Fabrication and Characterization of Novel Shape-Stabilized Phase Change Materials Based on P(TDA-co-HDA)/GO Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
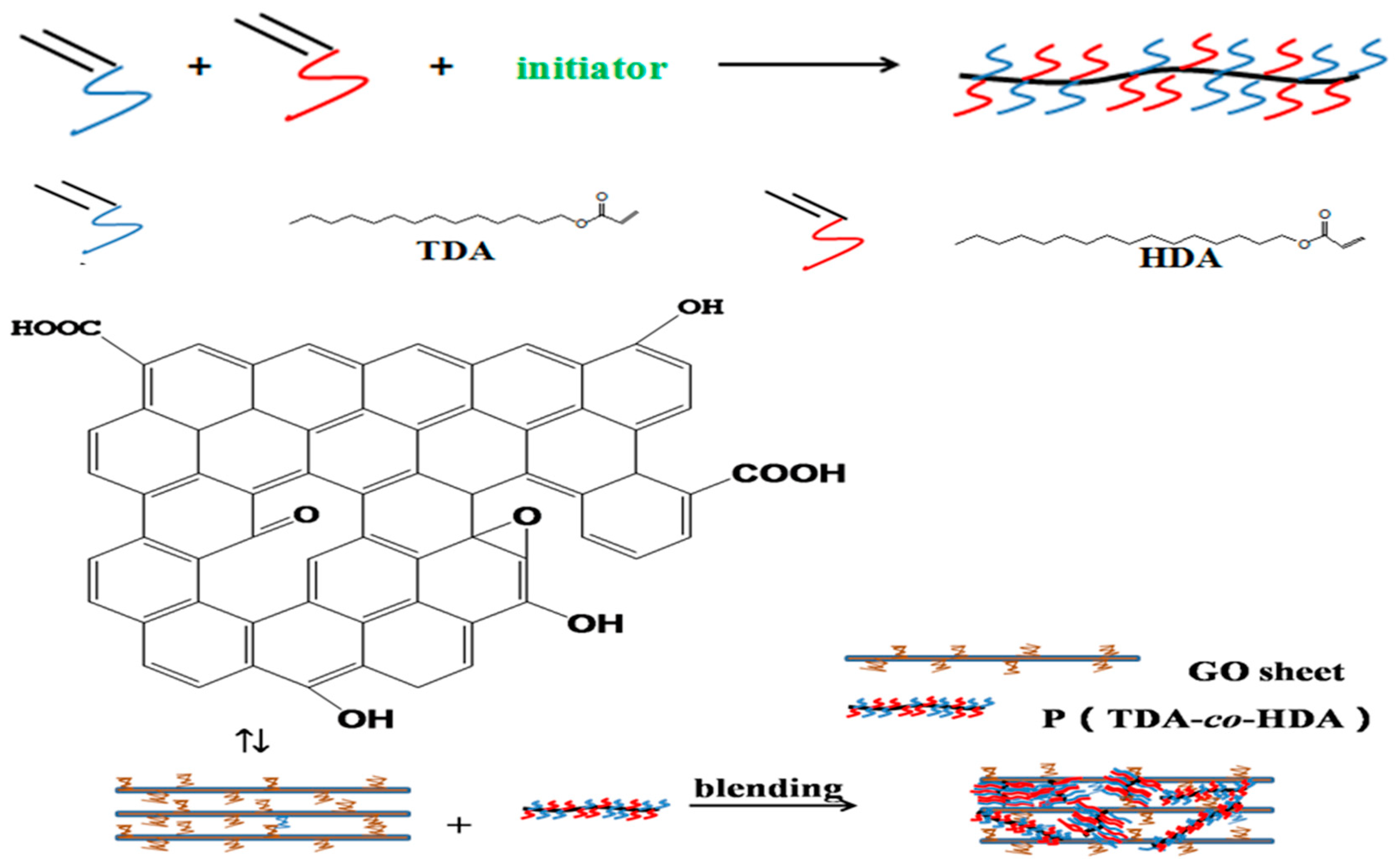
2.2. Synthesis of P(TDA-co-HDA)
2.3. Fabrication of SPCMs
2.4. Characterization
3. Results and Discussion
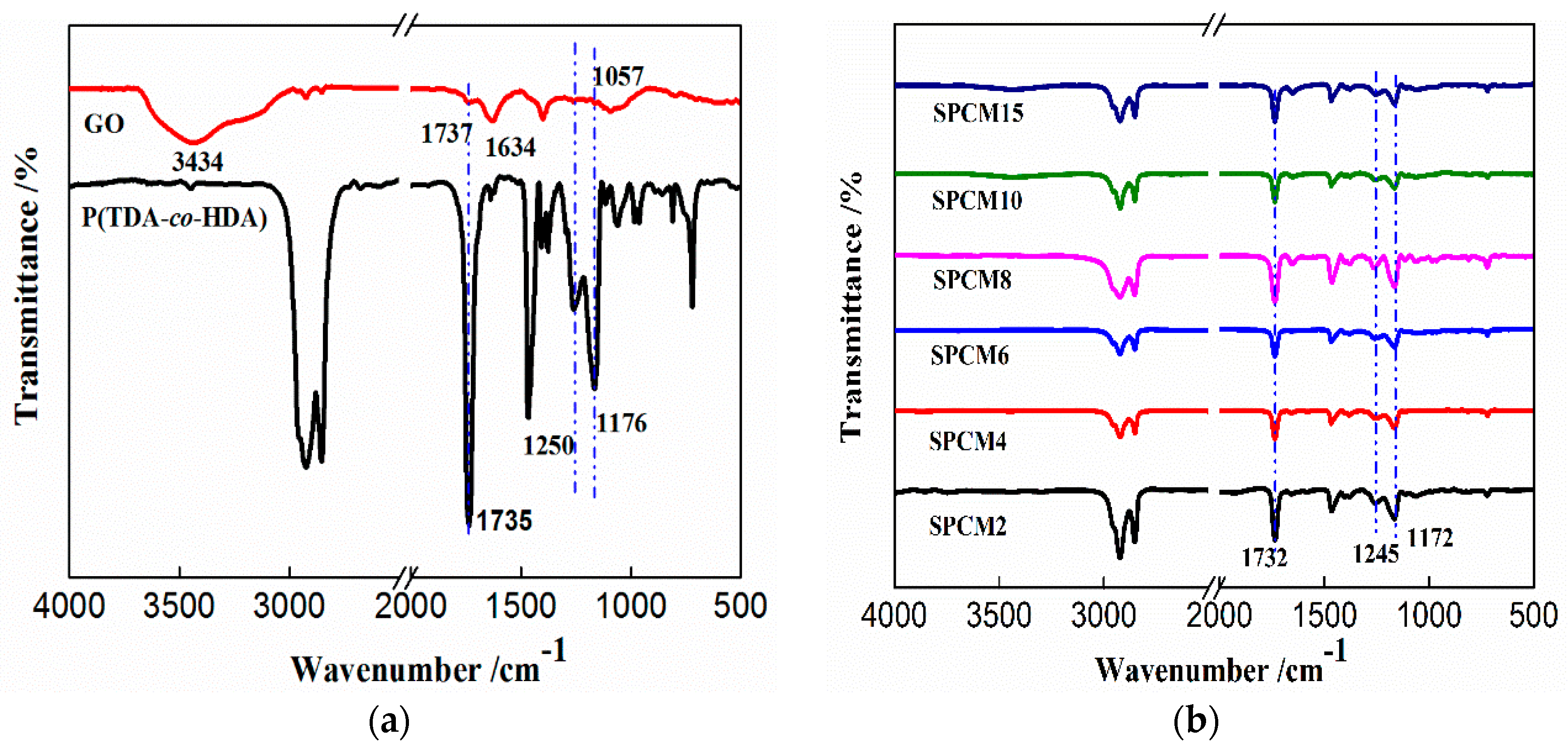
3.1. Morphologies and Chemical Structures of SPCMs
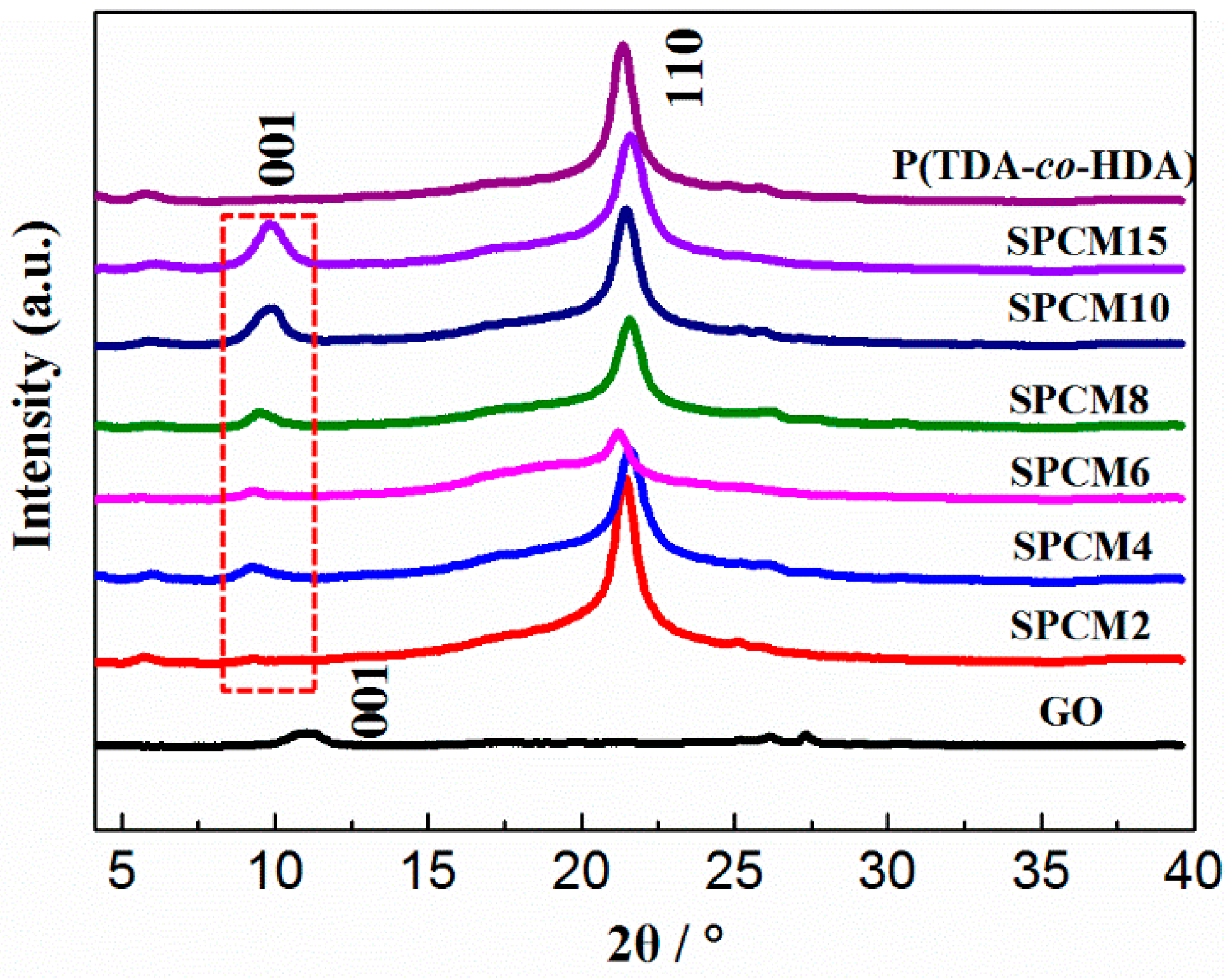
3.2. Thermal and Crystalline Properties of SPCMs
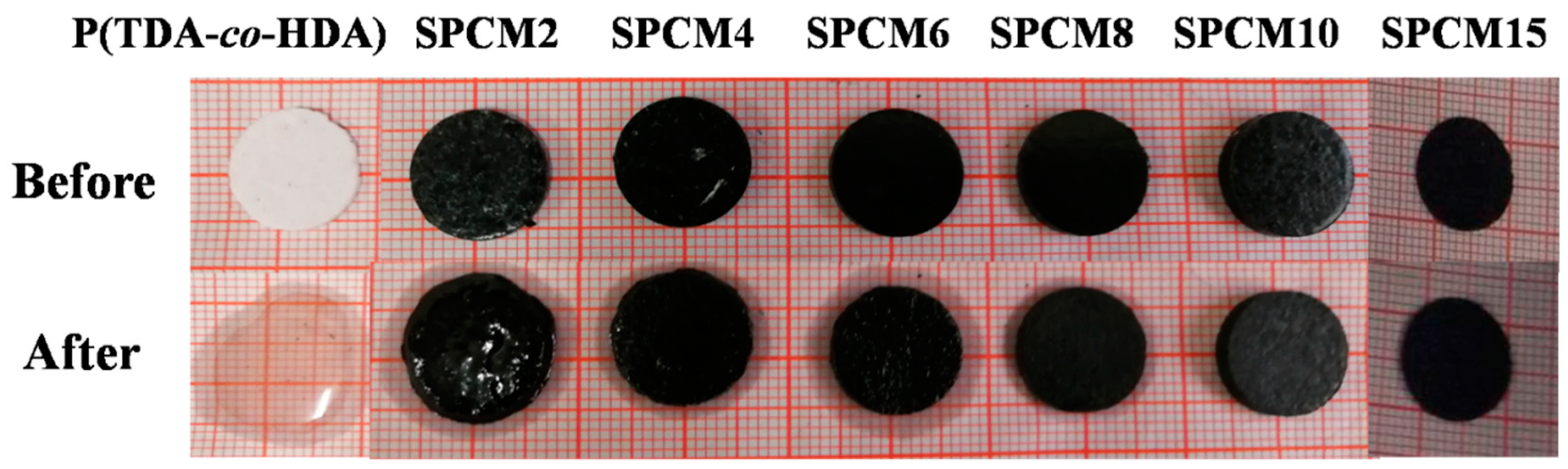
3.3. Shape-Stabilized Properties of SPCMs
3.4. Thermal Reliabilities and Structural Stabilities of the SPCMs
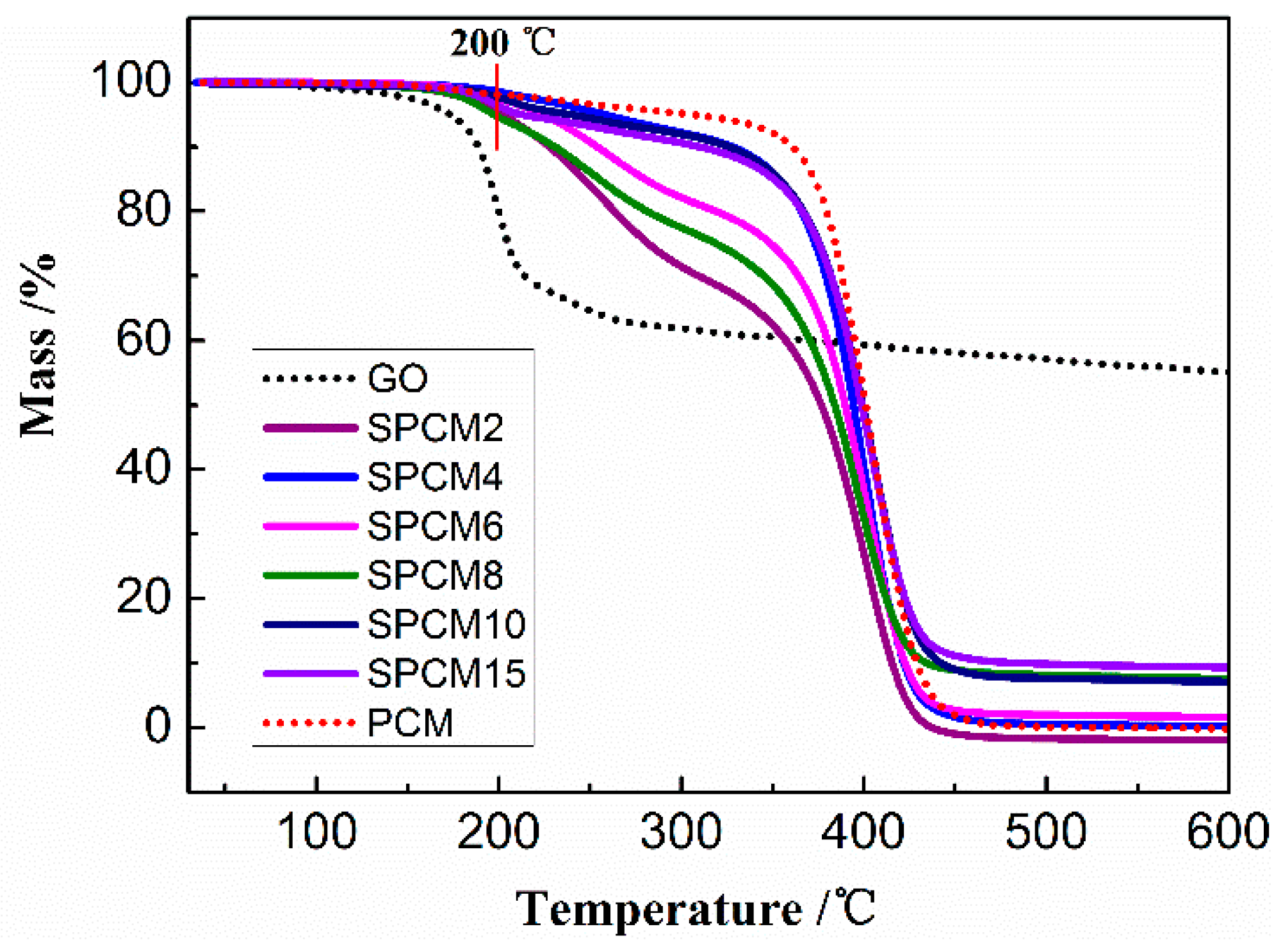
3.5. Thermal Stabilities of SPCMs
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Aydın, A.A.; Okutan, H. High-chain fatty acid esters of myristyl alcohol with even carbon number: Novel organic phase change materials for thermal energy storage-1. Sol. Energy Mater. Sol. Cells 2011, 95, 2752–2762. [Google Scholar] [CrossRef]
- Wang, C.Y.; Feng, L.L.; Xin, G.B.; Li, W.; Zheng, J.; Tian, W.H.; Li, X.G. Graphene oxide stabilized polyethylene glycol for heat storage. Phys. Chem. Chem. Phys. 2012, 14, 13233–13238. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, J.P.; Wang, Y.N.; Li, W.; Wang, X.C.; Shi, H.F.; Zhang, X.X. Effect of N-isopropylacrylamide on the preparation and properties of microencapsulated phase change materials. Energy 2016, 106, 221–230. