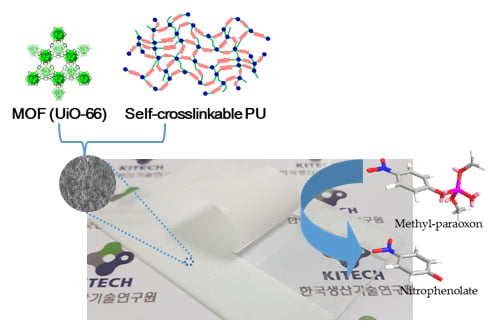
Preparation of Peelable Coating Films with a Metal Organic Framework (UiO-66) and Self-Crosslinkable Polyurethane for the Decomposition of Methyl Paraoxon
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Synthesis of UiO-66
2.3. Synthesis of Dimethylol Propionic Acid Based Silane Terminated Polyurethane Dispersions (DSPDs)
2.4. Preparation of UiO-66/DSPD Composite Films
2.5. Characterizations
3. Results and Discussion
3.1. Characterization of DSPD Films
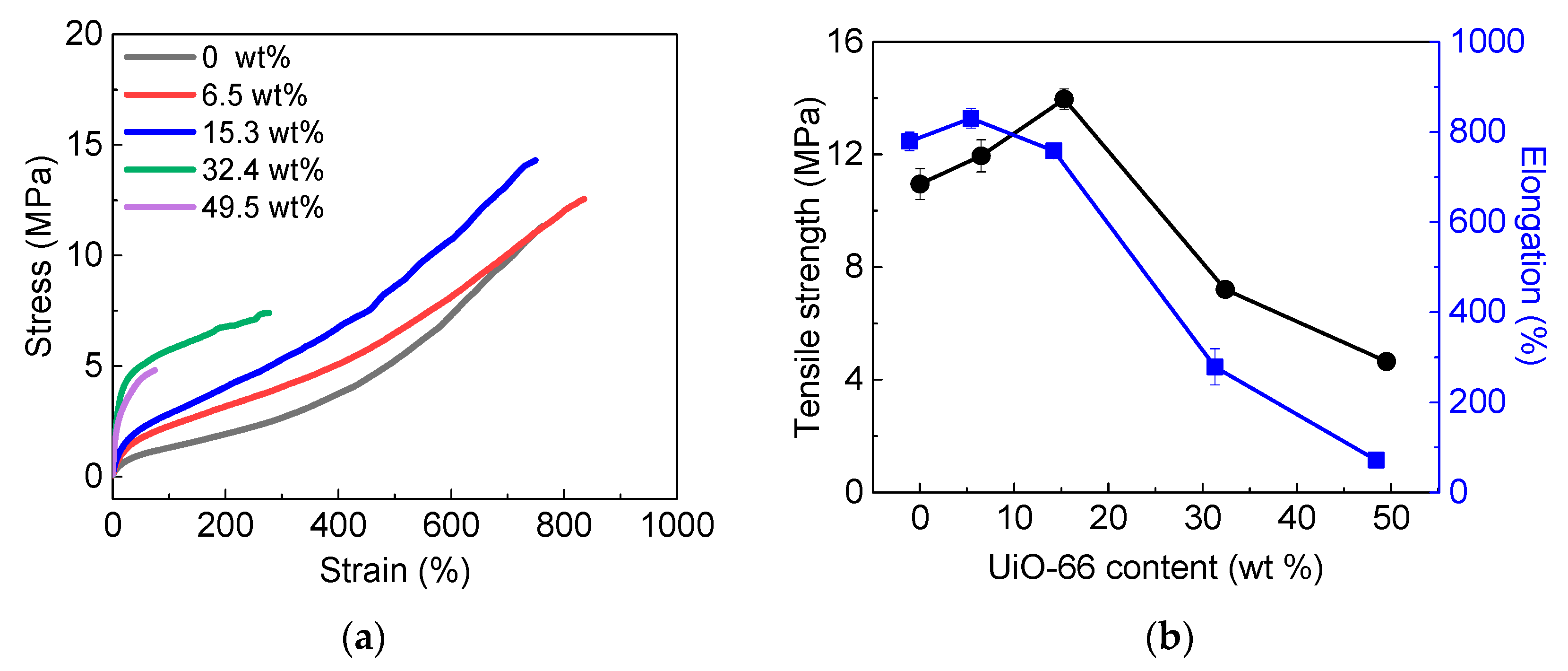
3.2. Characterization of UiO-66/DSPD Composite Films
3.3. MPO Decomposition of Composite Films
3.4. Peeling Properties of UiO-66/DSPD Composite Films
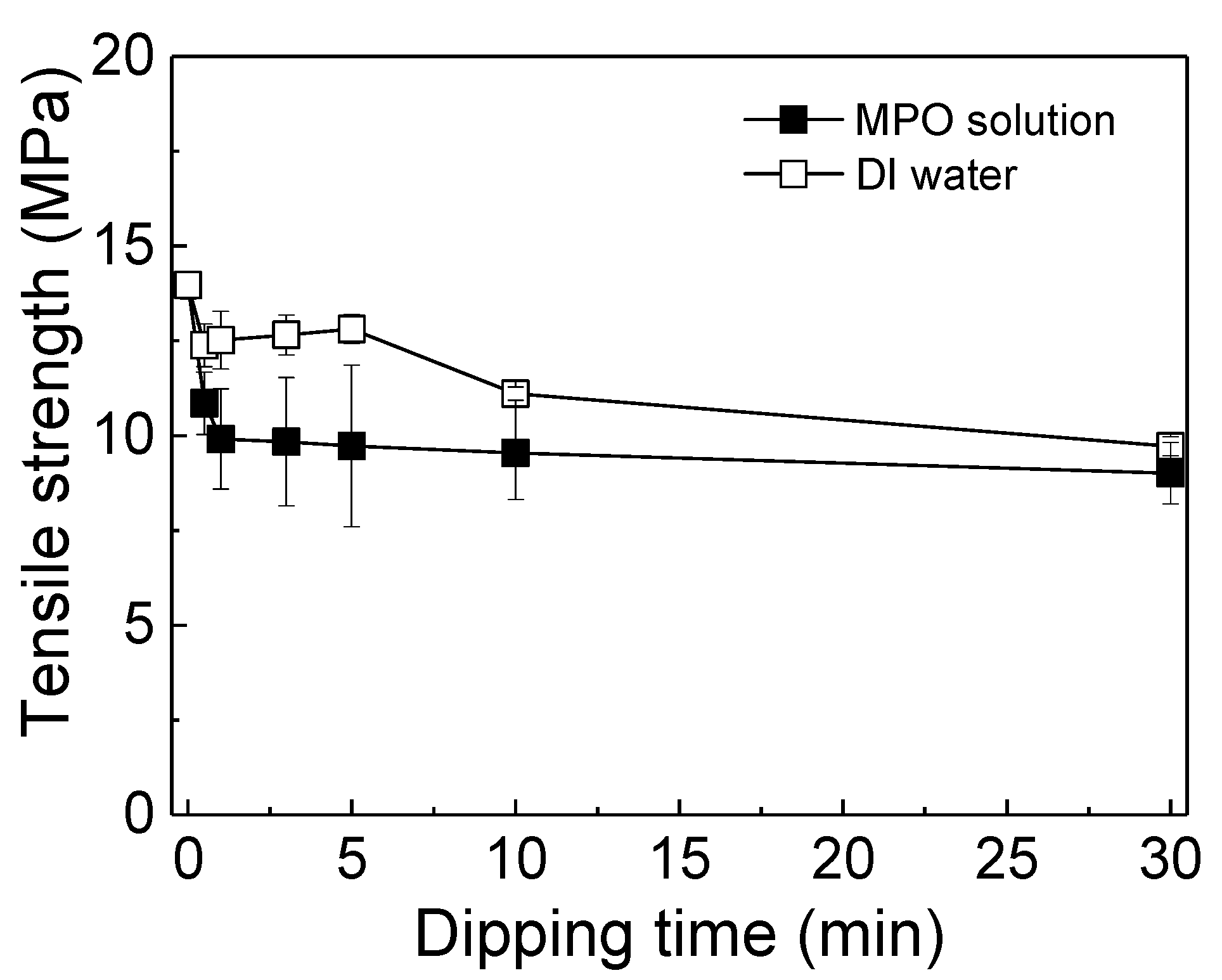
3.5. Mechanical Property Changes after MPO Decomposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Bandosz, T.J.; Laskoski, M.; Mahle, J.; Mogilevsky, G.; Peterson, G.W.; Rossin, J.A.; Wagner, G.W. Reactions of VX, GD, and HD with Zr(OH)4: Near Instantaneous Decontamination of VX. J. Phys. Chem. C 2012, 116, 11606–11614. [Google Scholar] [CrossRef]
- Katz, M.J.; Mondloch, J.E.; Totten, R.K.; Park, J.K.; Nguyen, S.T.; Farha, O.K.; Hupp, J.T. Simple and compelling biomimetic metal-organic framework catalyst for the degradation of nerve agent simulants. Angew. Chem. Int. Ed. 2014, 53, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Mondloch, J.E.; Katz, M.J.; Isley, W.C., 3rd; Ghosh, P.; Liao, P.; Bury, W.; Wagner, G.W.; Hall, M.G.; DeCoste, J.B.; Peterson, G.W.; et al. Destruction of chemical warfare agents using metal-organic frameworks. Nat. Mater. 