On the Origin of Alkali-Catalyzed Aromatization of Phenols
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparations of Sodium Phenolates
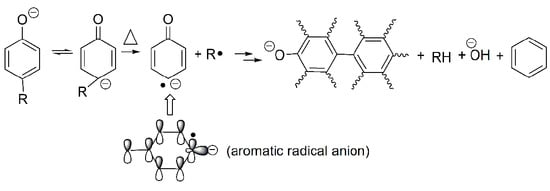
2.3. Characterizations
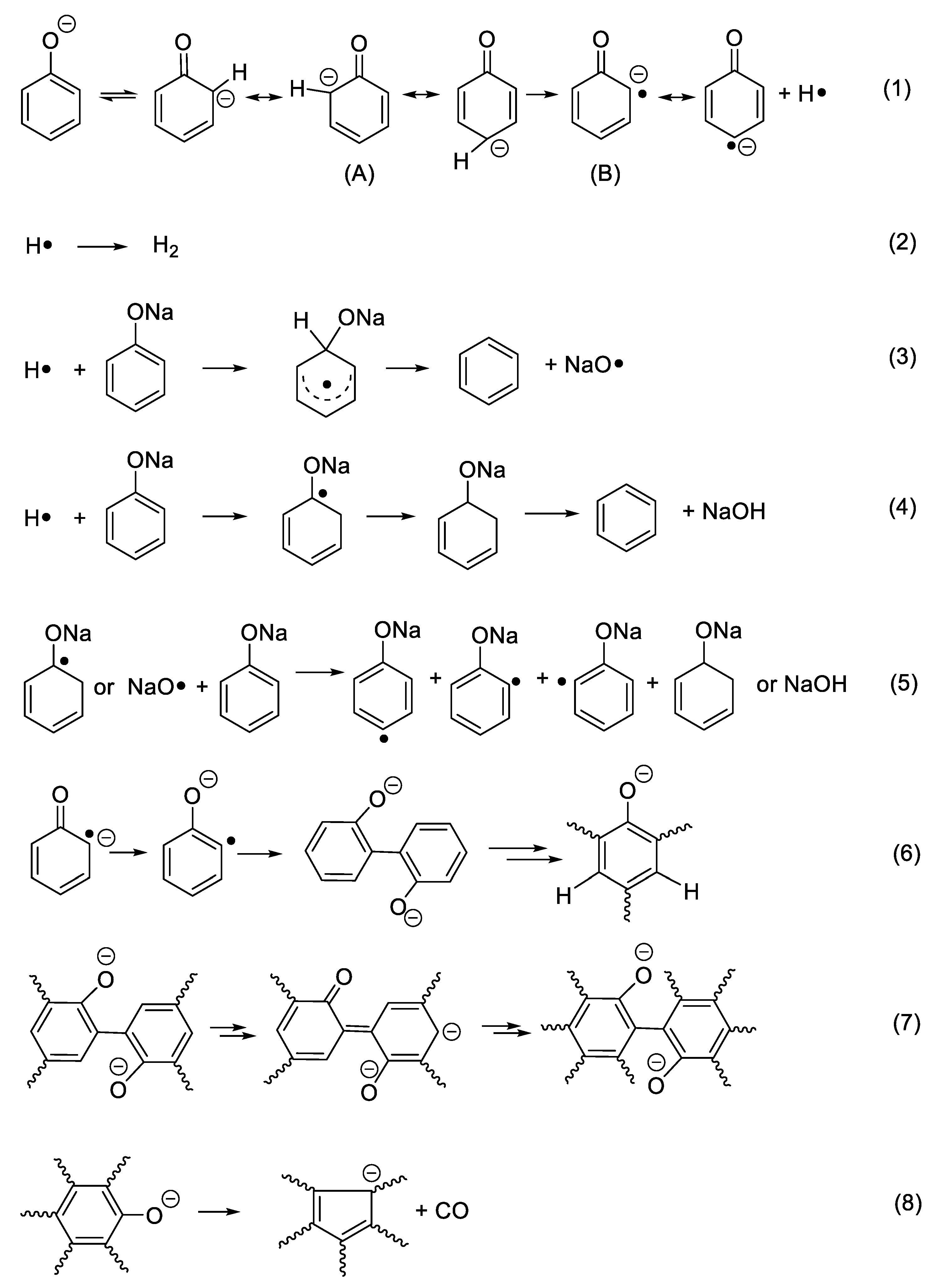
3. Results and Discussion
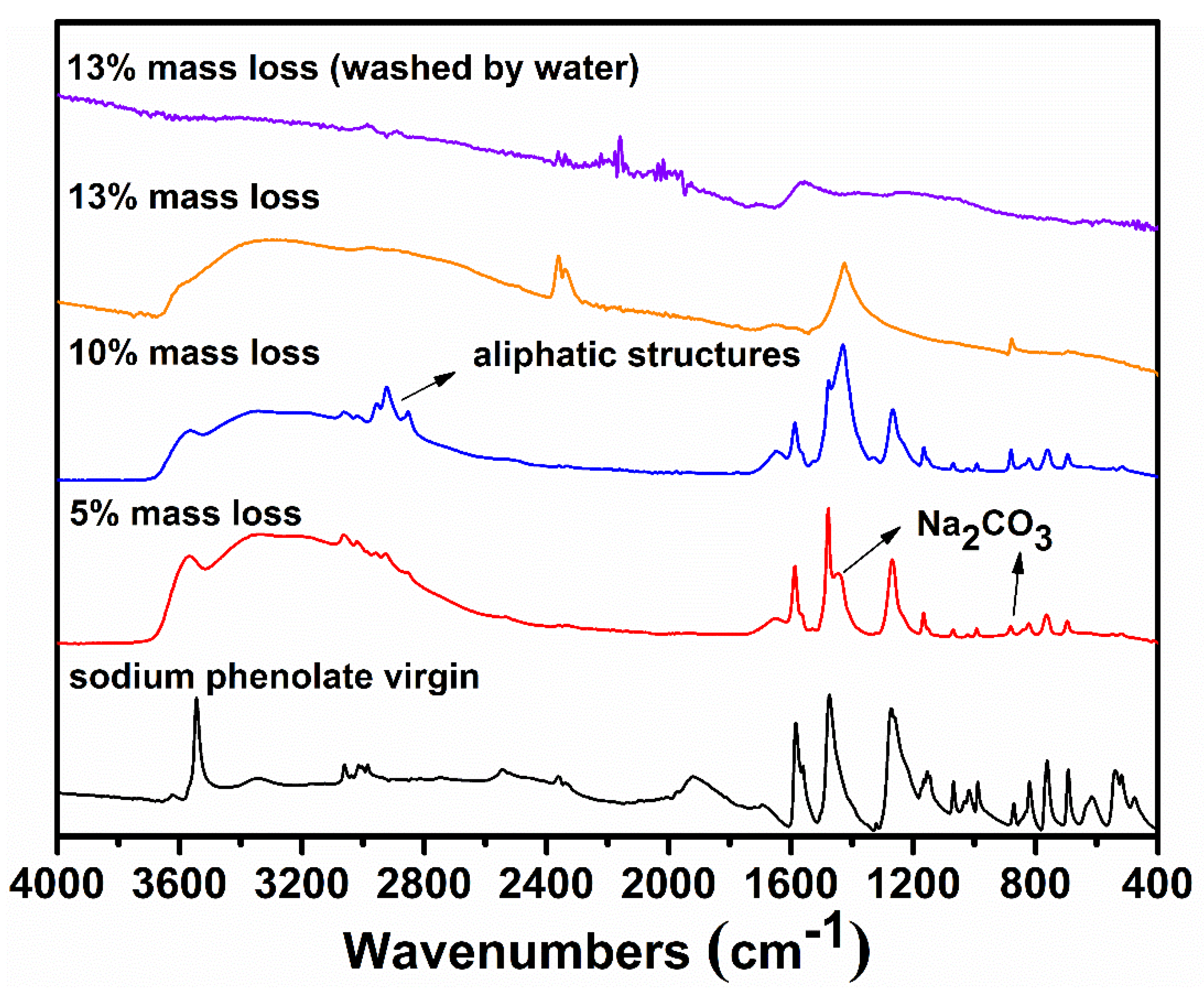
3.1. TGA of Sodium Phenolates
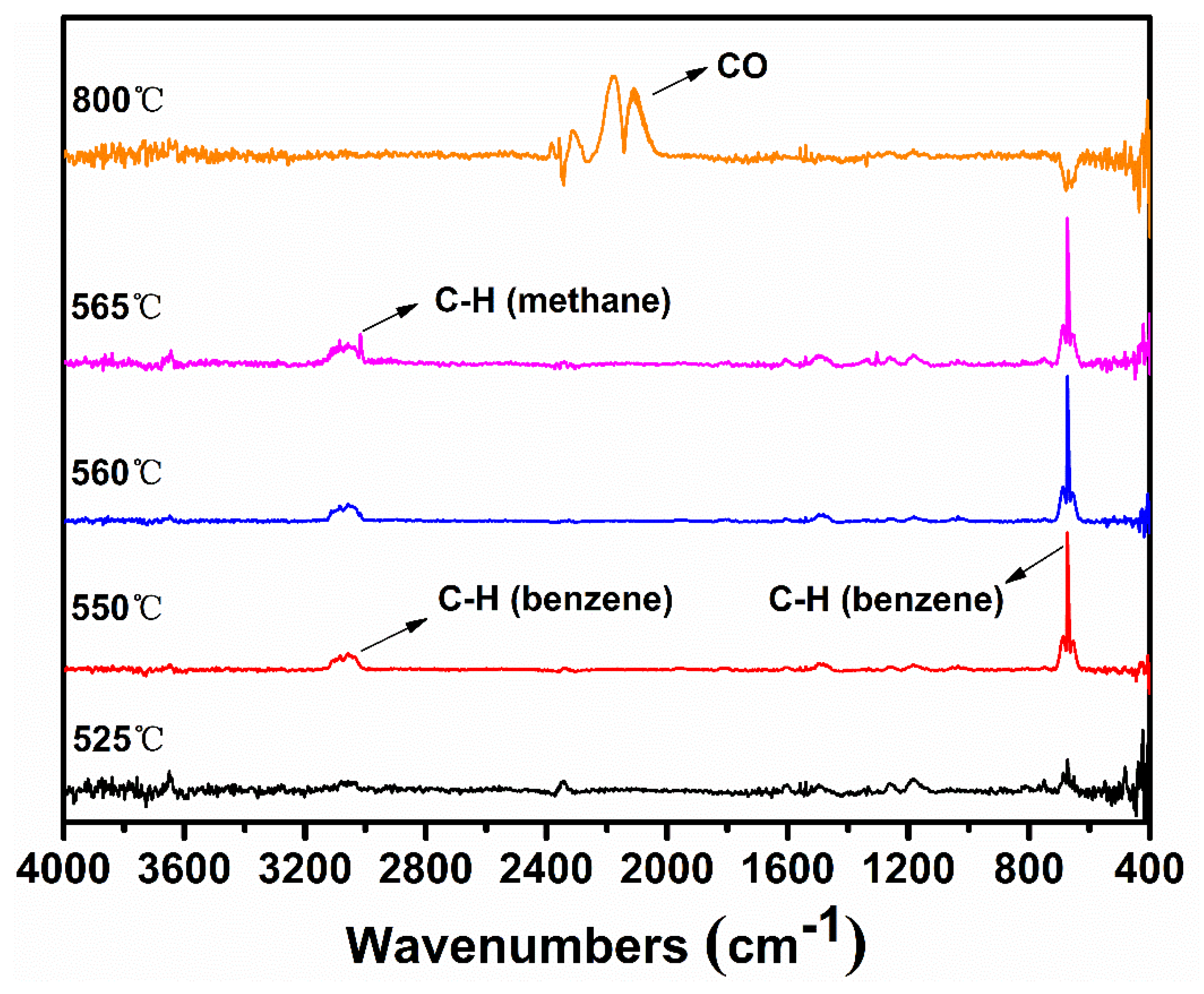
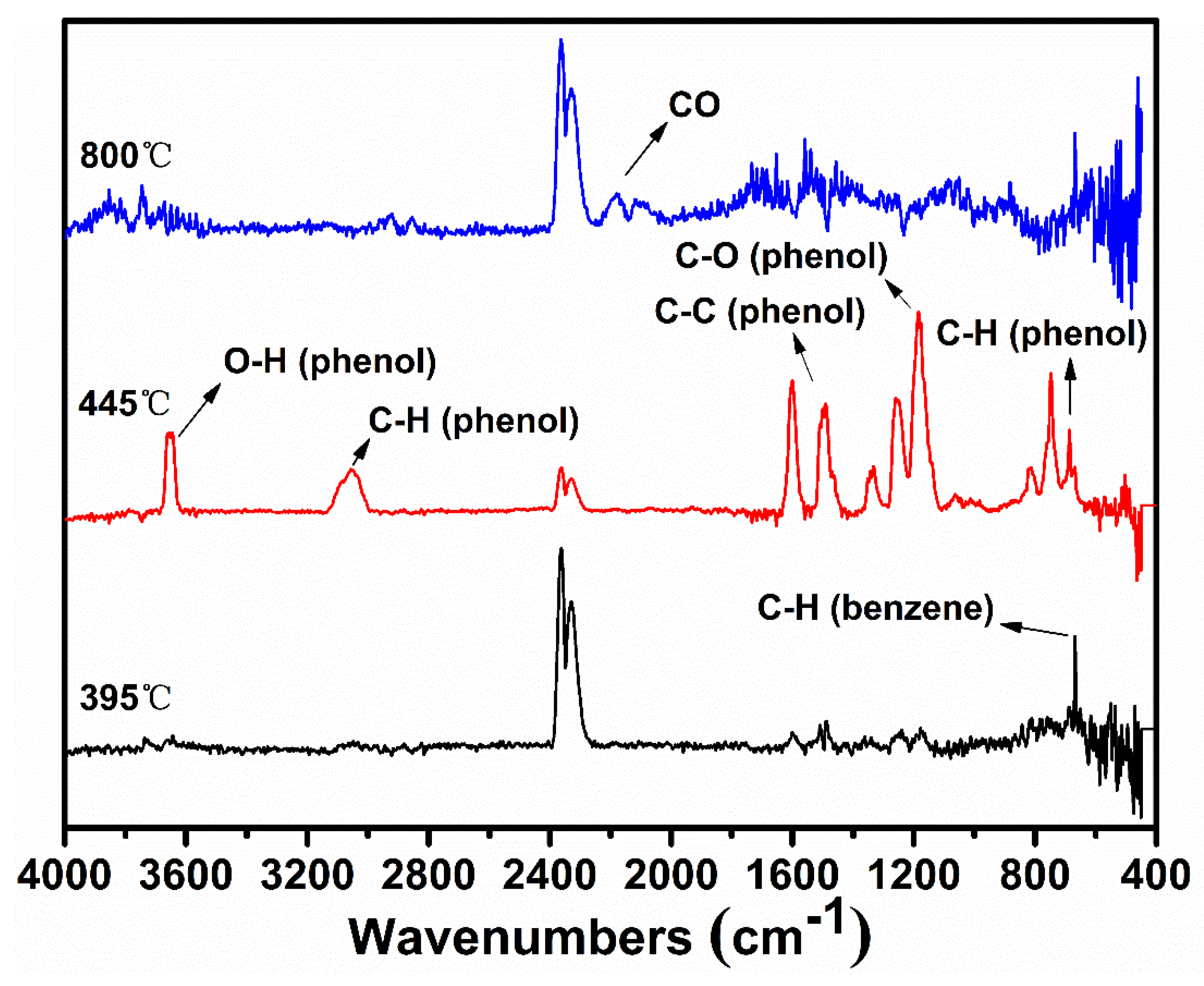
3.2. TGA-FTIR of Phenolates
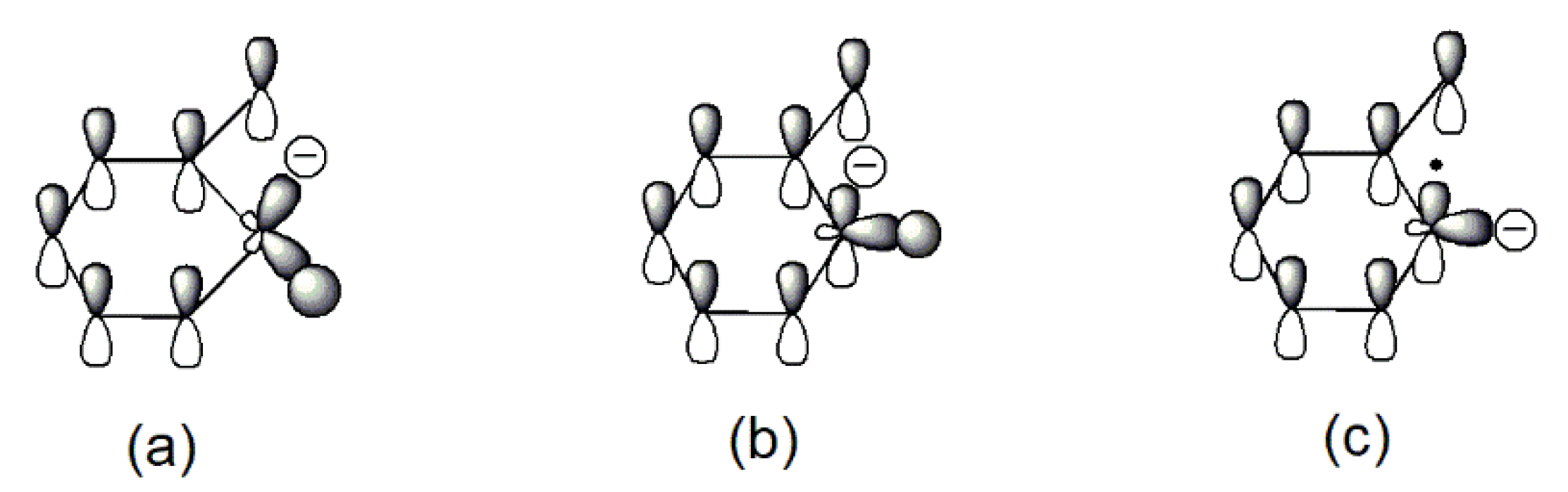
3.2.1. Sodium Phenolate
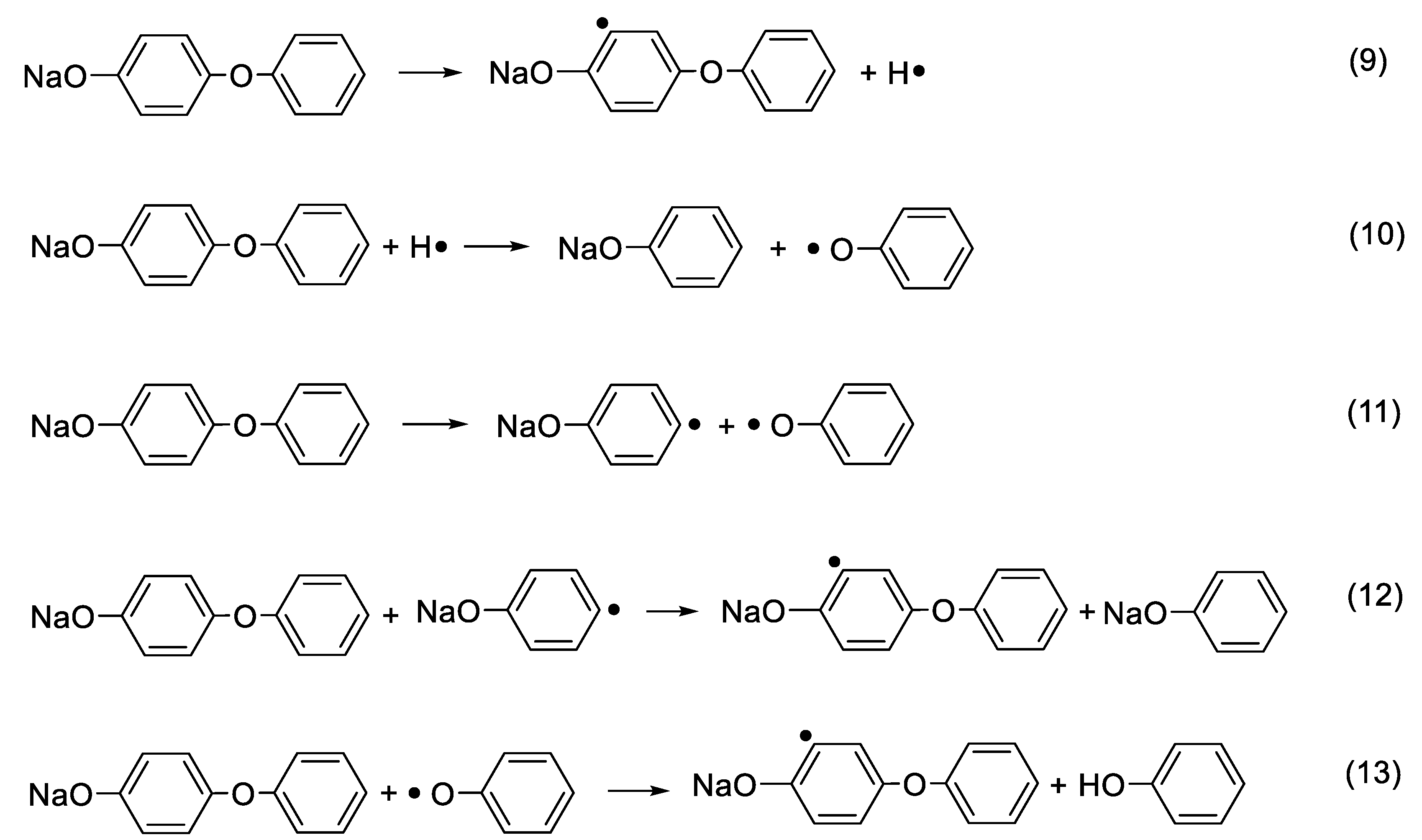
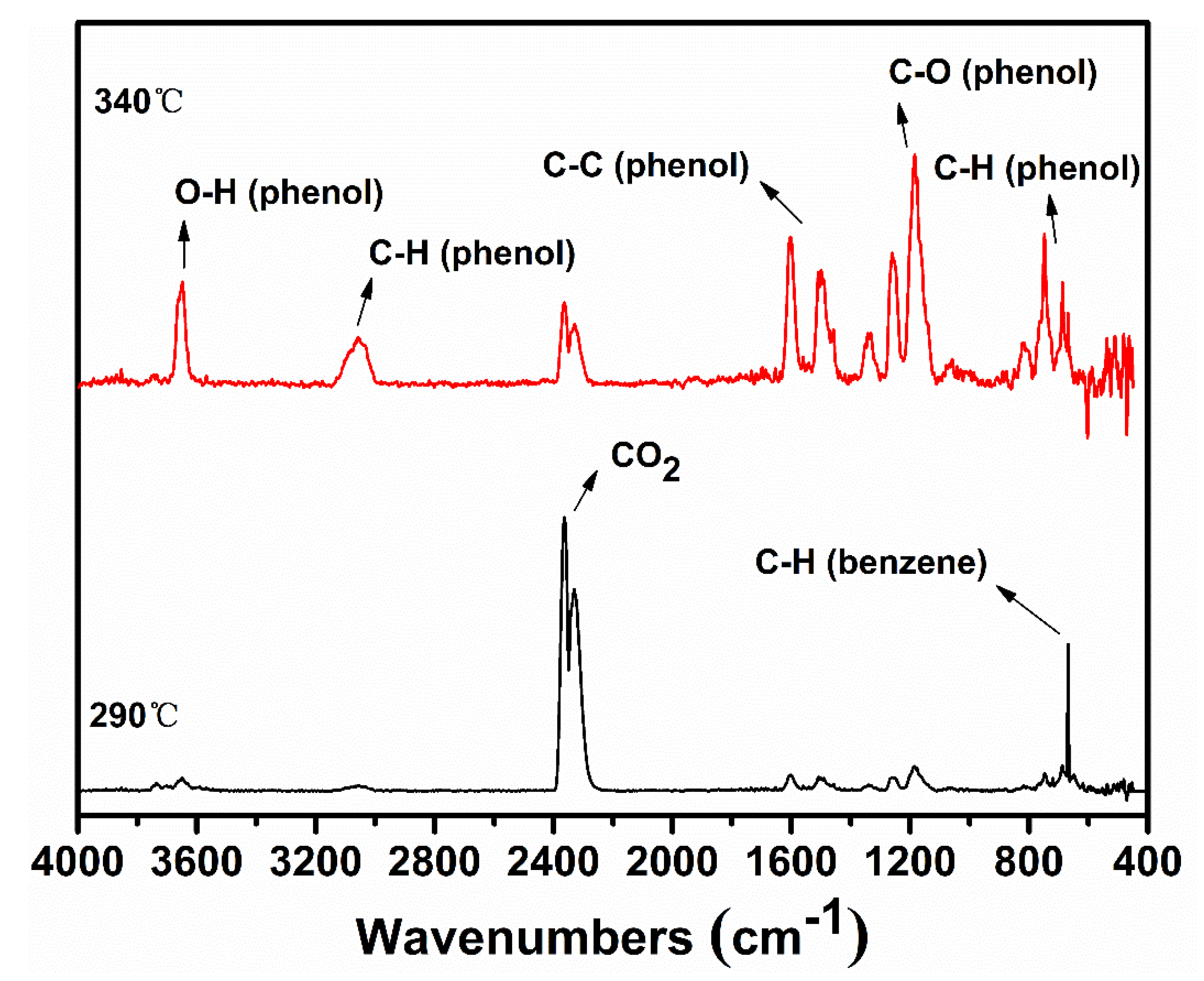
3.2.2. Sodium p-Phenoxyphenolate
3.2.3. Sodium p-Cresolate
3.2.4. Sodium m-Cresolate
3.2.5. Sodium Salicylate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gong, J.; Chen, X.C.; Tang, T. Recent progress in controlled carbonization of (waste) polymers. Prog. Polym. Sci. 2019, 94, 1–32. [Google Scholar] [CrossRef]
- Brech, Y.L.; Raya, J.; Delmotte, L.; Brosse, N.; Gadiou, R.; Dufour, A. Characterization of biomass char formation investigated by advanced solid state NMR. Carbon 2016, 108, 165–177. [Google Scholar] [CrossRef]
