A Composite Hydrogel with High Mechanical Strength, Fluorescence, and Degradable Behavior for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation
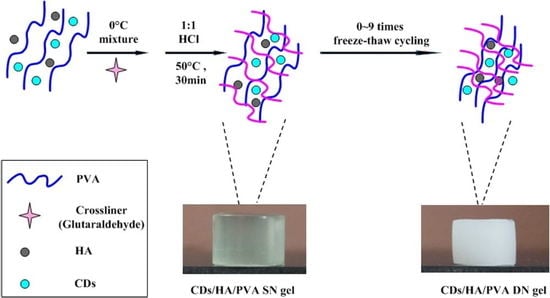
Preparation of the Carbon Dots (CDs)
Preparation of the CDs/HA/PVA Composite Hydrogel
2.2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.3. X-Ray Diffraction (XRD) Measurement
2.2.4. Swelling Behavior Characterization
2.2.5. Water Content of the Composite Hydrogel
2.2.6. Mechanical Property Measurements
2.2.7. Degradation Properties of the Hydrogel
3. Results
3.1. Preparation and Properties of the Hydrogels
3.2. Mechanical Properties of the Hydrogels
3.2.1. Mechanical Properties of the Hydrogels with Different Mass Ratios of CDs to PVA
3.2.2. Mechanical Properties of Hydrogels with Different Mass Ratios of HA to PVA
3.2.3. Mechanical Properties of the Hydrogels with Different Numbers of Freezing/Thawing Cycles
3.3. The Water Content and Swelling Behavior of the SN and DN Hydrogels
3.4. Degradation Behavior of the CDs/HA/PVA DN Hydrogels with Different Numbers of Freezing/Thawing Cycles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tozzi, G.; De Mori, A.; Oliveira, A.; Roldo, M. Composite Hydrogels for Bone Regeneration. Materials 2016, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Ionita, M.; Crica, L.E.; Tiainen, H.; Haugen, H.J.; Vasile, E.; Dinescu, S.; Costache, M.; Iovu, H. Gelatin–poly (vinyl alcohol) porous biocomposites reinforced with graphene oxide as biomaterials. J. Mater. Chem. B 2016, 4, 282–291. [Google Scholar] [CrossRef]
- Sourbh, T.; Jyoti, C.; Vinod, K.; Vijay, K.T. Progress in pectin based hydrogels for water purification: Trends and challenges. J. Environ. Manag. 2019, 238, 210–223. [Google Scholar] [Green Version]
- Sourbh, T.; Bhawna, S.; Ankit, V.; Jyoti, C.; Sigitas, T.; Vijay, K.T. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [Green Version]
- Gonzalez, J.S.; Alvarez, V.A. Mechanical properties of polyvinylalcohol/hydroxyapatite cryogel as potential artificial cartilage. J. Mech. Behav. Biomed. Mater. 2014, 34, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Jolanta, W.K.; Tomasz, R.; Iwona, M.P.; Vijay, K.T. Biopolymers for Biomedical and Pharmaceutical Applications: Recent Advances and Overview of Alginate Electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.K.; Stan, G.E.; Ciocan, L.T. Progress in Hydroxyapatite–Starch Based Sustainable Biomaterials for Biomedical Bone Substitution Applications. ACS Sustain. Chem. Eng. 2017, 5, 8491–8512. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Mallaiah, S.H. Swelling and mechanical properties of radiation crosslinked Au/PVA hydrogel nanocomposites. Radiat. Effects Defects Solids 2016, 171, 869–878. [Google Scholar] [CrossRef]
- Wang, Y.; Hsieh, Y.-L. Crosslinking of polyvinyl alcohol (PVA) fibrous membranes with glutaraldehyde and PEG diacylchloride. J. Appl. Polym. Sci. 2010, 116, 3249–3255. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Xu, C.; Li, Y.; Gao, J.; Wang, W.; Liu, Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater. Chem. 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Tudorachi, N.; Chiriac, A.P. Poly (vinyl alcohol-co-lactic acid) /Hydroxyapatite Composites: Synthesis and Characterization. J. Polym. Environ. 2011, 19, 546–558. [Google Scholar] [CrossRef]
