Electrospun Sesbania Gum-Based Polymeric N-Halamines for Antibacterial Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Epoxidized SG
2.3. Synthesis of Aminated SG
2.4. Synthesis of Chlorinated SG
2.5. Electrospinning
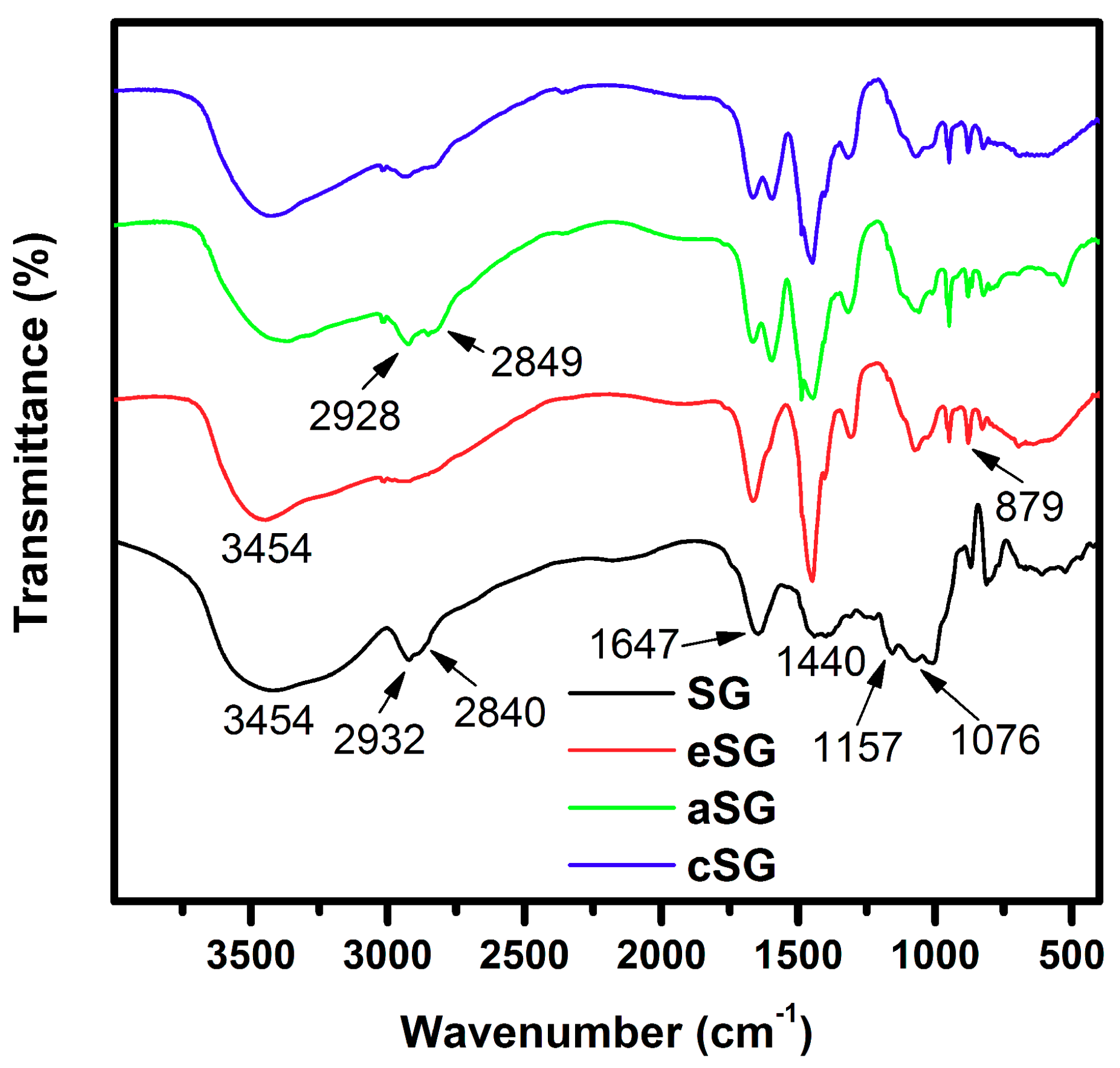
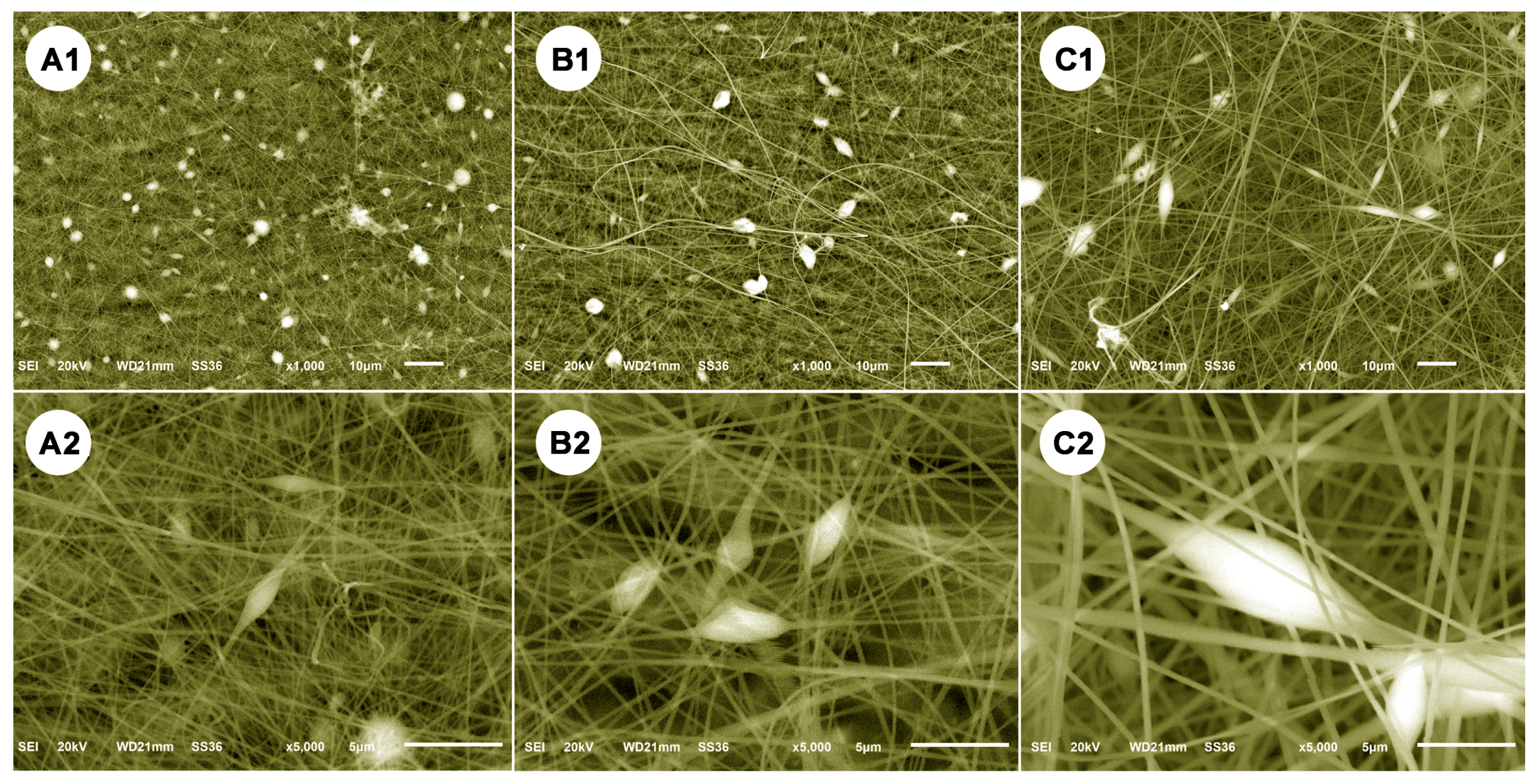
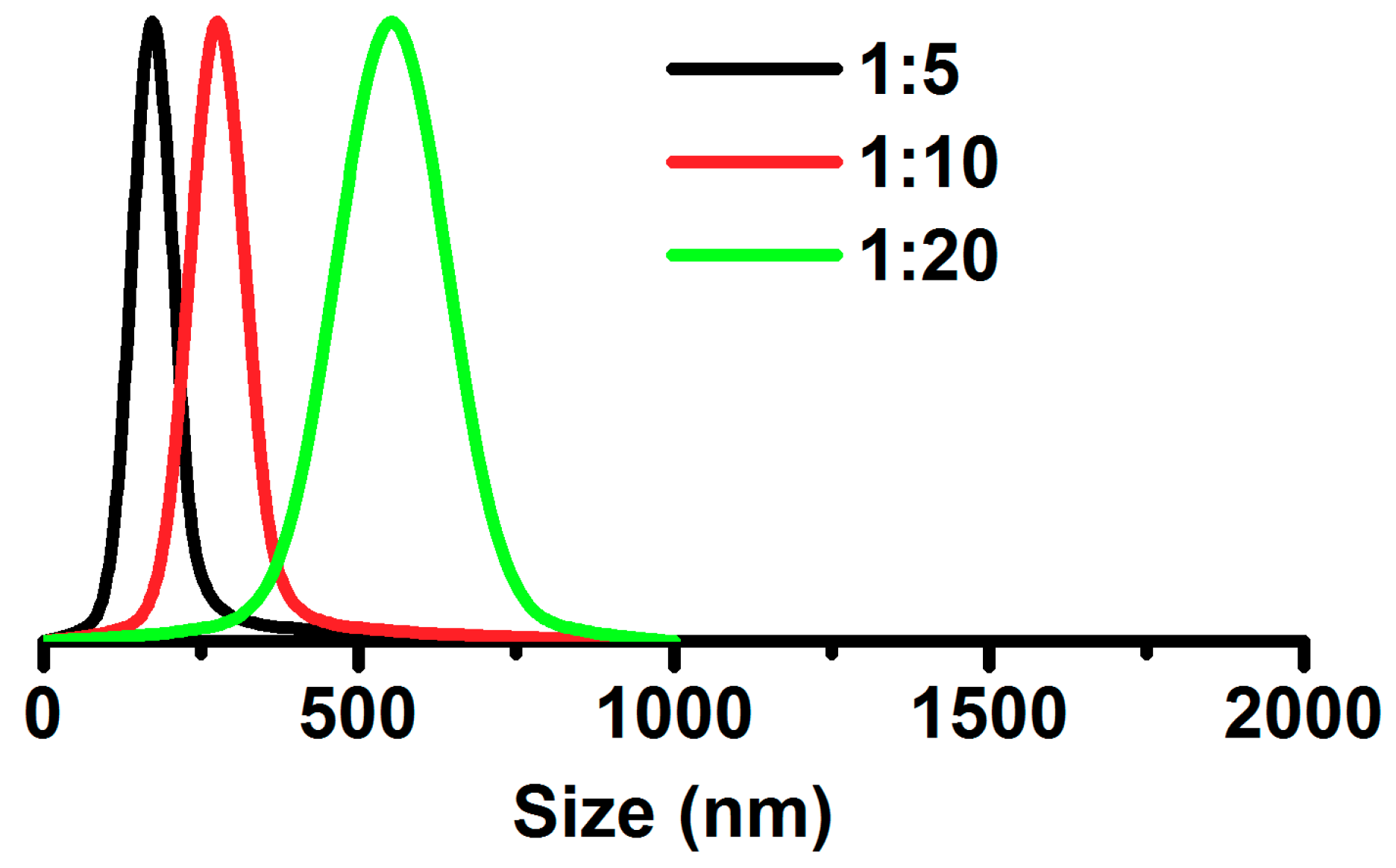
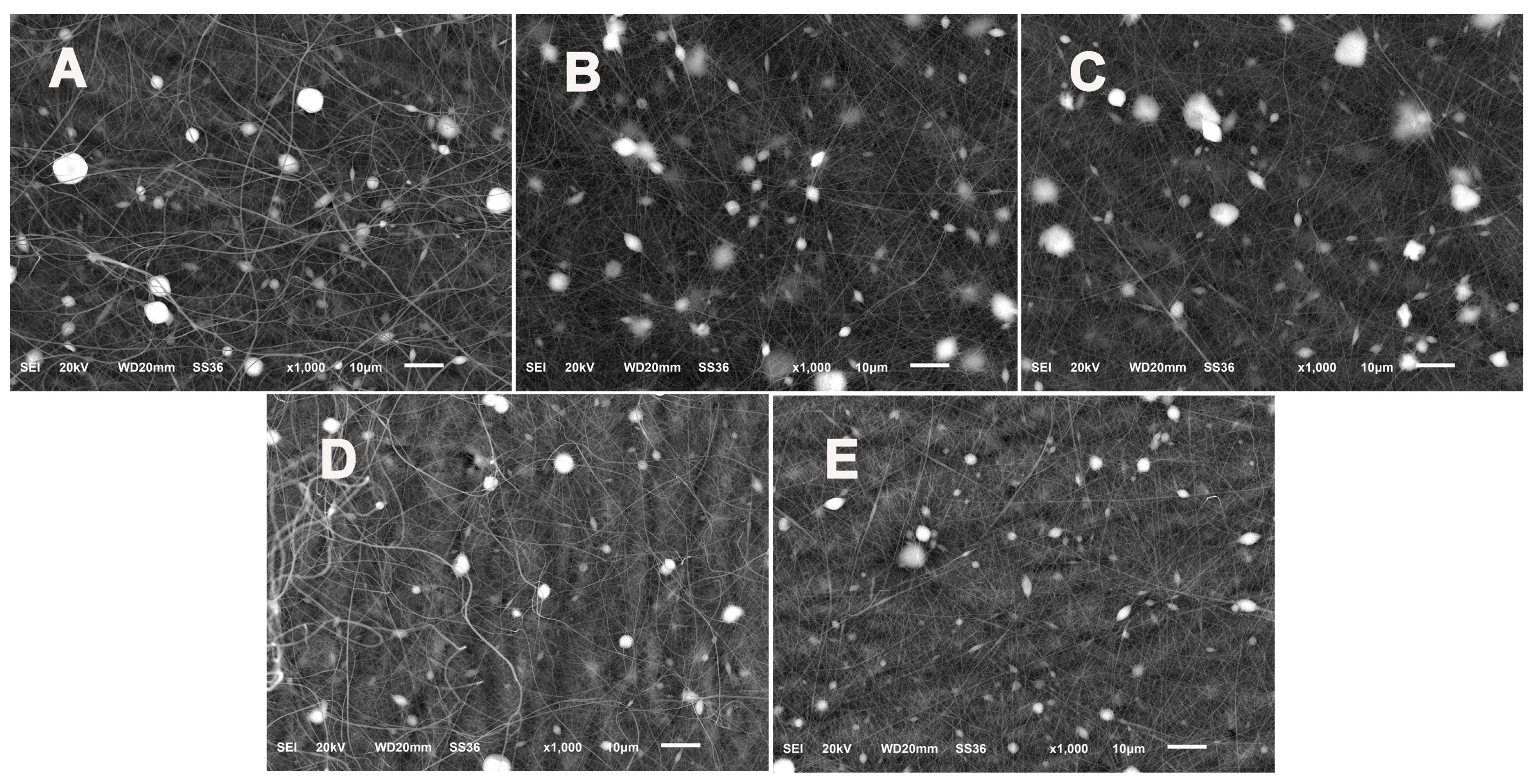
2.6. Characterization
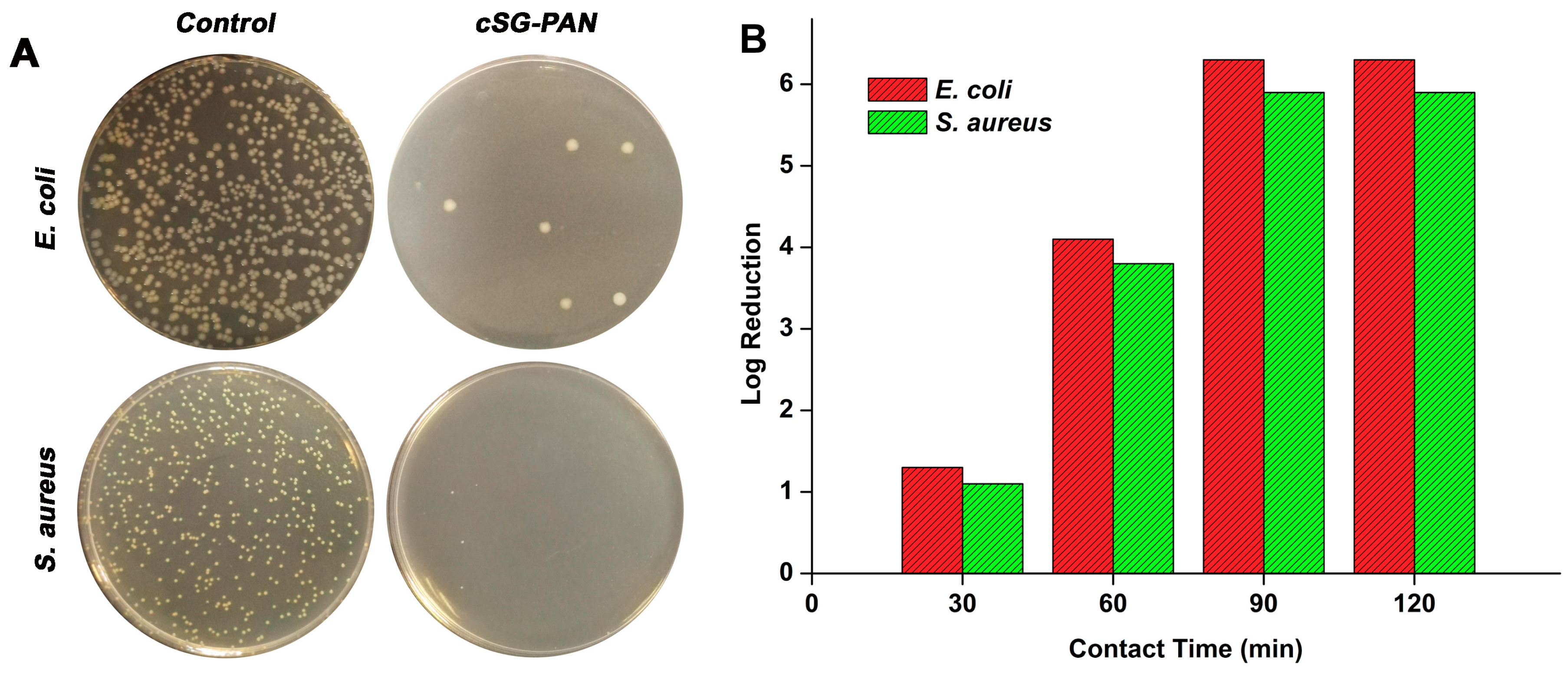
2.7. Antibacterial Test
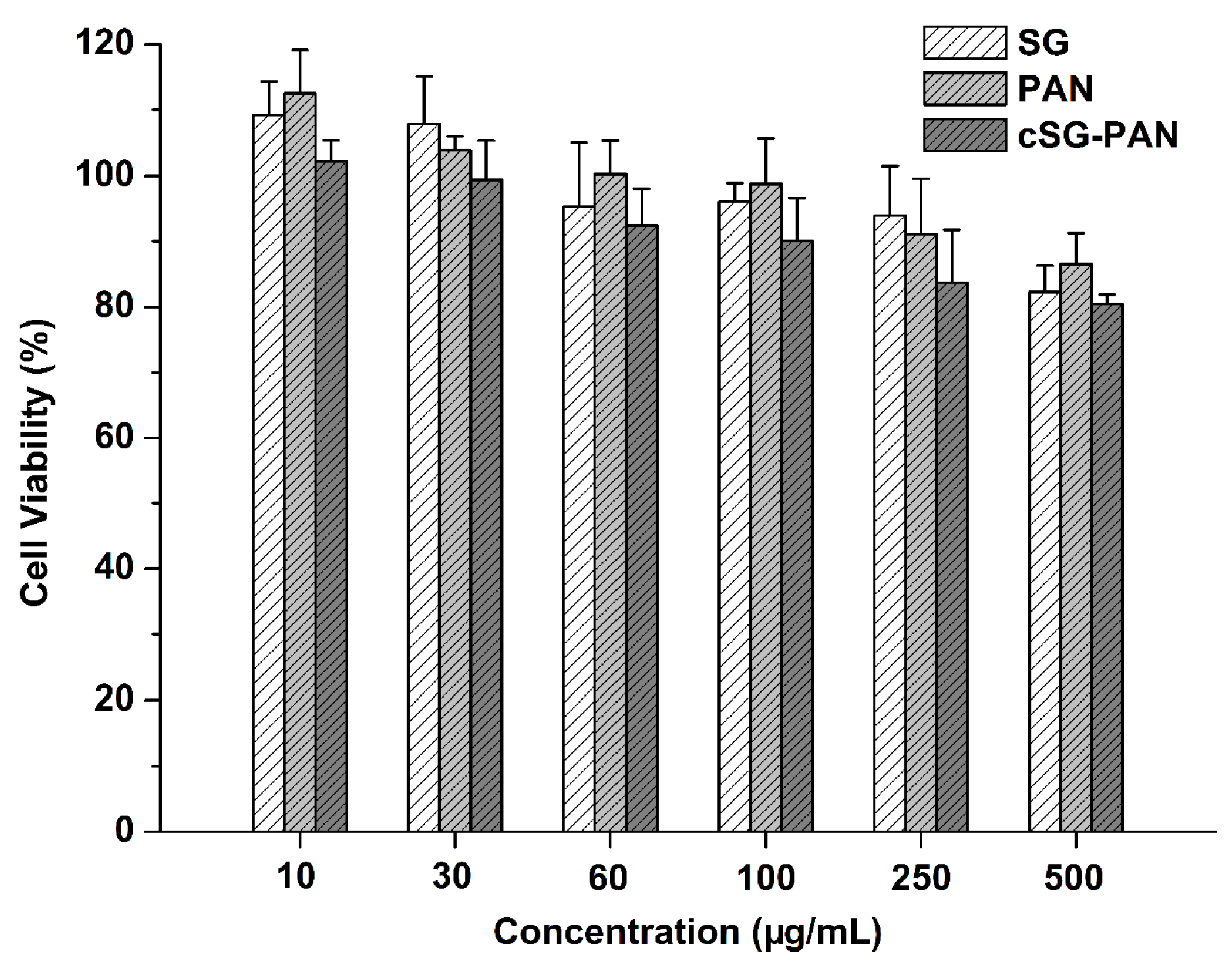
2.8. Cytotoxicity Test
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Munoz-Bonilla, A.; Fernandez-Garcia, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Bel, N.; Hornstra, L.M.; Veen, A.; Medema, G. Efficacy of flushing and chlorination in removing microorganisms from a pilot drinking water distribution system. Water 2019, 11, 903. [Google Scholar]
- Olasoji, S.O.; Oyewole, N.O.; Abiola, B.; Edokpayi, J.N. Water quality assessment of surface and groundwater sources using a water quality index method: A case study of a peri-urban town in southwest, Nigeria. Environments 2019, 6, 23. [Google Scholar] [CrossRef]
- Koscova, J.; Hurnikova, Z.; Pistl, J. Degree of bacterial contamination of mobile phone and computer keyboard surfaces and efficacy of disinfection with chlorhexidine digluconate and triclosan to its reduction. Int. J. Environ. Res. Public Health 2018, 15, 2238. [Google Scholar] [CrossRef] [PubMed]
- Girolamini, L.; Lizzadro, J.; Mazzotta, M.; Iervolino, M.; Dormi, A.; Cristino, S. Different trends in microbial contamination between two types of microfiltered water dispensers: From risk analysis to consumer health preservation. Int. J. Environ. Res. Public Health 2019, 16, 272. [Google Scholar] [CrossRef] [PubMed]
