Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Methods
2.2.1. Preparation of Liposomes
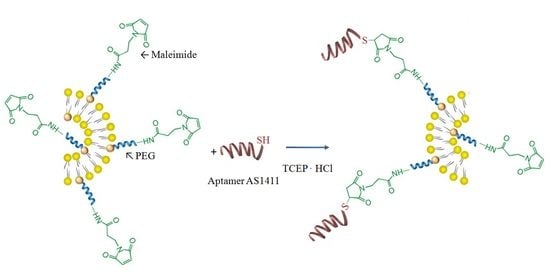
2.2.2. Conjugation of AS1411 to the Surface of Obtained Liposomes
2.3. Characterization
2.3.1. Apoptosis Assay
2.3.2. Uptake of the Tested Liposomes by Shift in Fluorescence Intensity
2.3.3. In Vitro Antitumoral Effect on Basal Cell Carcinoma
3. Results and Discussion
3.1. Size and Zeta Potential
3.2. 5-FU Encapsulation Efficiency
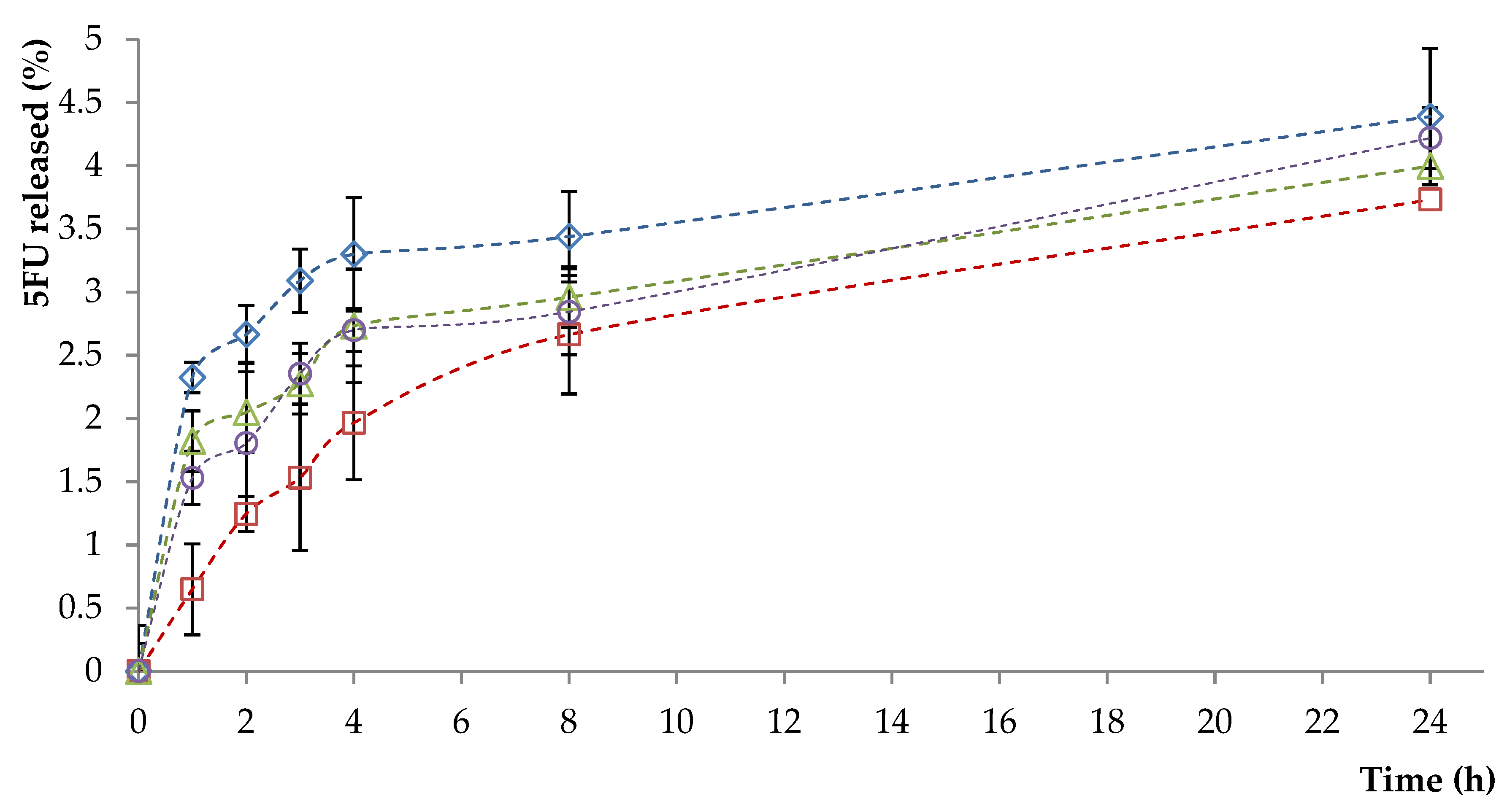
3.3. In Vitro Drug Release
3.4. In Vitro Evaluation of Liposomes Biocompatibility with Blood Components
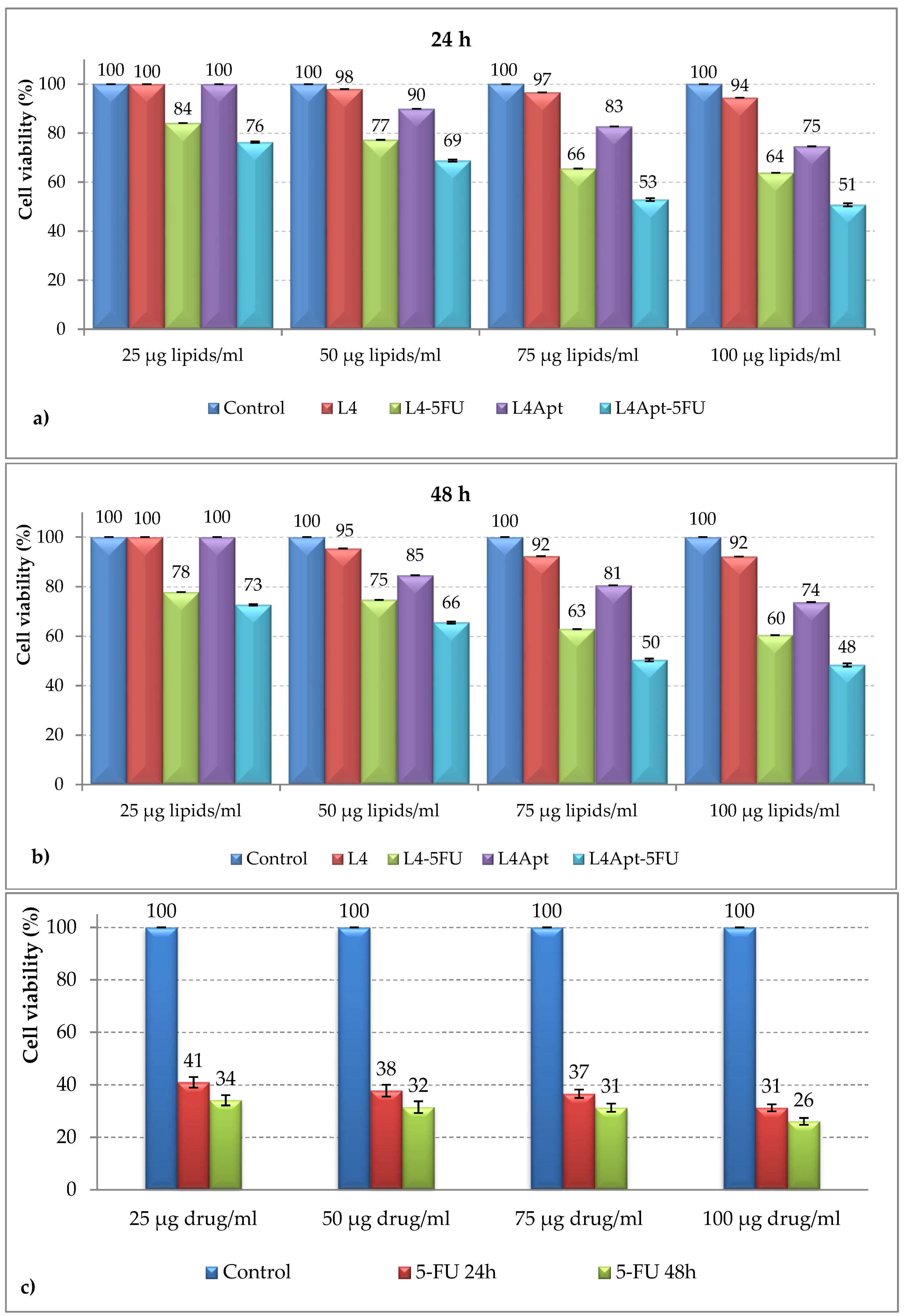
3.5. In Vitro Cytotoxic Effects
3.6. Apoptosis Evaluation
3.7. Uptake of the Tested Liposomes by Shift in Fluorescence Intensity
3.8. In Vitro Antitumoral Effect on Basal Cell Carcinoma
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dianzani, C.; Zara, G.P.; Maina, G.; Pettazzoni, P.; Pizzimenti, S.; Rossi, F.; Barrera, G. Drug Delivery Nanoparticles in Skin Cancers. BioMed Res. Int. 2014, 2014, 895986. [Google Scholar] [CrossRef] [PubMed]
- Raţă, D.M.; Chailan, J.F.; Peptu, C.A.; Costuleanu, M.; Popa, M. Chitosan: Poly (N-vinylpyrrolidone-alt-itaconic anhydride) nanocapsules—A promising alternative for the lung cancer treatment. J. Nanopart. Res. 2015, 17, 316. [Google Scholar] [CrossRef]
- Quynh, L.M.; Nam, N.H.; Kong, K.; Nhung, N.T.; Notingher, I.; Henini, M.; Luong, N.H. Surface-Enhanced Raman Spectroscopy Study of 4-ATP on Gold Nanoparticles for Basal Cell Carcinoma Fingerprint Detection. J. Electron. Mater. 2016, 45, 2563–2568. [Google Scholar] [CrossRef]
- Severino, P.; Fangueiro, J.F.; Ferreira, S.V.; Basso, R.; Chaud, M.V.; Santana, M.H.A.; Souto, E.B. Nanoemulsions and nanoparticles for non-melanoma skin cancer: Effects of lipid materials. Clin. Transl. Oncol. 2013, 15, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Barolet, D.; Boucher, A. No-needle jet intradermal aminolevulinic Acid photodynamic therapy for recurrent nodular Basal cell carcinoma of the nose: A case report. J. Skin Cancer 2011, 2011, 790509. [Google Scholar] [CrossRef]