- Ballistreri, A.; Montaudo, G.; Scamporrino, E.; Puglisi, C.; Vitalini, D.; Cucinella, S. Intumescent flame retardants for polymers. IV. The polycarbonate-aromatic sulfonates system. J. Polym. Sci., part A: Polym. Chem. 1988, 26, 2113–2127. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Sekiguchi, Y. Development of aromaticity in cellulosic chars. Carbon 1983, 21, 511–516. [Google Scholar] [CrossRef]
- Huang, X.B.; Ouyang, X.Y.; Ning, F.L.; Wang, J.Q. Mechanistic study on flame retardance of polycarbonate with a small amount of potassium perfluorobutane sulfonate by TGA-FTIR/XPS. Polym. Degrad. Stab. 2006, 91, 606–613. [Google Scholar] [CrossRef]
- Lv, D.Z.; Xu, M.H.; Liu, X.; Zhan, Z.H.; Li, Z.Y.; Yao, H. Effect of cellulose, lignin, alkali and alkaline earth metallic species on biomass pyrolysis and gasification. Fuel Process. Technol. 2010, 91, 903–909. [Google Scholar] [CrossRef]
- Chen, M.Q.; Wang, J.; Zhang, M.X.; Chen, M.G.; Zhu, X.F.; Min, F.F.; Tan, Z.C. Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J. Anal. Appl. Pyrolysis. 2008, 82, 145–150. [Google Scholar] [CrossRef]