- Accolti, M.D.; Soffritti, I.; Mazzacane, S.; Caselli, E. Fighting AMR in the healthcare environment: Microbiome-based sanitation approaches and monitoring tools. Int. J. Mol. Sci. 2019, 20, 1535. [Google Scholar] [CrossRef] [PubMed]
- Luz, G.; Sousa, B.; Guedes, A.; Barreto, C.; Brasil, L. Biocides used as additives to biodiesels and their risks to the environment and public health: A review. Molecules 2018, 23, 2698. [Google Scholar] [CrossRef]
- Kauppinen, A.; Miettinen, I.T. Persistence of norovirus GII genome in drinking water and wastewater at different temperatures. Pathogens 2017, 6, 48–59. [Google Scholar] [CrossRef]
- Xu, J.Q.; Bai, Y.J.; Wan, M.J.; Liu, Y.H.; Tao, L.; Wang, X. Antifungal paper based on a polyborneolacrylate coating. Polymers 2018, 10, 448. [Google Scholar] [CrossRef]
- Dastile, L.S.; Francis, J.; Muchenje, V. Consumers’ social representations of meat safety in two selected restaurants of raymond mhlaba municipality in the eastern cape, south Africa. Sustainability 2017, 9, 1651. [Google Scholar] [CrossRef]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic resistance increases with local temperature. Nat. Clim. Chang. 2018, 8, 510–514. [Google Scholar] [CrossRef]
- Hu, Y.F.; Yang, X.; Qin, J.; Lu, N.; Cheng, G.; Wu, N.; Pan, Y.L.; Li, J.; Zhu, L.Y.; Wang, X.; et al. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat. Commun. 2013, 4, 2151. [Google Scholar] [CrossRef] [Green Version]
- Crofts, T.S.; Gasparrini, A.J.; Dantas, G. Next-generation approaches to understand and combat the antibiotic resistome. Nature 2017, 15, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.J.; Akram, M.Z.; Batool, A.; Tian, L.Q.; Jan, S.U.; Boddula, R.; Guo, B.D.; et al. Antibacterial carbon-based nanomaterials. Adv. Mater. 2018, 30, 1804838. [Google Scholar] [CrossRef]