- Teskac, K.; Kristl, J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int. J. Pharm. 2010, 390, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic-Curic, N.; Winter, S.; Krajisnik, D.; Stupar, M.; Milic, J.; Graefe, S. Stability evaluation of temoporfin-loaded liposomal gels for topical application. J. Liposome Res. 2010, 20, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Henkin, R.I. Vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 367, 969–971. [Google Scholar] [CrossRef]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef]
- Minko, T.; Rodriguez-Rodriguez, L.; Pozharov, V. Nanotechnology approaches for personalized treatment of multidrug resistant cancers. Adv. Drug Deliv. Rev. 2013, 65, 1880–1895. [Google Scholar] [CrossRef] [Green Version]
- Dıaz, M.R.; Vivas-Mejia, P.E. Nanoparticles as drug delivery systems in cancer medicine: Emphasis on RNAi-containing nanoliposomes. Pharmaceuticals 2013, 6, 1361–1380. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shao, R.; Zhang, X.; Chen, C. Applications of nanotechnology for melanoma treatment, diagnosis, and theranostics. Int. J. Nanomed. 2013, 8, 2677–2688. [Google Scholar] [CrossRef] [PubMed]
- Holban, M.; Iurea (Raţă), D.; Popa, M. Polymeric Nanomedicines; Popa, M., Uglea, C.V., Eds.; Bentham Science Publishers: Sharjah, UAE, 2013; pp. 412–450. [Google Scholar]
- Iurea, D.M.; Peptu, C.A.; Chailan, J.F.; Carriere, P.; Popa, M. Sub-Micronic Capsules Based on Gelatin and Poly (maleic anhydride-alt-vinyl acetate) Obtained by Interfacial Condensation with Potential Biomedical Applications. J. Nanosci. Nanotechnol. 2013, 13, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Cadinoiu, A.N.; Peptu, C.A. Polymeric Nanomedicines; Popa, M., Uglea, C.V., Eds.; Bentham Science Publishers: Sharjah, UAE, 2013; pp. 293–317. [Google Scholar]