2015, 14, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; McCarthy, D.L.; Cowan, M.J.; Obuya, E.A.; DeCoste, J.B.; Skorenko, K.H.; Tong, L.; Boyer, S.M.; Bernier, W.E.; Jones, W.E., Jr. Photocatalytic activity of TiO2 polycrystalline sub-micron fibers with variable rutile fraction. Appl. Catal. B 2016, 187, 154–162. [Google Scholar] [CrossRef]
- Zhao, J.; Lee, D.T.; Yaga, R.W.; Hall, M.G.; Barton, H.F.; Woodward, I.R.; Oldham, C.J.; Walls, H.J.; Peterson, G.W.; Parsons, G.N. Ultra-Fast Degradation of Chemical Warfare Agents Using MOF-Nanofiber Kebabs. Angew. Chem. Int. Ed. 2016, 55, 13224–13228. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.X.; McEntee, M.; Browe, M.A.; Hall, M.G.; DeCoste, J.B.; Peterson, G.W. MOFabric: Electrospun Nanofiber Mats from PVDF/UiO-66-NH2 for Chemical Protection and Decontamination. ACS Appl. Mater. Interfaces 2017, 9, 13632–13636. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.W.; Lu, A.X.; Epps, T.H., 3rd. Tuning the Morphology and Activity of Electrospun Polystyrene/UiO-66-NH2 Metal-Organic Framework Composites to Enhance Chemical Warfare Agent Removal. ACS Appl. Mater. Interfaces 2017, 9, 32248–32254. [Google Scholar] [CrossRef]