- Gao, T.Y.; Fan, H.B.; Wang, X.J.; Gao, Y.Y.; Liu, W.X.; Chen, W.J.; Dong, A.; Wang, Y.J. Povidone−iodine-based polymeric nanoparticles for antibacterial applications. ACS Appl. Mater. Interfaces 2017, 9, 25738–25746. [Google Scholar] [CrossRef]
- Konai, M.M.; Bhattacharjee, B.; Ghosh, S.; Haldar, J. Recent progress in polymer research to tckle infections and antimicrobial resistance. Biomacromolecules 2018, 19, 1888–1917. [Google Scholar] [CrossRef]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef]
- Eegene, C.; Palermo, E.F. Self-immolative polymers with potent and selective antibacterial activity by hydrophilic side chain grafting. J. Mater. Chem. B 2018, 6, 7217–7229. [Google Scholar]
- Gour, N.; Ngo, K.X.; Vebert-Nardin, C. Anti-infectious surfaces achieved by polymer modification. Macromol. Mater. Eng. 2014, 299, 648–668. [Google Scholar] [CrossRef]
- Ghosh, C.; Yadav, V.; Younis, W.; Mohammad, H.; Hegazy, Y.A.; Seleem, M.N.; Sanyal, K.; Haldar, J. Aryl-alkyl-lysines: Membrane-active fungicides that act against biofilms of Candida albicans. ACS Infect. Dis. 2017, 3, 293–301. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Konai, M.M.; Paramanandham, K.; Nimita, V.C.; Shome, B.R.; Haldar, J. In vivo efficacy and pharmacological properties of a novel glycopeptides (YV4465) against vancomycin-intermediate Staphylococcus aureus. Int. J. Antimicrob. Agents 2015, 46, 446–450. [Google Scholar] [CrossRef]
- Song, J.; Jang, J. Antimicrobial polymer nanostructures: Synthetic route, mechanism of action and perspective. Adv. Colloid Interface Sci. 2014, 203, 37–50. [Google Scholar] [CrossRef]
- Dong, A.; Lan, S.; Huang, J.F.; Wang, T.; Zhao, T.Y.; Xiao, L.H.; Wang, W.W.; Zheng, X.; Liu, F.Q.; Gao, G.; et al. Modifying Fe3O4-functionalized nanoparticles with N-halamine and their magnetic/antibacterial properties. ACS Appl. Mater. Interfaces 2011, 3, 4228–4235. [Google Scholar] [CrossRef]