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhang, X.X.; Shi, H.F.; Li, W.; Meng, J.Y. Thermo-regulated sheath/core submicron fiber with poly(diethylene glycol hexadecyl ether acrylate) as a core. Text. Res. J. 2015, 86, 493–501. [Google Scholar] [CrossRef]
- Li, W.; Zhang, R.; Jiang, N.; Tang, X.F.; Shi, H.F.; Zhang, X.X.; Zhang, Y.K.; Dong, L.; Zhang, N.X. Composite macrocapsule of phase change materials/expanded graphite for thermal energy storage. Energy 2013, 57, 607–614. [Google Scholar] [CrossRef]
- Pei, D.F.; Chen, S.; Li, W.; Zhang, X.X. Poly(mono/diethylene glycol n-tetradecyl ether vinyl ether)s with Various Molecular Weights as Phase Change Materials. Polymers 2018, 10, 197. [Google Scholar] [Green Version]
- Behzadi, S.; Farid, M.M. Long term thermal stability of organic PCMs. Appl. Energy 2014, 122, 11–16. [Google Scholar] [CrossRef]
- Li, W.; Geng, X.Y.; Huang, R.; Wang, J.P.; Wang, N.; Zhang, X.X. Microencapsulated Comb-Like Polymeric Solid-Solid Phase Change Materials via In-Situ Polymerization. Polymers 2018, 10, 172. [Google Scholar] [CrossRef]
- Amin, M.; Putra, N.; Kosasih, E.A.; Prawiro, E.; Luanto, R.A.; Mahlia, T.M.I. Thermal propertiesof beeswax/graphene phase change material as energy storage for building applications. Appl. Therm. Eng. 2017, 112, 273–280. [Google Scholar] [CrossRef]