- McCarthy, D.L.; Liu, J.; Dwyer, D.B.; Troiano, J.L.; Boyer, S.M.; DeCoste, J.B.; Bernier, W.E.; Jones, J.W.E. Electrospun metal–organic framework polymer composites for the catalytic degradation of methyl paraoxon. New J. Chem. 2017, 41, 8748–8753. [Google Scholar] [CrossRef]
- Kaledin, A.L.; Troya, D.; Karwacki, C.J.; Balboa, A.; Gordon, W.O.; Morris, J.R.; Mitchell, M.B.; Frenkel, A.I.; Hill, C.L.; Musaev, D.G. Key mechanistic details of paraoxon decomposition by polyoxometalates: Critical role of para-nitro substitution. Chem. Phys. 2019, 518, 30–37. [Google Scholar] [CrossRef]
- Palomba, J.M.; Credille, C.V.; Kalaj, M.; DeCoste, J.B.; Peterson, G.W.; Tovar, T.M.; Cohen, S.M. High-throughput screening of solid-state catalysts for nerve agent degradation. Chem. Commun. 2018, 54, 5768–5771. [Google Scholar] [CrossRef]
- Pinto, M.L.; Dias, S.; Pires, J. Composite MOF foams: The example of UiO-66/polyurethane. ACS Appl. Mater. Interfaces 2013, 5, 2360–2363. [Google Scholar] [CrossRef]
- Semino, R.; Moreton, J.C.; Ramsahye, N.A.; Cohen, S.M.; Maurin, G. Understanding the origins of metal-organic framework/polymer compatibility. Chem. Sci. 2018, 9, 315–324. [Google Scholar] [CrossRef]
- Kalaj, M.; Denny, M.S., Jr.; Bentz, K.C.; Palomba, J.M.; Cohen, S.M. Nylon-MOF Composites through Postsynthetic Polymerization. Angew. Chem. Int. Ed. 2019, 58, 2336–2340. [Google Scholar] [CrossRef]