- Liu, B.-S.; Yao, C.-H.; Chen, Y.-S.; Hsu, S.-H. In vitro evaluation of degradation and cytotoxicity of a novel composite as a bone substitute. J. Biomed. Mater. Res. Part A 2010, 67A, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Boerman, O.C.; de Korte, C.; Rijpkema, M.; Heerschap, A.; Oosterwijk, E.; Jansen, J.A.; Walboomers, X.F. Preclinical imaging in bone tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 578–595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, J.; Bao, S.; Wu, Q.; Wang, Q. Semiconductor nanoparticle-based hydrogels prepared via self-initiated polymerization under sunlight, even visible light. Sci. Rep. 2013, 3, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.; Fei, X.; Tian, J.; Jing, M.; Xu, L.; Wang, X.; Liu, D.; Wang, Y.; Liu, J. A novel transparent luminous hydrogel with self-healing property. J. Mater. Chem. B 2017, 5, 5738–5744. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wu, M.; Wang, Y.Q.; He, X.W.; Li, W.Y.; Feng, X.Z. A new hydrothermal refluxing route to strong fluorescent carbon dots and its application as fluorescent imaging agent. Talanta 2013, 117, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Hu, M.; Gu, X.; Hu, Y.; Deng, Y.; Wang, C. PVA/Carbon Dot Nanocomposite Hydrogels for Simple Introduction of Ag Nanoparticles with Enhanced Antibacterial Activity. Macromol. Mater. Eng. 2016, 301, 1352–1362. [Google Scholar] [CrossRef]
- Hussain, R.; Tabassum, S.; Gilani, M.A.; Ahmed, E.; Sharif, A.; Manzoor, F.; Shah, A.T.; Asif, A.; Sharif, F.; Iqbal, F. In situ synthesis of mesoporous polyvinyl alcohol/hydroxyapatite composites for better biomedical coating adhesion. Appl. Surf. Sci. 2016, 364, 117–123. [Google Scholar] [CrossRef]
- Kumar, V.B.; Sahu, A.K.; Mohsin, A.S.M.; Li, X.; Gedanken, A. Refractive-Index Tuning of Highly Fluorescent Carbon Dots. ACS Appl. Mater. Interfaces 2017, 9, 28930–28938. [Google Scholar] [CrossRef]
- Ma, Y.; Bai, T.; Fei, W. The physical and chemical properties of the polyvinylalcohol/polyvinylpyrrolidone/hydroxyapatite composite hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ji, X.; Jing, M.; Hou, H.; Zhu, Y.; Fang, L.; Yang, X.; Chen, Q.; Banks, C.E. Carbon dots supported upon N-doped TiO2 nanorods applied into sodium and lithium ion batteries. J. Mater. Chem. A 2015, 3, 5648–5655. [Google Scholar] [CrossRef]
- Hu, M.; Gu, X.; Hu, Y.; Wang, T.; Huang, J.; Wang, C. Low Chemically Cross-Linked PAM/C-Dot Hydrogel with Robustness and Superstretchability in Both As-Prepared and Swelling Equilibrium States. Macromolecules 2016, 49, 3174–3183. [Google Scholar] [CrossRef]
- Maiolo, A.S.; Amado, M.N.; Gonzalez, J.S.; Alvarez, V.A. Development and characterization of Poly (vinyl alcohol) based hydrogels for potential use as an articular cartilage replacement. Mater. Sci. Eng. C 2012, 32, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, D.; He, Y. Graphene oxide/polyacrylamide/carboxymethyl cellulose sodium nanocomposite hydrogel with enhanced mechanical strength: Preparation, characterization and the swelling behavior. RSC Adv. 2014, 4, 44600–44609. [Google Scholar] [CrossRef]
- Qasim, S.B.; Husain, S.; Huang, Y.; Pogorielov, M.; Deineka, V.; Lyndin, M.; Rawlinson, A.; Rehman, I.U. In-vitro and in-vivo degradation studies of freeze gelated porous chitosan composite scaffolds for tissue engineering applications. Polym. Degrad. Stab. 2017, 136, 31–38. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xue, Y.; Wang, J.; Zhu, Y.; Zhu, Y.; Zhang, X.; Liao, J.; Li, X.; Wu, X.; Qin, Y.-X.; et al. A Composite Hydrogel with High Mechanical Strength, Fluorescence, and Degradable Behavior for Bone Tissue Engineering. Polymers 2019, 11, 1112. https://doi.org/10.3390/polym11071112
Wang Y, Xue Y, Wang J, Zhu Y, Zhu Y, Zhang X, Liao J, Li X, Wu X, Qin Y-X, et al. A Composite Hydrogel with High Mechanical Strength, Fluorescence, and Degradable Behavior for Bone Tissue Engineering. Polymers. 2019; 11(7):1112. https://doi.org/10.3390/polym11071112
Chicago/Turabian StyleWang, Yanqin, Yanan Xue, Jinghui Wang, Yaping Zhu, Yu Zhu, Xuehui Zhang, Jingwen Liao, Xiaona Li, Xiaogang Wu, Yi-Xian Qin, and et al. 2019. "A Composite Hydrogel with High Mechanical Strength, Fluorescence, and Degradable Behavior for Bone Tissue Engineering" Polymers 11, no. 7: 1112. https://doi.org/10.3390/polym11071112