Toughened Poly(lactic acid)/BEP Composites with Good Biodegradability and Cytocompatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA/BEP Composites
2.3. Characterization
2.3.1. Mechanical Properties
2.3.2. Surface Morphology of the Tensile Specimens and the Notched Impact Specimens
2.3.3. Crystallization Behavior and Isothermal Crystallization Kinetics
2.3.4. Crystallization Morphology
2.3.5. X-ray Diffraction Test
2.3.6. Biodegradation Test
Biodegradation Test in Soil
Biodegradation Test in Lipase Solution
2.3.7. Surface Morphology of PLA and PLA/BEP Composites after Biodegradation
2.3.8. Cytocompatibility Analysis
Cell Culture
MTT Assay
3. Results and Discussion
3.1. Effects of BEP on the Toughness of PLA/BEP Composites
3.2. Effects of BEP on the Crystallization Properties of PLA/BEP Composites
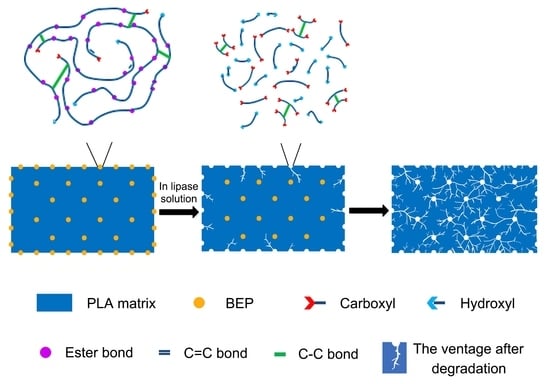
3.3. Effects of BEP on the Biodegradation of PLA/BEP Composites
3.4. Effects of BEP on the Cytocompatibility of PLA/BEP Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Felfel, R.M.; Ahmed, I.; Parsons, A.J.; Rudd, C.D. Bioresorbable composite screws manufactured via forging process: Pull-out, shear, flexural and degradation characteristics. J. Mech. Behav. Biomed. Mater. 2013, 18, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, M.; Kobayashi, S. Effect of extrusion drawing and twist-orientation on mechanical properties of self-reinforced poly(lactic acid) screws. Adv. Compos. Mater. 2016, 25, 443–456. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Wang, Y.W.; Maitz, P.K.; Dehghani, F. Reinforced poly(propylene carbonate) composite with enhanced and tunable characteristics, an alternative for poly(lactic acid). ACS Appl. Mater. Interfaces 2015, 7, 22421–22430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Lv, Z.R.; Ma, J.; Zhu, C.W.; Li, Q. Fabrication of hydrophilic small diameter vascular foam scaffolds of poly(ε-caprolactone)/polylactic blend by sodium hydroxide solution. Eur. Polym. J. 2019, 110, 31–40. [Google Scholar] [CrossRef]
- Lin, M.H.; Firoozi, N.; Tsai, C.T.; Wallace, M.B.; Kang, Y.Q. 3D-printed flexible polymer stents for potential applications in inoperable esophageal malignancies. Acta Biomater 2019, 83, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, J.; Nef, H.M.; Hamm, C.W. Current Status of Bioresorbable Scaffolds in the Treatment of Coronary Artery Disease. J. Am. Coll. Cardiol 2014, 64, 2541–2551. [Google Scholar] [CrossRef] [Green Version]
- Mi, H.Y.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.F.; Turng, L.S. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater. Sci. Eng. C. 2013, 33, 4767–4776. [Google Scholar] [CrossRef] [Green Version]
- Tomlin, E.M.; Nelson, S.J.; Rossmann, J.A. Ridge preservation for implant therapy: A review of the literature. Open Dent 2014, 8, 66–76. [Google Scholar] [CrossRef]
- Maharana, T.; Mohanty, B.; Negi, Y.S. Melt-solid polycondensation of lactic acid and its biodegradability. Prog. Polym. Sci. 2009, 34, 99–124. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Langer, R. Drug delivery and targeting. Nature 1998, 392, 5–10. [Google Scholar] [PubMed]
- Peres, C.; Matos, A.I.; Conniot, J.; Sainz, V.; Zupančič, E.; Silva, J.M.; Graça, L.; Gaspar, R.S.; Préat, V.; Florindo, H.F. Poly(lactic acid)-based particulate systems are promising tools for immune modulation. Acta Biomater. 2017, 48, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Maciel, R. Poly-lactic acid synthesis for application in biomedical devices - A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustainable Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Anderson, K.S.; Schreck, K.M.; Hillmyer, M.A. Toughening Polylactide. Polym. Rev. 2008, 48, 85–108. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.B.; Park, C.B. Poly (lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Zheng, Z.; Zhu, X.M.; Wang, H.T. Shape memory polymer hybrids of SBS/dl-PLA and their shape memory effects. Mater. Chem. Phys. 2013, 137, 750–755. [Google Scholar] [CrossRef]