- Kaasgaard, T.; Andresen, T.L. Liposomal cancer therapy: exploiting tumor characteristics. Exp. Opin. Drug Deliv. 2010, 7, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Huwyler, J.; Drewe, J.; Krähenbühl, S. Tumor targeting using liposomal antineoplastic drugs. Int. J. Nanomed. 2008, 3, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002, 4, 95. [Google Scholar] [CrossRef]
- Cadinoiu, A.N.; Darabă, O.; Merlușcă, P.; Anastasiu, D.; Vasiliu, M.; Chirap, A.M.; Bîrgăoanu, A.; Burlui, V. Liposomal formulations with potential dental applications. Int. J. Med. Dent. 2014, 18, 271–277. [Google Scholar]
- Ciobanu, B.C.; Cadinoiu, A.N.; Popa, M.; Desbrières, J.; Peptu, C.A. Modulated release from liposomes entrapped in chitosan/gelatin hydrogels. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 383–931. [Google Scholar] [CrossRef]
- Li, X.; Rao, X.; Cai, L.; Liu, X.; Wang, H.; Wu, W.; Zhu, C.; Chen, M.; Wang, P.G.; Yi, W. Targeting Tumor Cells by Natural Anti-Carbohydrate Antibodies Using Rhamnose-Functionalized Liposomes. ACS Chem. Biol. 2016, 11, 1205–1209. [Google Scholar] [CrossRef]
- Parolini, L.; Kotar, J.; DiMichele, L.; Mognetti, B.M. Controlling Self-Assembly Kinetics of DNA-Functionalized Liposomes Using Toehold Exchange Mechanism. ACS Nano 2016, 10, 2392–2398. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Lu, L.; Zhang, L.; Shi, K.; Cun, X.; Yang, Y.; Liu, Y.; Gao, H.; He, Q. Dual-functionalized liposomal delivery system for solid tumors based on RGD and a pH-responsive antimicrobial peptide. Sci. Rep. 2016, 6, 19800. [Google Scholar] [CrossRef] [PubMed]
- Jătariu, A.N.; Popa, M.; Peptu, C.A. Different particulate systems-bypass the biological barriers? J. Drug Target. 2010, 18, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Fathi, S.; Oyelere, A.K. Liposomal drug delivery systems for targeted cancer therapy: Is active targeting the best choice? Future Med. Chem. 2016, 8, 2091–2112. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Tang, L.; Yang, X.; Hwang, K.; Wang, W.; Yin, Q.; Wong, N.Y.; Dobrucki, L.W.; Yasui, N.; Katzenellenbogen, J.A.; et al. Selective Delivery of an Anticancer Drug with Aptamer-Functionalized Liposomes to Breast Cancer Cells in Vitro and in Vivo. J. Mater. Chem. B 2013, 1, 5288–5297. