Fabrication and Properties of a Bio-Based Biodegradable Thermoplastic Polyurethane Elastomer
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experimental Instruments
2.3. Experimental Methods
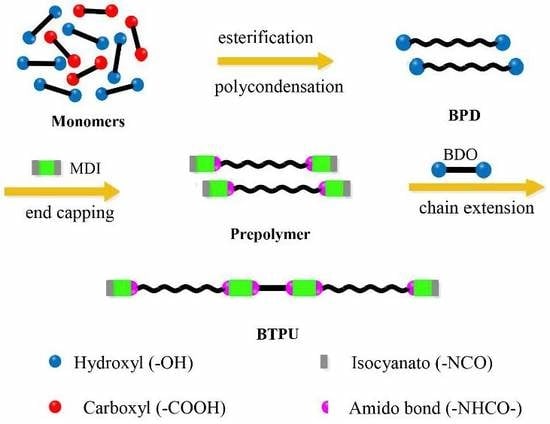
2.3.1. Synthesis of Biodegradable Polyester Diols (BPD)
2.3.2. Fabrication of BTPU
2.4. Characterization and Testing Methods
2.4.1. Analysis by Fourier Transform Infrared (FTIR) Spectrometer
2.4.2. Analysis by Proton Nuclear Magnetic Resonance (1H-NMR) Spectrometer
2.4.3. Analysis by Gel Permeation Chromatograph
2.4.4. Atomic Force Microscope (AFM) Observation
2.4.5. Mechanical Property Test
2.4.6. Thermal Property Test
2.4.7. Biodegradation Performance Test
3. Results and Discussion
3.1. Structural Characterization of BPD and BTPU
3.1.1. FTIR Analysis of BPD and BTPU
3.1.2. H-NMR Analysis of BPD and BTPU
3.1.3. Molecular Weight and Molecular Weight Distribution of BTPU
3.2. Micromorphological Structure of BTPU
3.3. Mechanical Properties of BTPU
3.4. Thermal Properties of BTPU
3.4.1. Glass Transition Temperature (Tg) and Melting Temperature (Tm) of BTPU
3.4.2. Thermal Stability of BTPU
3.5. Degradation Property of BTPU
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eceiza, A.; Larrañaga, M.; De La Caba, K.; Kortaberria, G.; Marieta, C.; Corcuera, M.; Mondragon, I. Structure-property relationships of thermoplastic polyurethane elastomers based on polycarbonate diols. J. Appl. Polym. Sci. 2010, 108, 3092–3103. [Google Scholar] [CrossRef]
- Eceiza, A.; Martin, M.; De La Caba, K.; Kortaberria, G.; Gabilondo, N.; Corcuera, M.; Mondragon, I. Thermoplastic polyurethane elastomers based on polycarbonate diols with different soft segment molecular weight and chemical structure: Mechanical and thermal properties. Polym. Eng. Sci. 2008, 48, 297–306. [Google Scholar] [CrossRef]
- Kultys, A.; Rogulska, M.; Głuchowska, H. The effect of soft-segment structure on the properties of novel thermoplastic polyurethane elastomers based on an unconventional chain extender. Polym. Int. 2011, 60, 652–659. [Google Scholar] [CrossRef]
- Buckley, C.P.; Prisacariu, C.; Martin, C. Elasticity and inelasticity of thermoplastic polyurethane elastomers: Sensitivity to chemical and physical structure. Polymer. 2010, 51, 3213–3224. [Google Scholar] [CrossRef]
- Qi, H.J.; Boyce, M.C. Stress-strain behavior of thermoplastic polyurethanes. Mech. Mater. 2005, 37, 817–839. [Google Scholar] [CrossRef]
- Hill, D.J.T.; Killeen, M.I.; O’Donnell, J.H.; Pomerya, P.J.; St John, D.; Whittaker, A.K. Laboratory wear testing of polyurethane elastomers. Wear 1997, 208, 155–160. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Nachman, M. The abrasive wear resistance of the segmented linear polyurethane elastomers based on a variety of polyols as soft segments. Polymers 2017, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Huang, Y.; Tang, L.; Hong, Y. Low-initial-modulus biodegradable polyurethane elastomers for soft tissue regeneration. ACS Appl. Mater. Interfaces 2017, 9, 2169–2180. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H.; Sienkiewicz, M. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater. Sci. Eng. C 2015, 46, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Schollenberger, C.S.; Dinbergs, K.; Stewart, F.D. Thermoplastic polyurethane elastomer melt polymerization study. Rubber Chem. Technol. 1982, 55, 137–150. [Google Scholar] [CrossRef]
- Im, H.G.; Ka, K.R.; Kim, C.K. Characteristics of polyurethane elastomer blends with poly(acrylonitrile-co-butadiene) rubber as an encapsulant for underwater sonar devices. Ind. Eng. Chem. Res. 2010, 49, 7336–7342. [Google Scholar] [CrossRef]