- Cao, Z.B.; Sun, Y.Y. N-halamine-based chitosan: Preparation, characterization, and antimicrobial function. J. Biomed. Mater. Res. Part A 2007, 85, 99–107. [Google Scholar] [CrossRef]
- Li, R.; Dou, J.F.; Jiang, Q.Y.; Li, J.; Xie, Z.W.; Liang, J.; Ren, X.H. Preparation and antimicrobial activity of β-cyclodextrin derivative copolymers/cellulose acetate nanofibers. Chem. Eng. J. 2014, 248, 264–272. [Google Scholar] [CrossRef]
- Kocer, H.B.; Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Polymeric antimicrobial N-halamine epoxides. ACS Appl. Mater. Interfaces 2011, 3, 2845–2850. [Google Scholar] [CrossRef]
- Hui, F.; Debiemme-Chouvy, C. Antimicrobial N-halamine polymers and coatings: A review of their synthesis, characterization, and applications. Biomacromolecules 2013, 14, 585–601. [Google Scholar] [CrossRef]
- Akdag, A.; Okur, S.; McKee, M.L.; Worley, S.D. The stabilities of N-Cl bonds in biocidal materials. J. Chem. Theory Comput. 2006, 2, 879–884. [Google Scholar] [CrossRef]
- Kocer, H.B.; Akdag, A.; Worley, S.D.; Acevedo, O.; Broughton, R.M.; Wu, Y. Mechanism of photolytic decomposition of N-halamine antimicrobial siloxane coatings. ACS Appl. Mater. Interfaces 2010, 2, 2456–2464. [Google Scholar] [CrossRef]
- Cerkez, I.; Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Epoxide tethering of polymeric N-halamine moieties. Cellulose 2012, 19, 959–966. [Google Scholar] [CrossRef]
- Ren, H.; Du, Y.G.; Su, Y.Y.; Guo, Y.C.; Zhu, Z.W.; Dong, A. A review on recent achievements and current challenges in antibacterial electrospun N-halamines. Colloid Interface Sci. Commun. 2018, 24, 24–34. [Google Scholar] [CrossRef]
- Dong, A.; Wang, Y.J.; Gao, Y.Y.; Gao, T.Y.; Gao, G. Chemical insights into antibacterial N-halamines. Chem. Rev. 2017, 117, 4806–4862. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, G. Novel refreshable N-halamine polymeric biocides: Grafting hydantoin-containing monomers onto high performance fibers by a continous process. J. Appl. Polym. Sci. 2003, 88, 1032–1039. [Google Scholar] [CrossRef]