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hou, J.; Liu, X.; Guo, Y.; Wu, Y.; Zhang, L.; Yang, Z. Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials 2014, 35, 3840–3850. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Tong, R.; Mishra, A.; Xu, W.; Wong, G.C.; Cheng, J.; Lu, Y. Reversible cell-specific drug delivery with aptamer-functionalized liposomes. Angew. Chem. Int. Ed. Engl. 2009, 48, 6494–6498. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, K.A.; Wang, Y.; Baeumner, A.J. Aptamer sandwich assays: Human a-thrombin detection using liposome enhancement. Anal. Bioanal. Chem. 2010, 398, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; O’Donoghue, M.B.; Liu, H.; Tan, W. A liposome-based nanostructure for aptamer directed delivery. Chem. Commun. 2010, 46, 249–251. [Google Scholar] [CrossRef]
- Kalantarian, P.; Najafabadi, A.R.; Haririan, I.; Vatanara, A.; Yamini, Y.; Darabi, M.; Gilani, K. Preparation of 5-fluorouracil nanoparticles by supercritical antisolvents for pulmonary delivery. Int. J. Nanomed. 2010, 5, 763–770. [Google Scholar] [CrossRef]
- Deepthi, S.; AbdulGafoor, A.A.; Sivashanmugam, A.; Naira, S.V.; Jayakumar, R. Nanostrontium ranelate incorporated injectable hydrogel enhanced matrix production supporting chondrogenesis in vitro. J. Mater. Chem. B 2016, 4, 4092–4103. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Ji, Q.; Qiu, L. Targeted delivery of anticancer drugs by aptamer AS1411 mediated Pluronic F127/cyclodextrin-linked polymer composite micelles. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, X.Y.; Liu, B.Y.; Xu, C.; Ai, S.L.; Zhuo, R.X.; Cheng, S.X. Aptamer-functionalized albumin-based nanoparticles for targeted drug delivery. Coll. Surf. B Biointerf. 