- Memon, S.A. Phase change materials integrated in building walls: A state of the art review. Renew. Sustain. Energy Rev. 2014, 31, 870–906. [Google Scholar] [CrossRef]
- Kaizawa, A.; Maruoka, N.; Kawai, A.; Kamano, H.; Jozuka, T.; Senda, T.; Akiyama, T. Thermo physical and heat transfer properties of phase change material candidate for waste heat transportation system. Heat Mass Transf. 2008, 44, 763–769. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Huang, Y. Electro spun phase change fibers based on polyethylene glycol/cellulose acetate blends. Appl. Energy 2011, 88, 3133–3139. [Google Scholar] [CrossRef]
- Cao, R.R.; Li, X.; Chen, S.; Yuan, H.R.; Zhang, X.X. Fabrication and characterization of novel shape-stabilized synergistic phase change materials based on PHDA/GO composites. Energy 2017, 138, 157–166. [Google Scholar] [CrossRef]
- Cao, R.R.; Liu, H.H.; Chen, S.; Pei, D.F.; Miao, J.L.; Zhang, X.X. Fabrication and properties of graphene oxide-grafted-poly(hexadecyl acrylate) as a solid-solid phase change material. Compos. Sci. Technol. 2017, 149, 262–268. [Google Scholar] [CrossRef]
- O’Leary, K.A.; Paul, D.R. Physical properties of poly(n-alkyl acrylate) copolymers. Part 2. Crystalline/non-crystalline combinations. Polymer 2006, 47, 1245–1258. [Google Scholar] [CrossRef]