- Dong, A.; Sun, Y.; Lan, S.; Wang, Q.; Cai, Q.; Qi, X.Z.; Zhang, Y.L.; Gao, G.; Liu, F.Q.; Harnoode, C. Barbituric acid-based magnetic N-halamine nanoparticles as recyclable antibacterial agents. ACS Appl. Mater. Interfaces 2013, 5, 8125–8130. [Google Scholar] [CrossRef]
- Dong, A.; Huang, Z.; Lan, S.; Wang, Q.; Bao, S.; Zhang, Y.L.; Gao, G.; Liu, F.Q.; Harnoode, C. N-Halamine-decorated polystyrene nanoparticles based on 5-allylbarbituric acid: from controllable fabrication to bactericidal evaluation. J. Colloid Interface Sci. 2014, 413, 92–99. [Google Scholar] [CrossRef]
- Dong, A.; Xue, M.; Lan, S.; Wang, Q.; Zhao, Y.; Wang, Y.; Zhang, Y.L.; Gao, G.; Liu, F.Q.; Harnoode, C. Bactericidal evaluation of N-halamine-functionalized silica nanoparticles based on barbituric acid. Colloids Surf. B 2014, 113, 450–457. [Google Scholar] [CrossRef]
- Zhou, C.E.; Kan, C.W. Plasma-assisted regenerable chitosan antimicrobial finishing for cotton. Cellulose 2014, 21, 2951–2962. [Google Scholar] [CrossRef]
- Patel, G.C.; Patel, M.M. Preliminary evaluation of sesbania seed gum mucilage as gelling agent. Int. J. PharmTech Res. 2009, 1, 840–843. [Google Scholar]
- Ma, X.D.; Pawlik, M. Effect of alkali metal cations on adsorption of guar gum onto quartz. J. Colloid Interface Sci. 2005, 289, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, P.Y.; Li, X.; Cui, Y.C. Sesbania gum xanthate supported palladium complex as an efficient catalyst for heck peaction. J. Appl. Polym. Sci. 2007, 105, 2198–2202. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Y.; Zhai, Y.A.; Liu, F.Q.; Gao, G. Synthesis of Sesbania gum supported dithiocarbamate chelating resin and studies on its adsorption performance for metal ions. Carbohydr. Polym. 2008, 73, 359–363. [Google Scholar] [CrossRef]