- Nowakowski, D.J.; Jones, J.M. Uncatalysed and potassium-catalysed pyrolysis of the cell-wall constituents of biomass and their model compounds. J. Anal. Appl. Pyrolysis. 2008, 83, 12–25. [Google Scholar] [CrossRef]
- Rutkowski, P. Pyrolysis of cellulose, xylan and lignin with the K2CO3 and ZnCl2 addition for bio-oil production. Fuel Process. Technol. 2011, 92, 517–522. [Google Scholar] [CrossRef]
- Oba, K.; Ishida, Y.; Ito, Y.; Ohtani, H.; Tsuge, S. Characterization of branching and/or cross-linking structures in polycarbonate by reactive pyrolysis-gas chromatography in the presence of organic alkali. Macromolecules 2000, 33, 8173–8183. [Google Scholar] [CrossRef]
- Bailly, C.; Daumerie, M.; Legras, R.; Mercier, J.P. Kinetics of the thermal degradation of anhydrous bisphenol-A polycarbonate/alkali metal arylcarboxylate systems in the melt. Makromol Chem. Phys. 1986, 187, 1197–1214. [Google Scholar] [CrossRef]
- Patwardhan, P.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef]
- Peng, C.N.; Zhang, G.Y.; Yue, J.R.; Xu, G.W. Pyrolysis of lignin for phenols with alkaline additive. Fuel Prococess. Technol. 2014, 124, 212–221. [Google Scholar] [CrossRef]
- Politou, A.S.; Morterra, C.; Low, M.J.D. Infrared studies of carbons. XII the formation of chars from a polycarbonate. Carbon 1990, 28, 529–538. [Google Scholar] [CrossRef]
- Hu, J.; Shen, D.K.; Wu, S.L.; Zhang, H.Y.; Xiao, R. Effect of temperature on structure evolution in char from hydrothermal degradation of lignin. J. Anal. Appl. Pyrolysis. 2014, 106, 118–124. [Google Scholar] [CrossRef]
- Xin, S.Z.; Yang, H.P.; Chen, Y.Q.; Yang, M.F.; Chen, L.; Wang, X.H.; Chen, H.P. Chemical structure evolution of char during the pyrolysis of cellulose. J. Anal. Appl. Pyrolysis. 2015, 116, 263–271. [Google Scholar] [CrossRef]
- McMillen, D.F.; Golden, D.M. Hydrocarbon bond dissociation energies. Annu. Rev. Phys. Chem. 1982, 33, 493–532. [Google Scholar] [CrossRef]
- Shi, J.; Huang, X.Y.; Wang, J.P.; Li, R. A theoretical study on C-COOH homolytic bond dissociation enthalpies. J. Phys. Chem. A. 2010, 114, 6263–6272. [Google Scholar] [CrossRef]