- Feng, F.; Ye, L. Morphologies and mechanical properties of polylactide/thermoplastic polyurethane elastomer blends. J. Appl. Polym. Sci. 2011, 119, 2778–2783. [Google Scholar] [CrossRef]
- Tan, L.-C.; Su, Q.-X.; Zhang, S.-D.; Huang, H.-X. Preparing thermoplastic polyurethane/thermoplastic starch with high mechanical and biodegradable properties. RSC Adv. 2015, 5, 80884–80892. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Zhang, C.; Kessler, M.R. Bio-based polyurethane foam made from compatible blends of vegetable-oil-based polyol and petroleum-based polyol. ACS Sustain. Chem. Eng. 2015, 3, 743–749. [Google Scholar] [CrossRef]
- Cregut, M.; Bedas, M.; Durand, M.J.; Thouand, G. New insights into polyurethane biodegradation and realistic prospects for the development of a sustainable waste recycling process. Biotechnol. Adv. 2013, 31, 1634–1647. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, F.; Su, R.; Qi, W.; He, Z. Ethanol production from high dry matter corncob using fed-batch simultaneous saccharification and fermentation after combined pretreatment. Bioresour. Technol. 2010, 10, 4959–4964. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Ryu, C.; Gang, K.S.; Yang, W.; Park, Y.K.; Jung, J.; Hyun, S. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Bioresour. Technol. 2013, 148, 196–201. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Jang, Y.-S.; Kim, B.; Shin, J.H.; Choi, Y.J.; Choi, S.; Song, C.-W.; Lee, J.; Park, H.G.; Lee, S.Y. Bio-based production of C2–C6 platform chemicals. Biotechnol. Bioeng. 2012, 109, 2437–2459. [Google Scholar] [CrossRef]
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: current state and prospects. Appl. Microbiol. Biotechnol. 2006, 69, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Christiane, N.; Yves, P.; Somerville, C. Plant polymers for biodegradable plastics: Cellulose, starch and polyhydroxyalkanoates. Mol. Breed. 1995, 1, 105–122. [Google Scholar] [CrossRef]
- Williams, C.K.; Hillmyer, M.A. Polymers from renewable resources: a perspective for a special issue of polymer reviews. Polym. Rev. 2008, 48, 1–10. [Google Scholar] [CrossRef]
- Amass, W.; Amass, A.; Tighe, B. A review of biodegradable polymers: uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Schmidt, S.; Ritter, B.S.; Kratzert, D.; Bruchmann, B.; Mülhanupt, R. Isocyanate-free route to poly (carbohydrate–urethane) thermosets and 100% bio-based coatings derived from glycerol feedstock. Macromolecules 2016, 49, 7268–7276. [Google Scholar] [CrossRef]
- Guo, W.-H.; Shen, Z.-L.; Guo, B.-C.; Zhang, L.-Q.; Jia, D.-M. Synthesis of bio-based copolyester and its reinforcement with zinc diacrylate for shape memory application. Polymer 2014, 55, 4324–4331. [Google Scholar] [CrossRef]
- Ferreira-Leitao, V.; Gottschalk, L.M.F.; Ferrara, M.A.; Nepomuceno, A.L.; Molinari, H.B.C.; Bon, E.P. Biomass residues in Brazil: availability and potential uses. Waste Biomass Valoriz. 2010, 1, 65–76. [Google Scholar] [CrossRef]
- Garcia-Nunez, J.A.; Ramirez-Contreras, N.E.; Rodriguez, D.T.; Silva-Lora, E.; Frear, C.S.; Stockle, C.; Garcia-Perez, M. Evolution of palm oil mills into bio-refineries: literature review on current and potential uses of residual biomass and effluents. Resour. Conserv. Recycl. 2016, 110, 99–114. [Google Scholar] [CrossRef]
- Ng, W.S.; Lee, C.S.; Chuah, C.H.; Cheng, S.F. Preparation and modification of water-blown porous biodegradable polyurethane foams with palm oil-based polyester polyol. Ind. Crop. Prod. 2017, 97, 65–78. [Google Scholar] [CrossRef]