- Pal, P.; Banerjee, A.; Halder, U.; Pandey, J.P.; Sen, G.; Bandopadhyay, R. Conferring antibacterial properties on sesbania gum via microwave-assisted graft copolymerization of DADMAC. J. Polym. Environ. 2018, 26, 3272–3282. [Google Scholar] [CrossRef]
- Lan, S.; Lu, Y.N.; Li, C.; Zhao, S.; Liu, N.R.; Sheng, X.L. Sesbania gum-supported hydrophilic electrospun fibers containing nanosilver with superior antibacterial activity. Nanomaterials 2019, 9, 592. [Google Scholar] [CrossRef]
- Bai, R.; Zhang, Q.; Li, L.; Li, P.; Wang, Y.; Simalou, O.; Zhang, Y.; Gao, G.; Dong, A. N-Halamine-containing electrospun fibers kill bacteria via a contact/release co-determined antibacterial pathway. ACS Appl. Mater. Interfaces 2016, 8, 31530–31540. [Google Scholar] [CrossRef]
- Bai, R.; Kang, J.; Simalou, O.; Liu, W.X.; Ren, H.; Gao, T.Y.; Gao, Y.Y.; Chen, W.J.; Dong, A.; Jia, R. Novel N−Br bond-containing N-halamine nanofibers with antibacterial activities. ACS Biomater. Sci. Eng. 2018, 4, 2193–2202. [Google Scholar] [CrossRef]
- Verma, S.; Ahuja, M. Carboxymethyl sesbania gum: Synthesis, characterization and evaluation for drug delivery. Int. J. Biol. Macromol. 2017, 98, 75–83. [Google Scholar] [CrossRef]
- Li, R.; Jia, X.; Wang, Y.; Li, Y.; Cheng, Y. The effects of extrusion processing on rheological and physicochemical properties of sesbania gum. Food Hydrocoll. 2019, 90, 35–40. [Google Scholar] [CrossRef]
- Dong, A.; Lan, S.; Huang, J.F.; Wang, T.; Zhao, T.Y.; Wang, W.W.; Xiao, L.H.; Zheng, X.; Liu, F.Q.; Gao, G.; et al. Preparation of magnetically separable N-halamine nanocomposites for the improved antibacterial application. J. Colloid Interface Sci. 2011, 364, 333–340. [Google Scholar] [CrossRef]