2018, 171, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.M.; Gaume, X.; Bouvet, P. The roles of nucleolin subcellular localization in cancer. Biochimie 2015, 113, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.M.; Gherghel, D.; Popa, M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Dias, F.V.; Querobino, S.M.; Alberto-Silva, C.; Fraceto, L.F.; de Paula, E.; de Araujo, D.R. Influence of hybrid polymeric nanoparticle/thermosensitive hydrogels systems on formulation tracking and in vitro artificial membrane permeation: A promising system for skin drug-delivery. Coll. Surf. B Biointerf. 2019, 174, 56–62. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Rajamanickam, V.M.; Yaspal, M.; Singh, S. Investigations on agglomeration and haemocompatibility of vitamin E TPGS surface modified berberine chloride nanoparticles. BioMed Res. Int. 2014, 2014, 951942. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef]
- ISO 10993-5. Biological Evaluation of Medical Devices; Part 5. Test for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Pozarowski, P.; Huang, X.; Halicka, D.H.; Lee, B.; Johnson, G.; Darzynkiewicz, Z. Interactions of fluorochrome-labeled caspase inhibitors with apoptotic cells: A caution in data interpretation. Cytom. A 2003, 55, 50–60. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Laville, N.; Aït-Aïssa, S.; Gomez, E.; Casellas, C.; Porcher, J.M. Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockert, J.C.; Blazquez-Castro, A.; Canete, M.; Horobin, R.W.; Villanueva, A. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.X.; Chuang, E.Y.; Lin, C.C.; Ho, Y.C.; Lin, K.J.; Cheng, P.Y.; Chen, K.J.; Wei, H.J.; Sung, H.W. An AS1411 aptamer-conjugated liposomal system containing a bubble-generating agent for tumor-specific chemotherapy that overcomes multidrug resistance. J. Control. Release 2015, 208, 42–51. [Google Scholar] [CrossRef] [PubMed]






| Sample | PC (mmol) | Chol (mmol) | DSPE-PEG-MAL (mmol) | PBS (mL) | 5-FU in PBS (mg/mL) | AS1411-SH (mmol) |
|---|---|---|---|---|---|---|
| L1-5FU-10 | 15 | 9 | 1 | 5 | 10 | - |
| L2-5FU-10 | 15 | 13.5 | 1 | 5 | 10 | - |
| L3-5FU-10 | 10 | 6 | 1 | 5 | 10 | - |
| L3-5FU-15 | 10 | 6 | 1 | 5 | 15 | - |
| L4-5FU-10 | 10 | 6 | 1.5 | 5 | 10 | - |
| L4-5FU-15 | 10 | 6 | 1.5 | 5 | 15 | - |
| L1Apt-5FU-10 | 15 | 9 | 1 | 5 | 10 | 1 |