- Qi, G.Q.; Liang, C.L.; Bao, R.Y.; Liu, Z.Y.; Yang, W.; Xie, B.H.; Yang, M.B. Polyethylene glycol based shape-stabilized phase change material for thermal energy storage with ultra-low content of graphene oxide. Sol. Energy Mater. Sol. Cells 2014, 123, 171–177. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.W.; An, J.H.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.J.; Tsao, N.; Tomovic, Z.; Li, J.L.; Mullen, K. Transparent carbon films as electrodes in organic solar cells. Angew. Chem. Int. Ed. 2008, 47, 2990–2992. [Google Scholar] [CrossRef]
- Qi, G.Q.; Yang, J.; Bao, R.Y.; Liu, Z.Y.; Yang, W.; Xie, B.H.; Yang, M.B. Enhanced comprehensive performance of polyethylene glycol based phase change material with hybrid graphenenanomaterials for thermal energy storage. Carbon 2015, 88, 196–205. [Google Scholar] [CrossRef]
- Tang, L.S.; Yang, J.; Bao, R.Y.; Liu, Z.Y.; Xie, B.H.; Yang, M.B.; Yang, W. Polyethylene glycol/graphene oxide aerogel shape-stabilized phase change materials for photo-to-thermal energy conversion and storage via tuning the oxidation degree of graphene oxide. Energy Convers. Manag. 2017, 146, 253–264. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Metselaar, H.S.C.; Silakhori, M. Shape-stabilized phase change materials with high thermal conductivity based on paraffin/grapheme oxide composite. Energy Convers. Manag. 2013, 67, 275–282. [Google Scholar] [CrossRef]