- Jie, Z.Q.; Yan, X.F.; Zhao, L.H.; Worley, S.D.; Liang, J. Eco-friendly synthesis of regenerable antimicrobial polymeric resin with N-halamine and quaternary ammonium salt groups. RSC Adv. 2014, 4, 6048–6054. [Google Scholar] [CrossRef]
- Jarusuwannapoom, T.; Hongrojjanawiwat, W.; Jitjaicham, S.; Wannatong, L.; Nithitanakul, M.; Pattamaprom, C.; Koombhongse, P.; Rangkupan, R.; Supaphol, P. Effects of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur. Polym. J. 2005, 41, 409–421. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Electrospinning of ultrafine cellulose acetate fibers: Studies of a new solvent system and deacetylation of ultrafine cellulose acetate fibers. J. Polym. Sci. B Polym. Phys. 2004, 42, 5–11. [Google Scholar] [CrossRef]
- Majumder, S.; Matin, M.A.; Sharif, A.; Arafat, M.T. Understanding solubility, spinnability and electrospinning behavior of cellulose acetate using different solvent systems. Bull. Mater. Sci. 2019, 42, 171. [Google Scholar] [CrossRef]
- Tungprapa, S.; Puangparn, T.; Weerasombut, M.; Jangchud, I.; Fakum, P.; Semongkhol, S.; Meechaisue, C.; Supaphol, P. Electrospun cellulose acetate fibers: Effect of solvent system on morphology and fiber diameter. Cellulose 2007, 14, 563–575. [Google Scholar] [CrossRef]
- Tiyek, I.; Gunduz, A.; Yalcinkaya, F.; Chaloupek, J. Influence of electrospinning parameters on the hydrophilicity of electrospun polycaprolactone nanofibers. J. Nanosci. Nanotechnol. 2019, 19, 7251–7260. [Google Scholar] [CrossRef]
- Ren, X.H.; Akdag, A.; Zhu, C.Y.; Kou, L.; Worley, S.D.; Huang, T.S. Electrospun polyacrylonitrile nanofibrous biomaterials. J. Biomed. Mater. Res. Part A 2009, 91, 385–390. [Google Scholar] [CrossRef]
- Sun, X.B.; Cao, Z.B.; Porteous, N.; Sun, Y.Y. An N-Halamine-based rechargeable antimicrobial and biofilm controlling polyurethane. Acta Biomater. 2012, 8, 1498–1506. [Google Scholar] [CrossRef]
