Tailor-Made Specific Recognition of Cyromazine Pesticide Integrated in a Potentiometric Strip Cell for Environmental and Food Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. MIPs Synthesis
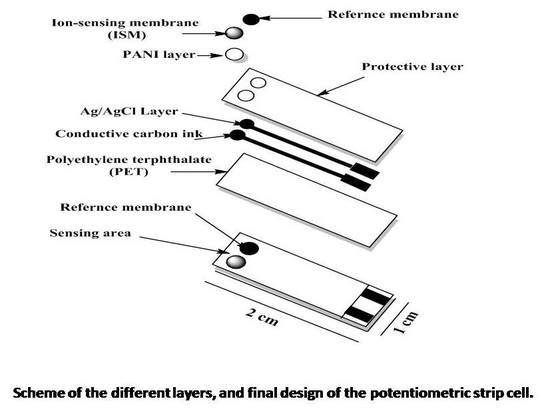
2.3. Planar Electrode Development
2.4. Potentiometric Measurements
2.5. Constant-Current Chronopotentiometry
2.6. Applications to Real Samples
3. Results and Discussions
3.1. Characterization of MIP Particles
3.2. Characteristics of the Proposed Sensors
3.3. Potential Stability and Conditioning Time
3.4. Analytical Applications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barceló, D.; Hennion, M.C. Trace Determination of Pesticides and Their Degradation Products in Water, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Patakioutas, G.; Savvas, D.; Matakoulis, C.; Sakellarides, T.; Albanis, T. Application and Fate of Cyromazine in a Closed-Cycle Hydroponic Cultivation of Bean (Phaseolus vulgarisL.). J. Agric. Food Chem. 2007, 55, 9928–9935. [Google Scholar] [CrossRef] [PubMed]
- Hyman, W. Propethampos, cyromazine and blowfly strike in sheep. J. S. Afr. Vet. Assoc. 1988, 59, 180. [Google Scholar] [PubMed]
- Lonsdale, B.; Tarry, D.W.; Bowen, F.I.; Stansfield, D.G. Cyromazine pour-on for the prevention of cutaneous myiasis of sheep. Vet. Rec. 1990, 126, 207–210. [Google Scholar] [PubMed]
- USEPA/Office of Pesticides Products Programs, Cyromazine Summary Document. Registration Review: Initial Docket, Document ID: EPA-HQ-OPP-2006-0108-0003, 2007, 41. Available online: http://www.regulations.gov (accessed on 19 September 2019).
- Shamshad, A.; Clift, A.D.; Mansfield, S. Effect of compost and casing treatments of insecticides against the sciarid Bradysia ocellaris (Diptera: Sciaridae) and on the total yield of cultivated mushrooms. Agaricus bisporus. Pest. Manag. Sci. 2009, 65, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Federal Register Environmental Documents, US Environmental Protection Agency, PA, USA. 2000. Available online: http://www.epa.gov/fedrgstr/ (accessed on 19 September 2019).
- Heikal, T.; Mossa, A.; Marei, G.; Abdel Rasoul, M. Cyromazine and Chlorpyrifos induced renal toxicity in rats: The ameliorating effects of green tea extract. J. Environ. Anal. Toxicol. 2012, 2, 146–152. [Google Scholar] [CrossRef]
- Lim, L.O.; Scherer, S.J.; Shuler, K.D.; Toth, J.P. Disposition of cyromazine in plants under environmental conditions. J. Agric. Food Chem. 1990, 38, 860–864. [Google Scholar] [CrossRef]
- Goutailler, G.; Valette, J.C.; Guillard, C.; Paїssé, O.; Faure, R. Photocatalysed degradation of cyromazine in aqueous titanium dioxide suspensions: Comparison with photolysis. J. Photoch. Photobio. A 2001, 141, 79–84. [Google Scholar] [CrossRef]
- Ricca, V.; Mannucci, E.; Calabrò, A.; Di Bernardo, M.; Cabras, P.L. Anorexia nervosa and celiac disease: Two case reports. Int. J. Eat. Disord. 2000, 27, 199–222. [Google Scholar] [CrossRef]
- Chan, E.; Griffiths, S.; Chan, C. Public-health risks of melamine in milk products. Lancet 2008, 372, 1444–1445. [Google Scholar] [CrossRef]
- Codex General Standard for Contaminants and Toxins in Food and Feed. Codex Standard 193-1995 (2013). Available online: http://www.codexalimentarius.org/download/standards/17/CXS_193e.Pdf (accessed on 19 September 2019).