- Plonka, A.M.; Wang, Q.; Gordon, W.O.; Balboa, A.; Troya, D.; Guo, W.; Sharp, C.H.; Senanayake, S.D.; Morris, J.R.; Hill, C.L.; et al. In situ probes of capture and decomposition of chemical warfare agent simulants by Zr-based metal organic frameworks. J. Am. Chem. Soc. 2017, 139, 599–602. [Google Scholar] [CrossRef]
- Swidler, R. Polymeric Peel-Off Coating Compositions and Methods of Use Thereof. U.S. Patent 6124044A, 26 September 2000. [Google Scholar]
- Salamon, P.A.; Conn, H. Temporary Protective Coatings for Precision Surfaces. U.S. Patent 5945462, 31 August 1999. [Google Scholar]
- Blaine, S.J.; Wilson, K.K. Protective Solvent Free Liquid Masking Compounds and Related Method. U.S. Patent 5494702, 27 Febuary 1996. [Google Scholar]
- Muller, H.P.; Gruttmann, H.; Petzoldt, J.; Muller, H.; Meixner, J.; Kurek, G. Coating Composition Comprising Diverse Anionic Polyurethane-Polyurea Dispersions. CA2314523, 25 July 2000. [Google Scholar]
- Muller, H.P.; Gruttmann, H.; Casselmann, H.; Muller, H.; Petzoldt, J.; Bock, M. Cosolvent-Free, Aqueous, Anionic Polyurethane Dispersions and Their Use as Peelable Coatings. U.S. Patent 6172126 B1, 9 January 2001. [Google Scholar]
- Lewandowski, K.; Krepski, L.R.; Mickus, D.E.; Roberts, R.R.; Heilmann, S.M.; Larson, W.K.; Purgett, M.D.; Koecher, S.D.; Johnson, S.A.; McGurran, D.J.; et al. Synthesis and properties of waterborne self-crosslinkable sulfo-urethane silanol dispersions. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3037–3045. [Google Scholar] [CrossRef]