- Li, C.H.; Hou, J.J.; Huang, Z.; Zhao, T.Y.; Xiao, L.H.; Gao, G.; Harnoode, C.; Dong, A. Assessment of 2,2,6,6-tetramethyl-4-piperidinol-base amine N-halamine-labeled silica nanoparticles as potent antibiotics for deactivating bacteria. Colloids Surf. B 2015, 126, 106–114. [Google Scholar] [CrossRef]
- Cerkez, I.; Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. N-halamine copolymers for biocidal coatings. React. Funct. Polym. 2012, 72, 673–679. [Google Scholar] [CrossRef]
- Cai, Q.; Bao, S.; Zhao, Y.; Zhao, T.Y.; Xiao, L.H.; Gao, G.; Chokto, H.; Dong, A. Tailored synthesis of amine N-halamine copolymerized polystyrene with capability of killing bacteria. J. Colloid Interface Sci. 2015, 444, 1–9. [Google Scholar] [CrossRef]
- Cai, Q.; Gao, Y.; Gao, T.; Lan, S.; Simalou, O.; Zhou, X.; Zhang, Y.; Harnoode, C.; Gao, G.; Dong, A. Insight into biological effects of zinc oxide nanoflowers on bacteria: Why morphology matters. ACS Appl. Mater. Interfaces 2016, 8, 10109–10120. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, S.; Lu, Y.; Zhang, J.; Guo, Y.; Li, C.; Zhao, S.; Sheng, X.; Dong, A. Electrospun Sesbania Gum-Based Polymeric N-Halamines for Antibacterial Applications. Polymers 2019, 11, 1117. https://doi.org/10.3390/polym11071117
Lan S, Lu Y, Zhang J, Guo Y, Li C, Zhao S, Sheng X, Dong A. Electrospun Sesbania Gum-Based Polymeric N-Halamines for Antibacterial Applications. Polymers. 2019; 11(7):1117. https://doi.org/10.3390/polym11071117
Chicago/Turabian StyleLan, Shi, Yaning Lu, Jinghua Zhang, Yanan Guo, Chun Li, Shuang Zhao, Xianliang Sheng, and Alideertu Dong. 2019. "Electrospun Sesbania Gum-Based Polymeric N-Halamines for Antibacterial Applications" Polymers 11, no. 7: 1117. https://doi.org/10.3390/polym11071117