- Maroufkhani, M.; Katbab, A.; Liu, W.C.; Zhang, J.W. Polylactide (PLA) and acrylonitrile butadiene rubber (NBR) blends: The effect of ACN content on morphology, compatibility and mechanical properties. Polymer 2017, 115, 37–44. [Google Scholar] [CrossRef]
- Talbamrung, T.; Kasemsook, C.; Sangtean, W.; Wachirahuttapong, S.; Thongpin, C. Effect of Peroxide and Organoclay on Thermal and Mechanical Properties of PLA in PLA/NBR Melted Blend. Energy Procedia 2016, 89, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Zaman, H.U.; Song, J.C.; Park, L.S.; Kang, I.K.; Park, S.Y.; Kwak, G.; Park, B.S.; Yoon, K.B. Poly (lactic acid) blends with desired end-use properties by addition of thermoplastic polyester elastomer and MDI. Polym. Bull 2011, 67, 187–198. [Google Scholar] [CrossRef]
- Raja, M.; Ryu, S.H.; Shanmugharaj, A.M. Thermal, mechanical and electroactive shape memory properties of polyurethane (PU)/poly (lactic acid) (PLA)/CNT nanocomposites. Eur. Polym. J. 2013, 49, 3492–3500. [Google Scholar] [CrossRef]
- Pinto, A.M.; Cabral, J.; Tanaka, D.A.P.; Mendes, A.M.; Magalhães, F.D. Effect of incorporation of graphene oxide and graphene nanoplatelets on mechanical and gas permeability properties of poly (lactic acid) films. Polym. Int. 2013, 62, 33–40. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, L.S.; Zhang, J.W. Manipulating Dispersion and Distribution of Graphene in PLA through Novel Interface Engineering for Improved Conductive Properties. ACS Appl. Mater. Interfaces 2014, 6, 14069–14075. [Google Scholar] [CrossRef]
- Seligra, P.G.; Lamanna, M.; Famá, L. PLA-fMWCNT Bionanofilms with High Modulus and Great Properties to Apply in Packaging and Biomedicine. Procedia Mater. Sci. 2015, 8, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Darie, R.N.; Pâslaru, E.; Sdrobis, A.; Pricope, G.M.; Hitruc, G.E.; Poiatǎ, A.; Baklavaridis, A.; Vasile, C. Effect of Nanoclay Hydrophilicity on the Poly (lactic acid)/Clay Nanocomposites Properties. Ind. Eng. Chem. Res. 2014, 53, 7877–7890. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Lei, L.; Yang, B.; Li, J.B.; Ren, J. Preparation of PLA-based nanocomposites modified by nano-attapulgite with good toughness-strength balance. Polym. Test 2017, 60, 78–83. [Google Scholar] [CrossRef]
- Ding, W.D.; Jahani, D.; Chang, E.; Alemdar, A.; Park, C.B.; Sain, M. Development of PLA/cellulosic fiber composite foams using injection molding: Crystallization and foaming behaviors. Comp. Part A 2016, 83, 130–139. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Ahmed, I.; Parsons, A.J.; Scotchford, C.A.; Walker, G.S.; Thielemans, W.; Rudd, C.D. Physico-chemical and mechanical properties of nanocomposites prepared using cellulose nanowhiskers and poly(lactic acid). J. Mater. Sci. 2012, 47, 2675–2686. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under composting conditions of plasticized PLA-PHB blends. Polym. Degrad. Stab 2014, 108, 307–318. [Google Scholar] [CrossRef]
- Piorkowska, E.; Kulinski, Z.; Galeski, A.; Masirek, R. Plasticization of semicrystalline poly(L-lactide) with poly(propylene glycol). Polymer 2006, 47, 7178–7188. [Google Scholar] [CrossRef]
- Courgneau, C.; Ducruet, V.; Avérous, L.; Grenet, J.; Domenek, S. Nonisothermal crystallization kinetics of poly(lactide)-effect of plasticizers and nucleating agent. Polym. Eng. Sci. 2013, 53, 1085–1098. [Google Scholar] [CrossRef]
- Wang, Q.G.; Zai, Y.Y.; Yang, D.J.; Qiu, L.Y.; Niu, C.Q. Bio-based elastomer nanoparticles with controllable biodegradability. RSC Adv. 2016, 6, 102142–102148. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Smith, R.R. Stress-whitening in high-impact polystyrenes. Polymer 1965, 6, 437–446. [Google Scholar] [CrossRef]