- Ministry of Health, Labour and Welfare. The Japanese Positive List System for Agricultural Chemical Residues in Foods (Enforcement on May 29, 2006): Maximum Residue Limits (MRLs) of Agricultural Chemicals in Foods. 2006. Available online: http://www.ffcr.or.jp/zaidan/ FFCRHOME.nsf/pages/MRLs-p (accessed on 29 May 2006).
- The European Parliament and the Council of the European Union. European Regulation (EC) No 396/2005 of the European parliament and of the council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Off. J. Eur. Union L70 2005, 48, 1–16. [Google Scholar]
- Yokley, R.A.; Mayer, L.C.; Rezaaiyan, R.; Manuli, M.E.; Cheung, M.W. Analytical method for the determination of cyromazine and melamine residues in soil using LC-UV and GCMSD. J. Agric. Food Chem. 2000, 48, 3352–3358. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, Q.; Li, D.; Wang, S.; Wang, X. Determination of residues of cyromazine and its metabolite melamine in chickens by gas chromatography-mass spectrometry. Se Pu 2009, 27, 401–405. [Google Scholar] [PubMed]
- Zhu, X.; Wang, S.; Liu, Q.; Xu, Q.; Xu, S.; Chen, H. Determination of residues of cyromazine and its metabolite, melamine, in animal-derived food by gas chromatography-mass spectrometry with derivatization. J. Agric. Food Chem. 2009, 57, 11075–11080. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Chen, Y.; Wang, Z.; Yang, W.; Zhang, L. Development and validation of a gas chromatography-mass spectrometry method for the simultaneous determination of melamine and cyromazine in animal feeds. J. Anim. Veter. Adv. 2011, 10, 73–80. [Google Scholar]
- Wei, R.; Wang, R.; Zeng, Q.; Chen, M.; Liu, T. High-performance liquid chromatographic method for the determination of cyromazine and melamine residues in milk and pork. J. Chromatogr. Sci. 2009, 47, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Qin, X.; Ge, X.; Wang, L. Effective separation and sensitive determination of cyanuric acid, melamine and cyromazine in environmental water by reversed phase high-performance liquid chromatography. Environ. Technol. 2011, 32, 317–323. [Google Scholar] [CrossRef]
- Ge, X.; Wu, X.; Liang, S.; Su, M.; Sun, H. Trace residue analysis of dicyandiamide, cyromazine, and melamine in animal tissue foods by ultra-performance liquid chromatography. J. Food Drug Anal. 2016, 24, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Chen, L.; Bai, B.; Yang, X.; Tan, Y.; Wang, J.; Zhao, X.; Zhou, C. Liquid chromatography-mass spectrometry method for evaluating the dissipation dynamics of cyromazine and its metabolite in Agaricus bisporus and dietary risk assessment. Environ. Sci. Pollut. Res. 2018, 25, 2285–2292. [Google Scholar] [CrossRef]
- Hou, X.; Zhou, D.; Huai, W.; Beier, R.C.; Sun, Y.; Lu, Y.; Wu, G.; Sun, Z.; Wu, Y. Simultaneous determination of cyromazine and dicyclanil in animal edible tissues using UPLC-MS/MS. Food Addit. Contam. Part A 2013, 30, 660–665. [Google Scholar] [CrossRef]
- Wang, P.C.; Lee, R.J.; Chen, C.Y.; Chou, C.C.; Lee, M.R. Determination of cyromazine and melamine in chicken eggs using quick, easy, cheap, effective, rugged and safe (QuEChERS) extraction coupled with liquid chromatographytandem mass spectrometry. Anal. Chim. Acta 2012, 752, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Chen, L.; Sun, B.; Ai, S. Fluorescence detection of cyromazine using gallic acid-reduced gold nanoparticles. Sens. Actuators B 2012, 174, 458–464. [Google Scholar] [CrossRef]