- Lewandowski, K.; Krepski, L.R.; Mickus, D.E. Dry-peelable temporary protective coatings from waterborne self-crosslinkable sulfourethane-silanol dispersions. J. Appl. Polym. Sci. 2004, 91, 1443–1449. [Google Scholar] [CrossRef]
- Sardon, H.; Irusta, L.; Fernández-Berridi, M.J.; Lansalot, M.; Bourgeat-Lami, E. Synthesis of room temperature self-curable waterborne hybrid polyurethanes functionalized with (3-aminopropyl)triethoxysilane (APTES). Polymer 2010, 51, 5051–5057. [Google Scholar] [CrossRef]
- Kim, K.; Seo, J.Y.; Baek, K.Y.; Bae, J.Y.; Shin, S. Metal–organic framework (UiO--66)--dispersed polyurethane composite films for the decontamination of methyl paraoxon. Polym. Int. 2019, 68, 1502–1508. [Google Scholar] [CrossRef]
- Bakuru, V.R.; Churipard, S.R.; Maradur, S.P.; Kalidindi, S.B. Exploring the Bronsted acidity of UiO-66 (Zr, Ce, Hf) metal-organic frameworks for efficient solketal synthesis from glycerol acetalization. Dalton Trans. 2019, 48, 843–847. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Nik, O.G.; Chen, X.Y.; Kaliaguine, S. Functionalized metal organic framework-polyimide mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2012, 413–414, 48–61. [Google Scholar] [CrossRef]

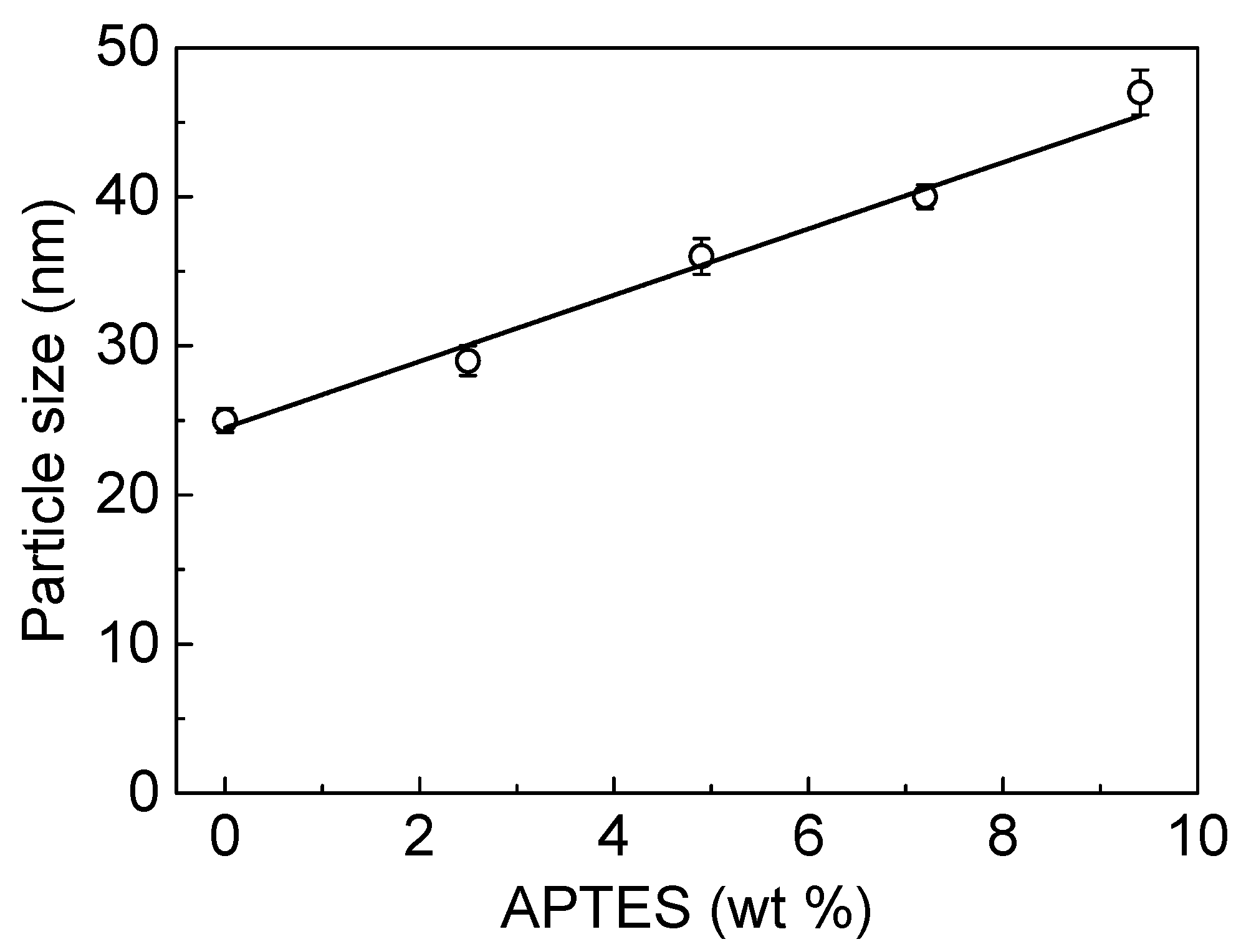