- Cai, Y.; Ke, H.; Dong, J.; Wei, Q.; Lin, J.; Zhao, Y.; Song, L.; Hu, Y.; Huang, F.L.; Gao, W.D.; et al. Effects of nano-SiO2 on morphology, thermal energy storage, thermal stability, and combustion properties of electrospunlauric acid/PET ultrafine composite fibers as form-stable phase change materials. Appl. Energy 2011, 88, 2106–2112. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C.; Naghavi, M.S.; Sadeghinezhad, E.; Akhiani, A.R. Preparation and characterization of palmitic acid/grapheme nanoplatelets composite with remarkable thermal conductivity as a novel shape stabilized phase change material. Appl. Therm. Eng. 2013, 61, 633–640. [Google Scholar] [CrossRef]
- Kim, D.; Jung, J.; Kim, Y.; Lee, M.; Seo, J.; Khan, S.B. Structure and thermal properties of octadecane/expanded graphite composites as shape-stabilized phase change materials. Int. J. Heat Mass Transf. 2016, 95, 735–741. [Google Scholar] [CrossRef]
- Tang, B.; Wang, Y.; Qiu, M.; Zhang, S. A full-band sunlight-driven carbon nanotube/PEG/SiO2 composites for solar energy storage. Sol. Energy Mater. Sol. Cells 2014, 123, 7–12. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Effect of carbon nanospheres on shape stabilization and thermal behavior of phase change materials for thermal energy storage. Energy Convers. Manag. 2014, 88, 206–213. [Google Scholar] [CrossRef]
- Li, S.Q.; Kong, L.; Wang, H.X.; Xu, H.X.; Li, J.; Shi, H.F. Thermal performance and shape-stabilization of comb-like polymeric phase change materials enhanced by octadecylamine-functionalized graphene oxide. Energy Convers. Manag. 2018, 168, 119–127. [Google Scholar] [CrossRef]