- Manion, J.A.; McMillen, D.F.; Malhotra, R. Decarboxylation and coupling reactions of aromatic acids under coal-liquefaction conditions. Energy Fuels 1996, 10, 776–788. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Pyrolysis of organic molecules with applications to health and environmental Issues. In Pyrolysis of Alcohols and Phenols; Moldoveanu, S.C., Ed.; Elsevier: Amsterdam, the Netherlands, 2010; pp. 259–313. [Google Scholar]
- Cypres, R.; Bettens, B. Mecanismes de fragmentation pyrolytique du phenol et des cresols. Tetrahedron 1974, 30, 1253–1260. [Google Scholar] [CrossRef]
- Scheer, A.M.; Mukarakate, C.; Robichaud, D.J.; Nimlos, M.R.; Carstensen, H.H.; Ellison, G.B. Unimolecular thermal decomposition of phenol and d(5)-phenol: Direct observation of cyclopentadiene formation via cyclohexadienone. J. Chem. Phys. 2012, 136, 044309. [Google Scholar] [CrossRef] [PubMed]
- Egger, K.W.; Cocks, A.T. Homopolar- and heteropolar bond dissociation energies and heats of formation of radicals and ions in the gas phase. I. Data on organic molecules. Helv. Chim. Acta. 1973, 56, 1516–1536. [Google Scholar] [CrossRef]