- Mat Desa, M.S.Z.; Hassan, A.; Arsad, A.; Arjmandi, R. Effect of core–shell rubber toughening on mechanical, thermal, and morphological properties of poly (lactic acid)/multiwalled carbon nanotubes nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47756. [Google Scholar] [CrossRef]
- Sun, Y.; He, C. Biodegradable “core–shell” rubber nanoparticles and their toughening of poly (lactides). Macromolecules 2013, 46, 9625–9633. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Clayton, D.; Keast, W.E. Rubber-toughening of plastics. 2. creep mechanisms in HIPS/PPO blends. J. Mater. Sci. 1972, 7, 1443–1453. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.L.; Ramy-Ratiarison, R.; Paint, Y.; Raquez, J.M.; Dubois, P. Recent advances in production of poly (lactic acid) (PLA) nanocomposites: A versatile method to tune crystallization properties of PLA. Nanocomposites 2015, 1, 71–82. [Google Scholar] [CrossRef]
- Zhang, J.M.; Duan, Y.X.; Sato, H.; Tsuji, H.; Noda, I.; Yan, S.; Ozaki, Y. Crystal Modifications and Thermal Behavior of Poly (l-lactic acid) Revealed by Infrared Spectroscopy. Macromolecules 2005, 38, 8012–8021. [Google Scholar] [CrossRef]
- Nam, J.Y.; Ray, S.S.; Okamoto, M. Crystallization Behavior and Morphology of Biodegradable Polylactide/Layered Silicate Nanocomposite. Macromolecules 2003, 36, 7126–7131. [Google Scholar] [CrossRef]
- Nitta, K.H.; Takayanagi, M. Tensile yield of isotactic polypropylene in terms of a lamellar-cluster model. J. Polym. Sci. Part B: Polym. Phys. 2000, 38, 1037–1044. [Google Scholar]
- Karamanlioglu, M.; Houlden, A.; Robson, G.D. Isolation and characterization of fungal communities associated with degradation and growth on the surface of poly(lactic) acid (PLA) in soil and compost. Int. Biodeterior. Biodegrad. 2014, 95, 301–310. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Robson, G.D. The influence of biotic and abiotic factors on the rate of degradation of poly(lactic) acid (PLA) coupons buried in compost and soil. Polym. Degrad. Stab. 2013, 98, 2063–2071. [Google Scholar] [CrossRef]
- Ho, K.L.G.; Pometto, A.L.; Gadea-Rivas, A.; Briceño, J.A.; Rojas, A. Degradation of Polylactic Acid (PLA) Plastic in Costa Rican Soil and Iowa State University Compost Rows. J. Environ. Polym. Degrad. 1999, 7, 173–177. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, S.H.; Han, Y.K.; Kim, Y.H. Synthesis and Degradation of End-Group-Functionalized Polylactide. J. Polym. Sci. Part A: Polym. Chem. 2001, 39, 973–985. [Google Scholar] [CrossRef]
- VandeVord, P.J.; Matthew, H.W.T.; DeSilva, S.P.; Mayton, L.; Wu, B.; Wooleyl, P.H. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 2002, 59, 585–590. [Google Scholar] [CrossRef]













| Sample Numbers | PLA | PLA-2 | PLA-4 | PLA-6 | PLA-8 | PLA-10 |
|---|---|---|---|---|---|---|
| PLA/phr | 100 | 100 | 100 | 100 | 100 | 100 |
| BEP/phr | 0 | 2 | 4 | 6 | 8 | 10 |
| HMV-8CA/phr | 0 | 0.06 | 0.12 | 0.18 | 0.24 | 0.3 |
| Sample Numbers | t1/2/min | n | K | Tm/°C | ΔHm/J·g−1 | Crystallinity/% | tm/min |
|---|---|---|---|---|---|---|---|
| PLA | 12.07 | 2.69 | 0.047 | 165.4 | 28.41 | 30.55 | 16 |
| PLA-4 | 10.22 | 2.81 | 0.051 | 159.7, 167.0 | 40.67 | 45.48 | 14 |
| PLA-8 | 7.11 | 2.89 | 0.075 | 160.3, 167.3 | 39.62 | 46.01 | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Li, Y.; Zhou, X.; Wang, T.; Qiu, L.; Gu, Y.; Chang, J. Toughened Poly(lactic acid)/BEP Composites with Good Biodegradability and Cytocompatibility. Polymers 2019, 11, 1413. https://doi.org/10.3390/polym11091413
Wang Q, Li Y, Zhou X, Wang T, Qiu L, Gu Y, Chang J. Toughened Poly(lactic acid)/BEP Composites with Good Biodegradability and Cytocompatibility. Polymers. 2019; 11(9):1413. https://doi.org/10.3390/polym11091413
Chicago/Turabian StyleWang, Qingguo, Yongxuan Li, Xue Zhou, Tongyao Wang, Liyan Qiu, Yuanchun Gu, and Jiabing Chang. 2019. "Toughened Poly(lactic acid)/BEP Composites with Good Biodegradability and Cytocompatibility" Polymers 11, no. 9: 1413. https://doi.org/10.3390/polym11091413