- Liu, H.H.; Hou, L.C.; Peng, W.W.; Zhang, Q.; Zhang, X.X. Fabrication and characterization of polyamide 6-functionalized graphenenanocomposite fiber. J. Mater. Sci. 2012, 47, 8052–8060. [Google Scholar] [CrossRef]
- Miao, J.L.; Liu, H.H.; Li, W.; Zhang, X.X. Mussel-inspired polydopamine functionalized graphene as a conductive adhesion promoter and protective layer for silver nanowire transparent electrodes. Langmuir 2016, 32, 5365–5372. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Ke, K.; Luo, Y.; Yang, W.; Xie, B.H.; Yang, M.B. Crystallization, rheological behavior and mechanical properties of poly(vinylidene fluoride) composites containing graphitic fillers: A comparative study. Polym. Int. 2012, 61, 1031–1040. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, X.; Hsuan, Y.G.; Li, C.Y. Reduced grapheme oxide-induced polyethylene crystallization in solution and nanocomposites. Macromolecules 2011, 45, 993–1000. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, W.; Li, G.L.; Tian, W.H.; Li, X.G. The influence of interactions between polyethylene glycol and graphene oxide in shape-stabilized PCMs on their phase change behaviors. Adv. Mater. Res. 2013, 800, 459–463. [Google Scholar] [CrossRef]





| Samples | ΔHm(J∙g−1) | Tmo(°C) | Tmp(°C) | ΔHc (J∙g−1) | Tco(°C) | Tcp(°C) |
|---|---|---|---|---|---|---|