- Kucharczyk, P.; Pavelková, A.; Stloukal, P.; Sedlarík, V. Degradation behaviour of PLA-based polyesterurethanes under abiotic and biotic environments. Polym. Degrad. Stabil. 2016, 129, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Acik, G.; Kamaci, M.; Altinkok, C.; Karabulut, H.F.; Tasdelen, M.A. Synthesis and properties of soybean oil-based biodegradable polyurethane films. Prog. Org. Coat. 2018, 123, 261–266. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.-H. Microbial Succinic Acid, Its Polymer Poly (Butylene Succinate), and Applications. M. Plastics from Bacteria; Springer: Berlin, Germany, 2010; pp. 347–388. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Patel Martin, K. Plastics derived from biological sources: present and future: A technical and environmental review. Chem. Rev. 2011, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.; Kolinski, A.; Skolnick, J.; Levine, Y.K. Effect of double bonds on the dynamics of hydrocarbon chains. J. Chem. Phys. 1992, 97, 1240–1249. [Google Scholar] [CrossRef]
- Wang, Q.-G.; Zai, Y.-Y.; Yang, D.-J.; Qiu, L.-Y.; Niu, C.-Q. Bio-based elastomer nanoparticles with controllable biodegradability. RSC Adv. 2016, 6, 102142–102148. [Google Scholar] [CrossRef]
- Hoshino, A.; Isono, Y. Degradation of aliphatic polyester films by commercially available lipases with special reference to rapid and complete degradation of poly(L-lactide) film by lipase PL derived from Alcaligenes sp. Biodegradation 2002, 13, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Rosu, D.; Tudorachi, N.; Rosu, L. Investigations on the thermal stability of a MDI based polyurethane elastomer. J. Anal. Appl. Pyrolysis 2010, 89, 152–158. [Google Scholar] [CrossRef]
- Askari, F.; Barikani, M.; Barmar, M.; Shokrolahi, F.; Vafayan, M. Study of thermal stability and degradation kinetics of polyurethane–ureas by thermogravimetry. Iran. Polym. J. 2015, 24, 783–789. [Google Scholar] [CrossRef]
- Umare, S.S.; Chandure, A.S. Synthesis, characterization and biodegradation studies of poly(ester urethane)s. Chem. Eng. J. 2008, 142, 65–77. [Google Scholar] [CrossRef]








| Sample Name | Soft Segment (BPD) Content (wt%) | Hard Segment Content (wt%) | |
|---|---|---|---|
| MDI | BDO | ||
| BTPU-1 | 60 | 30.45 | 9.55 |
| BTPU-2 | 70 | 23.27 | 6.73 |
| BTPU-3 | 80 | 16.09 | 3.91 |
| Sample Name | Soft Segment (BPD) CONTENT (wt%) | |||
|---|---|---|---|---|
| BTPU-1 | 60 | 40,004 | 90,809 | 2.27 |
| BTPU-2 | 70 | 51,236 | 167,541 | 3.27 |
| BTPU-3 | 80 | 63,545 | 240,200 | 3.78 |
| Sample Name | Soft Segment (wt%) | Tensile Strength (MPa) | Elongation at Break (%) | Hardness (A) |
|---|---|---|---|---|
| BTPU-1 | 60 | 30.2 ± 1.5 | 802 ± 15 | 87 ± 1 |
| BTPU-2 | 70 | 21.3 ± 1.2 | 1008 ± 17 | 80 ± 1 |
| BTPU-3 | 80 | 13.5 ± 1.2 | 1405 ± 20 | 60 ± 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yan, J.; Wang, T.; Zai, Y.; Qiu, L.; Wang, Q. Fabrication and Properties of a Bio-Based Biodegradable Thermoplastic Polyurethane Elastomer. Polymers 2019, 11, 1121. https://doi.org/10.3390/polym11071121
Wang Z, Yan J, Wang T, Zai Y, Qiu L, Wang Q. Fabrication and Properties of a Bio-Based Biodegradable Thermoplastic Polyurethane Elastomer. Polymers. 2019; 11(7):1121. https://doi.org/10.3390/polym11071121
Chicago/Turabian StyleWang, Zhaoshan, Jieqiong Yan, Tongyao Wang, Yingying Zai, Liyan Qiu, and Qingguo Wang. 2019. "Fabrication and Properties of a Bio-Based Biodegradable Thermoplastic Polyurethane Elastomer" Polymers 11, no. 7: 1121. https://doi.org/10.3390/polym11071121