- Sun, H.; Liu, N.; Wang, L.; Wu, Y. Effective separation and simultaneous detection of cyromazine and melamine in food by capillary electrophoresis. Electrophoresis 2010, 31, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhong, Y.; Liu, J.; Zhang, H.; Xi, J.; Wang, J. An enzyme linked immunosorbent assay for the determination of cyromazine and melamine residues in animal muscle tissues. Food Control 2010, 21, 1482–1487. [Google Scholar] [CrossRef]
- Le, T.; Yan, P.; Xu, J.; Hao, Y. A novel colloidal gold-based lateral flow immunoassay for rapid simultaneous detection of cyromazine and melamine in foods of animal origin. Food Chem. 2013, 138, 1610–1615. [Google Scholar] [CrossRef]
- Armenta, S.; Quintás, G.; Garrigues, S.; Guardia, M. Determination of cyromazine in pesticide commercial formulations by vibrational spectrometric procedures. Anal. Chim. Acta 2004, 524, 257–264. [Google Scholar] [CrossRef]
- Crespo, G.A. Recent Advances in Ion-selective membrane electrodes for in situ environmental water analysis. Electrochim. Acta 2017, 245, 1023–1034. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Kamel, A.H.; Soror, T.Y.; Al-Romian, F.M. Graphite Solid-Contact Mepiquat Potentiometric Sensors Based on Molecularly Imprinted Polymers and Their Application to Flow Through Analysis. Anal. Methods 2012, 4, 3007–3012. [Google Scholar] [CrossRef]
- Kamel, A.H.; Hassan, A.M. E Solid Contact Potentiometric Sensors Based on Host-Tailored Molecularly Imprinted Polymers for Creatine Assessment. Int. J. Electrochem. Sci. 2016, 11, 8938–8949. [Google Scholar] [CrossRef]
- El-Kosasy, A.; Kamel, A.H.; Hussin, L.; Ayad, M.F.; Fares, N. Mimicking new receptors based on molecular imprinting and their application to potentiometric assessment of 2,4-dichlorophenol as a food taint. Food Chem. 2018, 250, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.H.; Jiang, X.; Li, P.; Liang, R. A paper-based potentiometric sensing platform based on molecularly imprinted nanobeads for determination of bisphenol A. Anal. Methods 2018, 10, 3890–3895. [Google Scholar] [CrossRef]
- Abdalla, N.S.; Youssef, M.A.; Algarni, H.; Awwad, N.S.; Kamel, A.H. All Solid-State Poly(Vinyl Chloride) Membrane Potentiometric Sensor Integrated with Nano-Beads Imprinted Polymers for Sensitive and Rapid Detection of Bispyribac Herbicide as Organic Pollutant. Molecules 2019, 24, 712. [Google Scholar] [CrossRef] [PubMed]
- Rubinova, N.; Chumbimuni-Torres, K.Y.; Bakker, E. Solid-contact potentiometric polymer membrane microelectrodes for the detection of silver ions at the femtomole level. Sens. Actuators B 2007, 12, 135–141. [Google Scholar] [CrossRef]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable potentiometric ion sensors. Trends Anal. Chem. 2019, 110, 303–320. [Google Scholar] [CrossRef]
- Lindner, E.; Gyurcsányi, R.J. Quality control criteria for solid-contact, solvent polymeric membrane ion-selective electrodes. J. Solid State Electrochem. 2009, 13, 51–68. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Li, P.; Liang, R.; Yang, X.; Qin, W. Imprinted nanobead-based disposable screen-printed potentiometric sensor for highly sensitive detection of 2-naphthoic acid. Mater. Lett. 2018, 25, 138–141. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recommendations for nomenclature of ion selective electrodes (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Lindner, E.; Umezawa, Y. Performance evaluation criteria for preparation and measurement of macro- and microfabricated ion-selective electrodes (IUPAC Technical Report). Pure Appl. Chem. 2008, 80, 85–104. [Google Scholar] [CrossRef]
- FSIS. Cyromazine and Melamine. United States Department of Agriculture, Food Safety and Inspection Service. 1991 July; pp. 1–29. Available online: http://www.fsis.usda.gov/ophs/clg/Cyromazine.pdf (accessed on 18 July 1991).