| PTDA | 72 | 14.8 | 22.3 | −71 | 12.3 | 6.2 |
| PHDA | 109 | 21.7 | 36.4 | −109 | 34.1 | 24.6 |
| P(TDA-co-HDA) | 99 | 21.2 | 30.8 | −98 | 20.4 | 13.4 |
| SPCM2 | 96 | 21.7 | 29.6 | −96 | 20.5 | 13.5 |
| SPCM4 | 90 | 23.4 | 30.8 | −90 | 25.1 | 13.9 |
| SPCM6 | 81 | 22.1 | 35.2 | −80 | 27.9 | 15.8 |
| SPCM8 | 75 | 23.0 | 34.1 | −75 | 22.8 | 13.1 |
| SPCM10 | 70 | 20.0 | 29.9 | −70 | 20.8 | 12.1 |
| SPCM15 | 67 | 24.6 | 32.0 | −67 | 21.0 | 13.3 |
| Samples | ΔHm(J∙g−1) | Tmo(°C) | Tmp(°C) | ΔHc (J∙g−1) | Tco(°C) | Tcp(°C) |
|---|---|---|---|---|---|---|
| 1 cycle | 70 | 20.0 | 29.9 | −70 | 20.8 | 12.1 |
| 100 cycles | 70 | 20.2 | 30.0 | −71 | 20.7 | 12.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Yu, Y.; Cao, R.; Liu, H.; Zhang, X. Fabrication and Characterization of Novel Shape-Stabilized Phase Change Materials Based on P(TDA-co-HDA)/GO Composites. Polymers 2019, 11, 1113. https://doi.org/10.3390/polym11071113
Chen S, Yu Y, Cao R, Liu H, Zhang X. Fabrication and Characterization of Novel Shape-Stabilized Phase Change Materials Based on P(TDA-co-HDA)/GO Composites. Polymers. 2019; 11(7):1113. https://doi.org/10.3390/polym11071113
Chicago/Turabian StyleChen, Sai, Yue Yu, Ruirui Cao, Haihui Liu, and Xingxiang Zhang. 2019. "Fabrication and Characterization of Novel Shape-Stabilized Phase Change Materials Based on P(TDA-co-HDA)/GO Composites" Polymers 11, no. 7: 1113. https://doi.org/10.3390/polym11071113