- Lovell, A.B.; Brezinsky, K.; Glassman, I. The gas phase pyrolysis of phenol. Int. J. Chem. Kinet. 1989, 21, 547–560. [Google Scholar] [CrossRef]
- Manion, J.A.; Louw, R. Rates, products, and mechanisms in the gas-phase hydrogenolysis of phenol between 922 and 1175K. J. Phys. Chem. 1989, 93, 3563–3574. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Bresch, S.; Gomes, G.D.P. Orbital hybridization: A key electronic factor in control of structure and reactivity. J. Phys. Org. Chem. 2015, 28, 147–162. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. Advanced Organic Chemistry, 5th ed.; A Wiley-Interscience Publication: New York, NY, USA, 2001; p. 42. [Google Scholar]
- Smith, M.B.; March, J. Advanced Organic Chemistry, 5th ed.; A Wiley-Interscience Publication: New York, NY, USA, 2001; p. 244. [Google Scholar]
- Gomez-Serrano, V.; Villegas, J.P.; Perez-Florindo, A.; Duran-Valle, C.; Valenzuela-Calahorro, C. FT-IR study of rockrose and of char and activated carbon. J. Anal. Appl. Pyrolysis. 1996, 36, 71–80. [Google Scholar] [CrossRef]
- Horrocks, A.R. Developments in flame retardants for heat and fire resistant textiles–the role of char formation and intumescence. Polym. Degrad. Stab. 1996, 54, 143–154. [Google Scholar] [CrossRef]
- Innes, J.; Innes, A. Flame retardants for polycarbonate–new and classical solutions. Plast. Addit. Compd. 2006, 8, 26–29. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. A TGA/FTIR and mass spectral study on the thermal degradation of bisphenol A polycarbonate. Polym. Degrad. Stab. 2004, 86, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Brezinsky, K.; Pecullan, M.; Glassman, I. Pyrolysis and oxidation of phenol. J. Phys. Chem. A. 1998, 102, 8614–8619. [Google Scholar] [CrossRef]
- Horn, C.; Roy, K.; Frank, P.; Just, T. Shock-tube study on the high-temperature pyrolysis of phenol. Symp. (Int.) Combust., [Proc.] 1998, 27, 321–328. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Pyrolysis of organic molecules with applications to health and environmental Issues. In Pyrolysis of Ethers; Moldoveanu, S.C., Ed.; Elsevier: Amsterdam, the Netherlands, 2010; Volume 28, pp. 315–338. [Google Scholar]