- Available online: https://Pubchem.ncbi.nlm.nih.gov (accessed on 19 September 2019).
- Bakker, E. Determination of Unbiased Selectivity Coefficients of Neutral Carrier-Based Cation Selective Electrodes. Anal. Chem. 1997, 69, 1061–1069. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]






| Parameter | MIP/PANI-ISE | NIP/PANI-ISE | TPB/PAN-ISE |
|---|---|---|---|
| Slope (mV/decade) | 28.1 ± 2.1 | 12.7 ± 0.5 | 36.4 ± 1.6 |
| Correlation coefficient (r2) | 0.9999 | 0.9957 | 0.99916 |
| Detection limit (M) | 2.2 × 10−6 | 5.0 × 10−6 | 8.13 × 10−6 |
| Linear range (M) | 5.2 × 10−6 | 1.0 × 10−5 | 5.7 × 10−5 |
| Working pH range (pH) | 3–4.5 | 3–4.5 | 3.0–4.5 |
| Response time (s) | <10 | <10 | <10 |
| Accuracy (mV%) | 99.2 | 98.2 | 99.1 |
| Precision (mV%) | 1.1 | 0.9 | 1.3 |
| Interfering Ion, X | log KpotM,X | |
|---|---|---|
| MIP/PANI-ISE | TPB/PAN-ISE | |
| Diaquat | −2.03 | −3.74 |
| Acetamipride | −2.80 | −4.63 |
| Dinotefuran | −4.02 | −6.07 |
| Imidachloprid | −4.35 | −6.44 |
| Bispyribac | −4.26 | −6.53 |
| Flucarbazone | −6.01 | −7.27 |
| Melamine | −5.32 | −4.12 |
| Atrazine | −4.11 | −3.91 |
| Na+ | −7.51 | −2.43 |
| K+ | −6.62 | −2.34 |
| Cu2+ | −8.1 | −4.13 |
| Fe3+ | −9.31 | −5.34 |
| Commercial Product | Label (w%/v%) | a Found | |||
|---|---|---|---|---|---|
| Potentiometry | RSD, % | Official Standard Method [45] | RSD, % | ||
| Nomenee-kz, Kafr El-Zayat Pesticides and Chemicals Company (Gharbia, Egypt) | 3 | 2.78 ± 0.2 | 92.6 | 2.83 ± 0.05 | 94.3 |
| Sample | Amount of Cyromazine (µg/g) | |
|---|---|---|
| Potentiometry | Official Standard Method [45] a | |
| Sample 1 | 5.6 ± 0.7 | 4.7 ± 0.6 |
| Sample 2 | 8.3 ± 0.3 | 8.7 ± 0.2 |
| Sample 3 | 10.2 ± 0.5 | 9.3 ± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, N.S.; E. Amr, A.E.-G.; S. M. El-Tantawy, A.; A. Al-Omar, M.; H. Kamel, A.; Khalifa, N.M. Tailor-Made Specific Recognition of Cyromazine Pesticide Integrated in a Potentiometric Strip Cell for Environmental and Food Analysis. Polymers 2019, 11, 1526. https://doi.org/10.3390/polym11091526
Abdalla NS, E. Amr AE-G, S. M. El-Tantawy A, A. Al-Omar M, H. Kamel A, Khalifa NM. Tailor-Made Specific Recognition of Cyromazine Pesticide Integrated in a Potentiometric Strip Cell for Environmental and Food Analysis. Polymers. 2019; 11(9):1526. https://doi.org/10.3390/polym11091526
Chicago/Turabian StyleAbdalla, Nashwa S., Abd El-Galil E. Amr, Aliaa S. M. El-Tantawy, Mohamed A. Al-Omar, Ayman H. Kamel, and Nagy M. Khalifa. 2019. "Tailor-Made Specific Recognition of Cyromazine Pesticide Integrated in a Potentiometric Strip Cell for Environmental and Food Analysis" Polymers 11, no. 9: 1526. https://doi.org/10.3390/polym11091526