse nmr diffusion measurements. J. Phys. Chem. A 1999, 103, 448–450. [Google Scholar] [CrossRef]
- Sarkar, S.; Mondal, A.; Tiwari, A.K.; Shunmugam, R. Unique emission from norbornene derived terpyridine-a selective chemodosimeter for G-type nerve agent surrogates. Chem. Commun. 2012, 48, 4223–4225. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, R.; Pan, D.; Yang, R.; Xu, Y.; Wang, L.; Yang, M. Thiolactone-maleimide: A functional monomer to synthesize fluorescent aliphatic poly(amide-imide) with excellent solubility via in situ pegylation. Polym. Chem. 2016, 7, 6241–6249. [Google Scholar] [CrossRef]
- Xu, Q.; Lee, K.M.; Wang, F.; Yoon, J. Visual detection of copper ions based on azide- and alkyne-functionalized polydiacetylene vesicles. J. Mater. Chem. 2011, 21, 15214–15217. [Google Scholar] [CrossRef]



| Polymer Code a | Mn × 10−3 b | Mn × 10−3 c | Mw/Mnc |
|---|---|---|---|
| EG18-b-AP4 | 1.1 | 1.5 | 1.26 |
| EG18-b-AP12 | 1.8 | 3.3 | 1.98 |
| EG45-b-AP6 | 2.5 | 2.7 | 1.40 |
| EG45-b-AP22 | 3.6 | 2.5 | 2.37 |
| Solvent | EG18-b-AP4 | EG18-b-AP12 | EG45-b-AP6 | EG45-b-AP22 |
|---|---|---|---|---|
| water | ++ | + | ++ | + |
| methanol | + | – | ++ | + |
| acetic acid | ++ | ++ | ++ | ++ |
| NMP | ++ | ++ | ++ | ++ |
| DMSO | ++ | ++ | ++ | ++ |
| DMF | ++ | ++ | ++ | ++ |
| acetonitrile | ++ | + | ++ | + |
| acetone | ++ | – | + | – |
| dicholromethane | ++ | ++ | ++ | + |
| chloroform | ++ | ++ | ++ | + |
| pyridine | ++ | ++ | ++ | + |
| THF | + | – | + | – |
| ethyl acetate | + | – | – | – |
| toluene | – | – | – | – |
| Polymer Code | Φ | ||
|---|---|---|---|
| In DMF | In Acetonitrile | In Water | |
| EG18-b-AP4 | 0.034 | 0.020 | 0.018 |
| EG18-b-AP12 | 0.020 | — | — |
| EG45-b-AP6 | 0.029 | 0.019 | 0.014 |
| EG45-b-AP22 | 0.024 | — | — |
| Model | Abs. 1/nm | Emission 2/nm |
|---|---|---|
| EG2-b-AP4 | 231.1 | 456.6 |
| EG5-b-AP4 | 231.4 | 455.7 |
| EG5-b-AP7 | 237.1 | 473.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Mori, A.; Hashidzume, A. Emission Properties of Diblock Copolymers Composed of Poly(ethylene glycol) and Dense 1,2,3-Triazole Blocks. Polymers 2019, 11, 1086. https://doi.org/10.3390/polym11071086
Yang Y, Mori A, Hashidzume A. Emission Properties of Diblock Copolymers Composed of Poly(ethylene glycol) and Dense 1,2,3-Triazole Blocks. Polymers. 2019; 11(7):1086. https://doi.org/10.3390/polym11071086
Chicago/Turabian StyleYang, Yanqiong, Asami Mori, and Akihito Hashidzume. 2019. "Emission Properties of Diblock Copolymers Composed of Poly(ethylene glycol) and Dense 1,2,3-Triazole Blocks" Polymers 11, no. 7: 1086. https://doi.org/10.3390/polym11071086