- Olivella, S.; Sole, A.; Garcia-Raso, A. Ab-initio calculations of the potential surface for the thermal decomposition of the phenoxyl radical. J. Phys. Chem. 1995, 99, 10549–10556. [Google Scholar] [CrossRef]
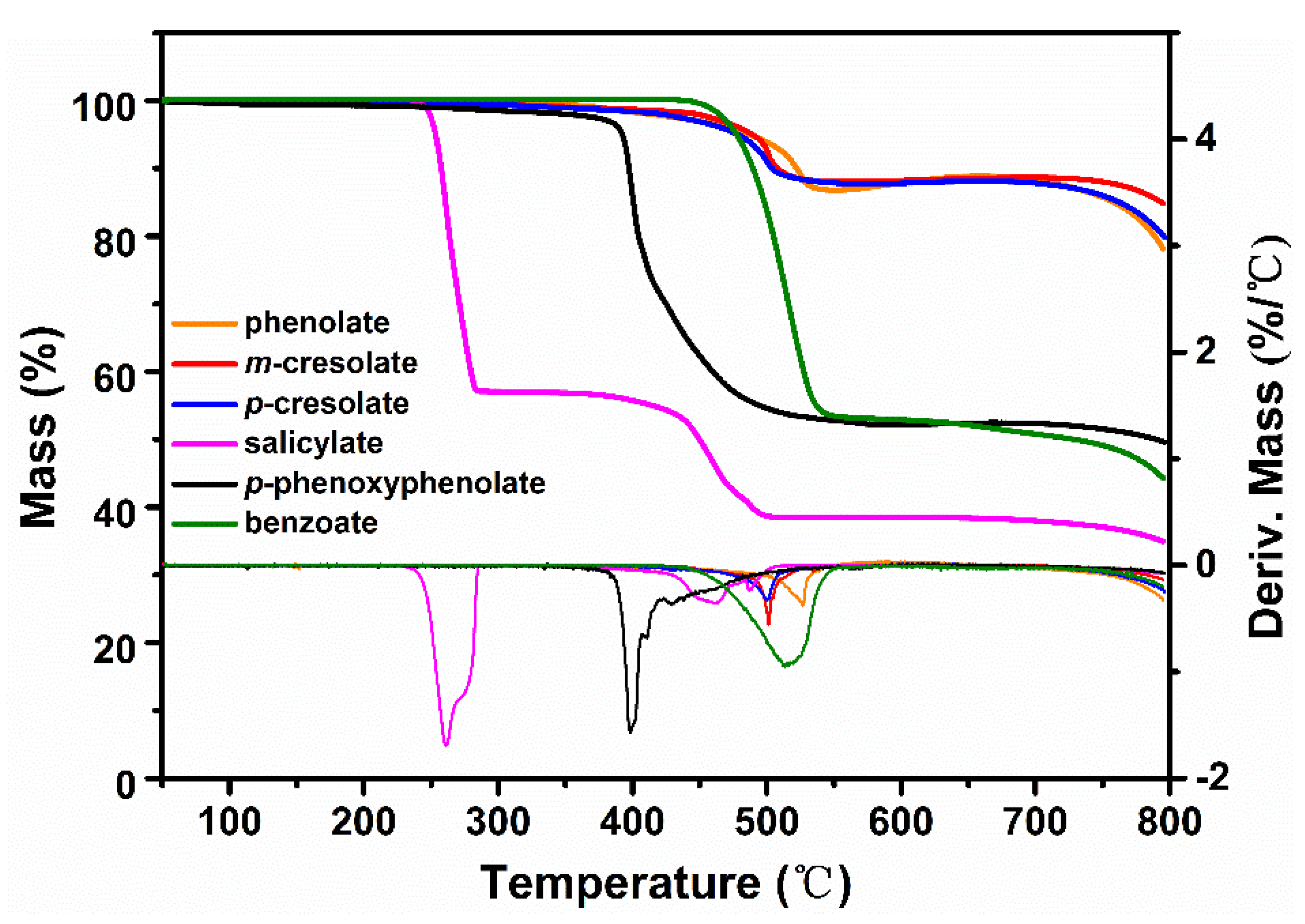



| Sodium salts | T1% (°C) | T5% (°C) | Tmax (°C) | Mass Loss (%) |
|---|---|---|---|---|
| phenolate | 375 | 487 | 527 | 13.2 |
| m-cresolate | 359 | 489 | 501 | 13.0 |
| p-cresolate | 344 | 478 | 500 | 13.2 |
| p-phenoxyphenolate | 253 | 392 | 400 | 47.0 |
| salicylate | 246 | 253 | 261 | 42.9 |
| benzoate | 457 | 477 | 516 | 46.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Yao, Q.; Zhao, Y.; Cao, W. On the Origin of Alkali-Catalyzed Aromatization of Phenols. Polymers 2019, 11, 1119. https://doi.org/10.3390/polym11071119
Ji Y, Yao Q, Zhao Y, Cao W. On the Origin of Alkali-Catalyzed Aromatization of Phenols. Polymers. 2019; 11(7):1119. https://doi.org/10.3390/polym11071119
Chicago/Turabian StyleJi, Yu, Qiang Yao, Yueying Zhao, and Weihong Cao. 2019. "On the Origin of Alkali-Catalyzed Aromatization of Phenols" Polymers 11, no. 7: 1119. https://doi.org/10.3390/polym11071119