| L2Apt-5FU-10 | 15 | 13.5 | 1 | 5 | 10 | 1 |
| L3Apt-5FU-10 | 10 | 6 | 1 | 5 | 10 | 1 |
| L4Apt-5FU-10 | 10 | 6 | 1.5 | 5 | 10 | 1.5 |
| L4Apt-5FU-15 | 10 | 6 | 1.5 | 5 | 15 | 1.5 |
| Sample | Conjugation Efficiency (%) |
|---|---|
| L1Apt-5FU-10 | 36.2 ± 3.5 |
| L2Apt-5FU-10 | 37.3 ± 3.8 |
| L3Apt-5FU-10 | 28.0 ± 3.0 |
| L4Apt-5FU-10 | 39.6 ± 2.9 |
| L4Apt-5FU-15 | 37.2 ± 3.7 |
| Sample | Mean Diameter (d.nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| L1 | 182 ± 10 | 0.12 | −0.4 ± 0.1 |
| L2 | 187 ± 22 | 0.18 | −0.4 ± 0.5 |
| L3 | 182 ± 12 | 0.15 | −0.5 ± 0.2 |
| L1-5FU-10 | 184 ± 12 | 0.10 | −4.5 ± 0.3 |
| L2-5FU-10 | 189 ± 23 | 0.18 | −0.4 ± 0.9 |
| L3-5FU-10 | 170 ± 11 | 0.08 | −5.6 ± 0.7 |
| L3-5FU-15 | 185 ± 12 | 0.15 | −0.4 ± 0.1 |
| L4-5FU-10 | 164 ± 33 | 0.11 | −5.3 ± 0.3 |
| L4-5FU-15 | 170 ± 18 | 0.14 | −6.4 ± 0.9 |
| L1Apt-5FU-10 | 200 ± 16 | 0.36 | −9.6 ± 1.5 |
| L2Apt-5FU-10 | 197 ± 15 | 0.13 | −12.0 ± 2.4 |
| L3Apt-5FU-10 | 190 ± 9 | 0.20 | −11.1 ± 1.2 |
| L4Apt-5FU-10 | 172 ± 21 | 0.09 | −12.4 ± 1.6 |
| L4Apt-5FU-15 | 182 ± 27 | 0.16 | −12.9 ± 1.3 |
| Sample | Encapsulation Efficiency of 5-FU (%) |
|---|---|
| L1-5FU-10 | 7.8 ± 0.9 |
| L3-5FU-10 | 7.5 ± 0.9 |
| L3-5FU-15 | 8.5 ± 1.1 |
| L4-5FU-15 | 8.7 ± 1.4 |
| L1Apt-5FU-10 | 7.2 ± 0.2 |
| L3Apt-5FU-10 | 7.1 ± 1.2 |
| L3Apt-5FU-15 | 8.1 ± 0.8 |
| L4Apt-5FU-15 | 8.3 ± 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Daraba, O.M.; Gherghel, D.; Vochita, G.; Popa, M. Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma. Polymers 2019, 11, 1515. https://doi.org/10.3390/polym11091515
Cadinoiu AN, Rata DM, Atanase LI, Daraba OM, Gherghel D, Vochita G, Popa M. Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma. Polymers. 2019; 11(9):1515. https://doi.org/10.3390/polym11091515
Chicago/Turabian StyleCadinoiu, Anca N., Delia M. Rata, Leonard I. Atanase, Oana M. Daraba, Daniela Gherghel, Gabriela Vochita, and Marcel Popa. 2019. "Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma" Polymers 11, no. 9: 1515. https://doi.org/10.3390/polym11091515