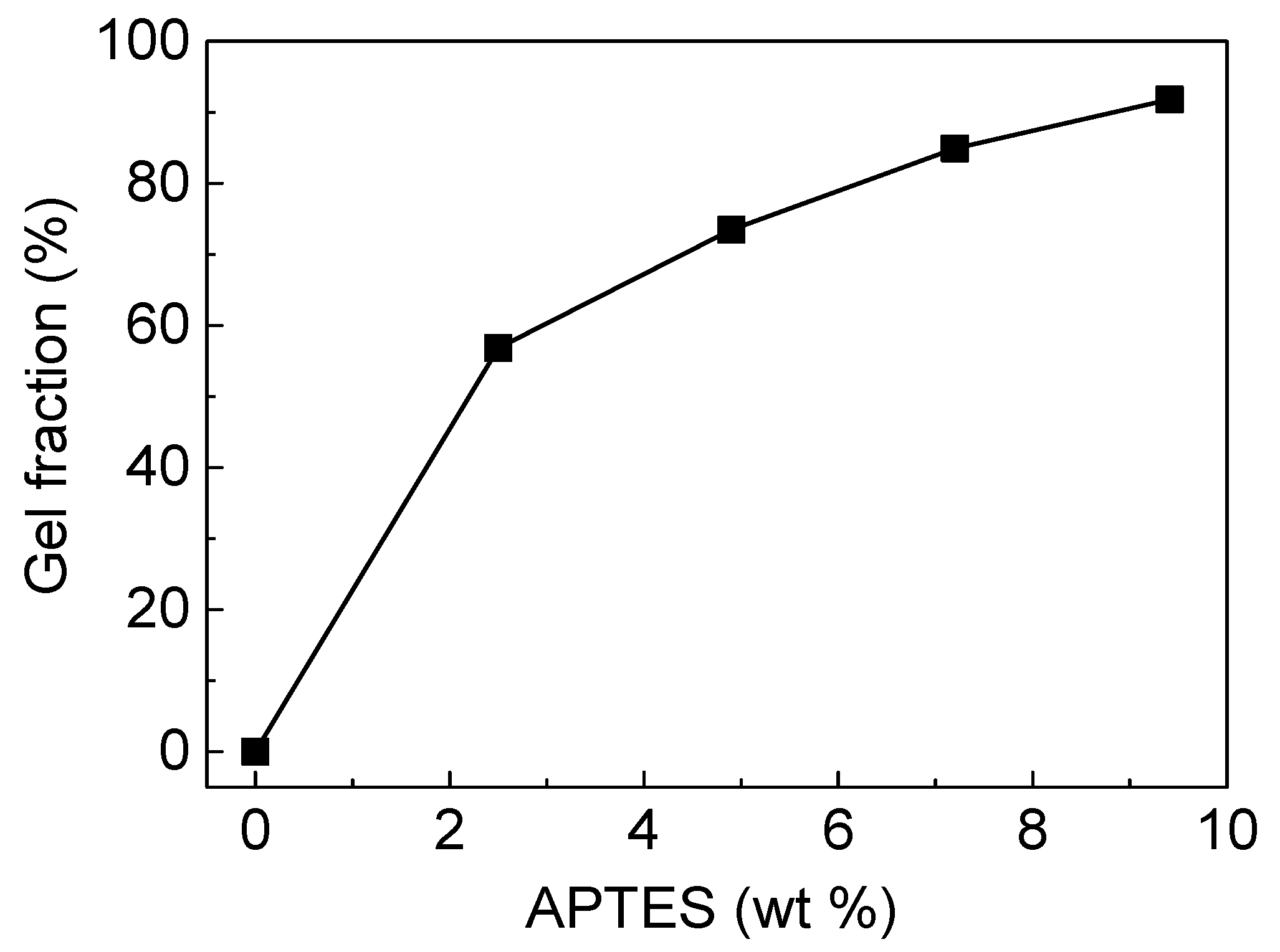






| Sample | Polyol (g) | DMPA (g) | IPDI (g) | EG (g) | APTES (g) | EG/APTES (mol ratio) | APTES in Feed (wt.%) | APTES by TGA (wt.%) |
|---|---|---|---|---|---|---|---|---|
| DSPD1 | 224.32 | 12.00 | 66.76 | 4.96 | 8.84 | 2 | 2.79 | 2.50 |
| DSPD2 | 3.72 | 17.68 | 0.75 | 5.45 | 4.90 | |||
| DSPD3 | 2.48 | 26.52 | 0.33 | 7.99 | 7.20 | |||
| DSPD4 | 1.24 | 35.36 | 0.125 | 10.41 | 9.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, N.H.; Park, H.-w.; Chae, G.-s.; Lee, J.H.; Bae, S.W.; Shin, S. Preparation of Peelable Coating Films with a Metal Organic Framework (UiO-66) and Self-Crosslinkable Polyurethane for the Decomposition of Methyl Paraoxon. Polymers 2019, 11, 1298. https://doi.org/10.3390/polym11081298
Long NH, Park H-w, Chae G-s, Lee JH, Bae SW, Shin S. Preparation of Peelable Coating Films with a Metal Organic Framework (UiO-66) and Self-Crosslinkable Polyurethane for the Decomposition of Methyl Paraoxon. Polymers. 2019; 11(8):1298. https://doi.org/10.3390/polym11081298
Chicago/Turabian StyleLong, Ngo Hoang, Hee-woong Park, Gyeong-seok Chae, Jung Hyun Lee, Se Won Bae, and Seunghan Shin. 2019. "Preparation of Peelable Coating Films with a Metal Organic Framework (UiO-66) and Self-Crosslinkable Polyurethane for the Decomposition of Methyl Paraoxon" Polymers 11, no. 8: 1298. https://doi.org/10.3390/polym11081298