Polysaccharide Extracted from Bletilla striata Promotes Proliferation and Migration of Human Tenocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of Polysaccharide from BS
2.3. Cytotoxicity of BSP
2.4. Isolation and Culture of HTs
2.5. Gene Expression of HTs
2.6. Western Blot Analysis
2.7. Gap Closure Migration Assay
2.8. Transwell Migration Assay
2.9. ECM Synthesis Test
2.10. Statistical Analysis
3. Results
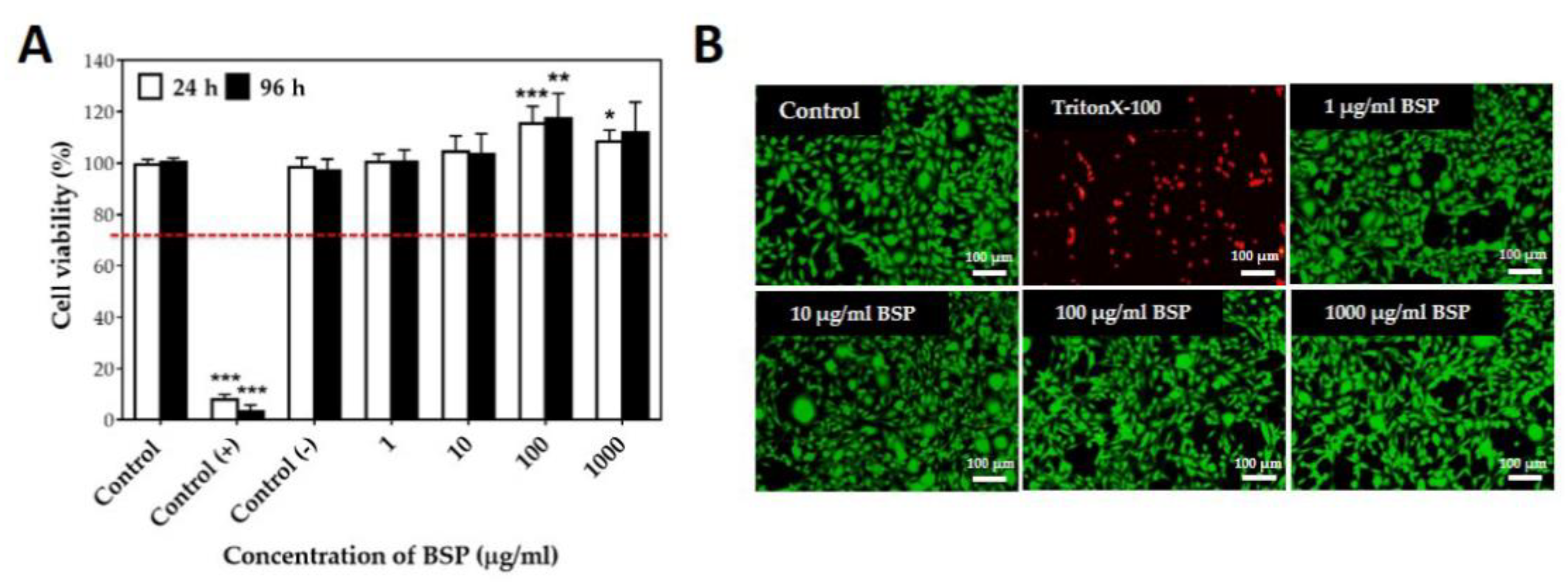
3.1. Characterization and Cytotoxicity of BSP
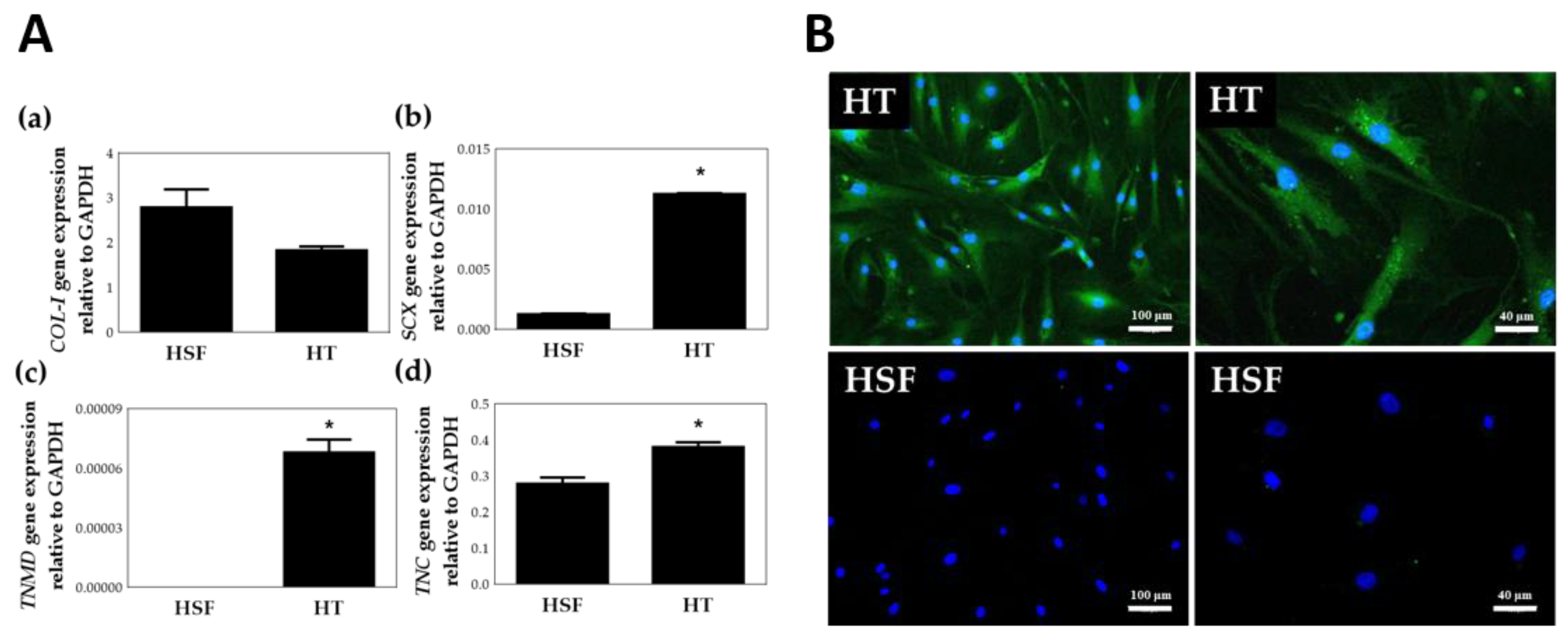
3.2. Identification of HTs
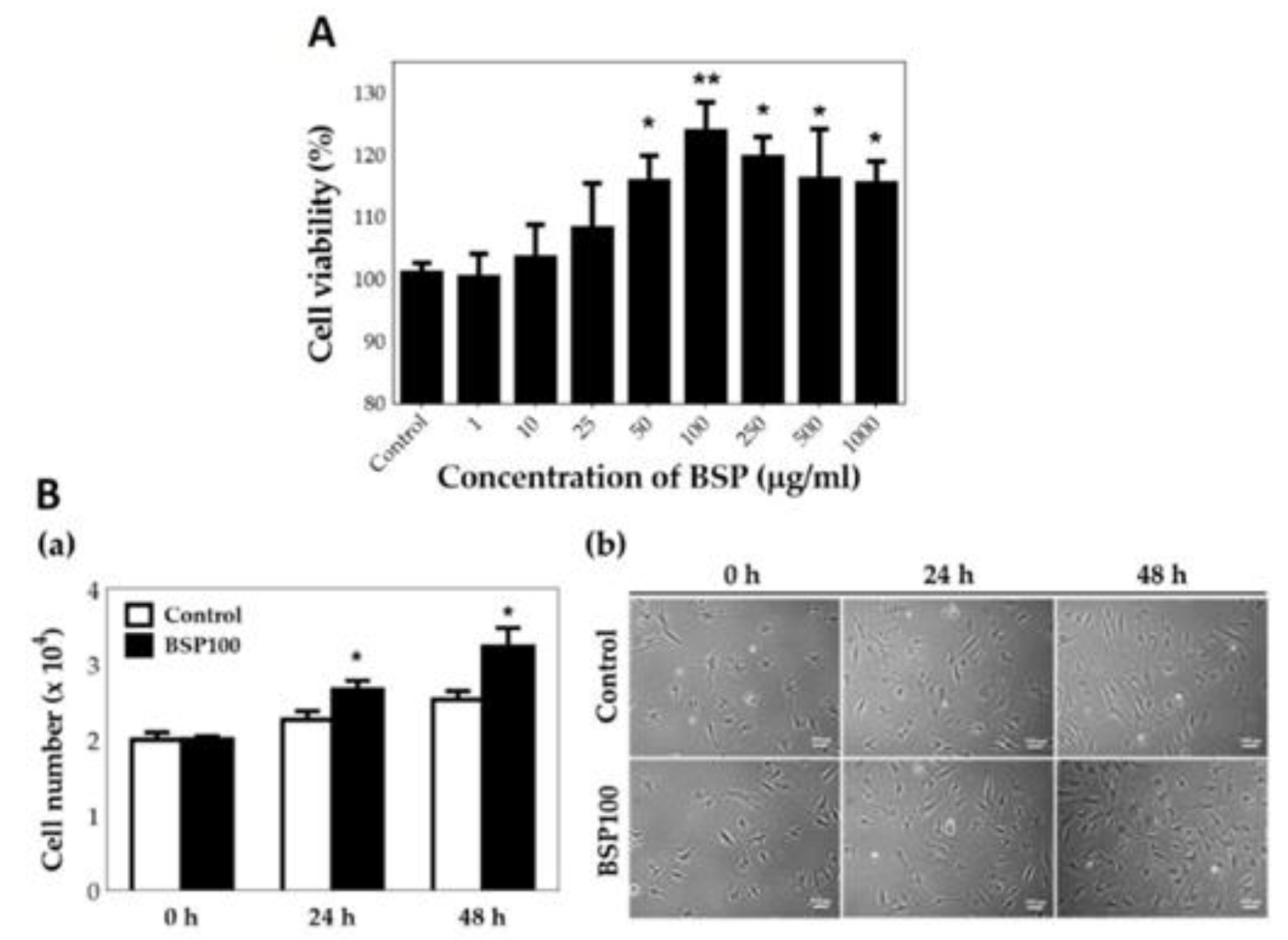
3.3. HT Proliferation Test
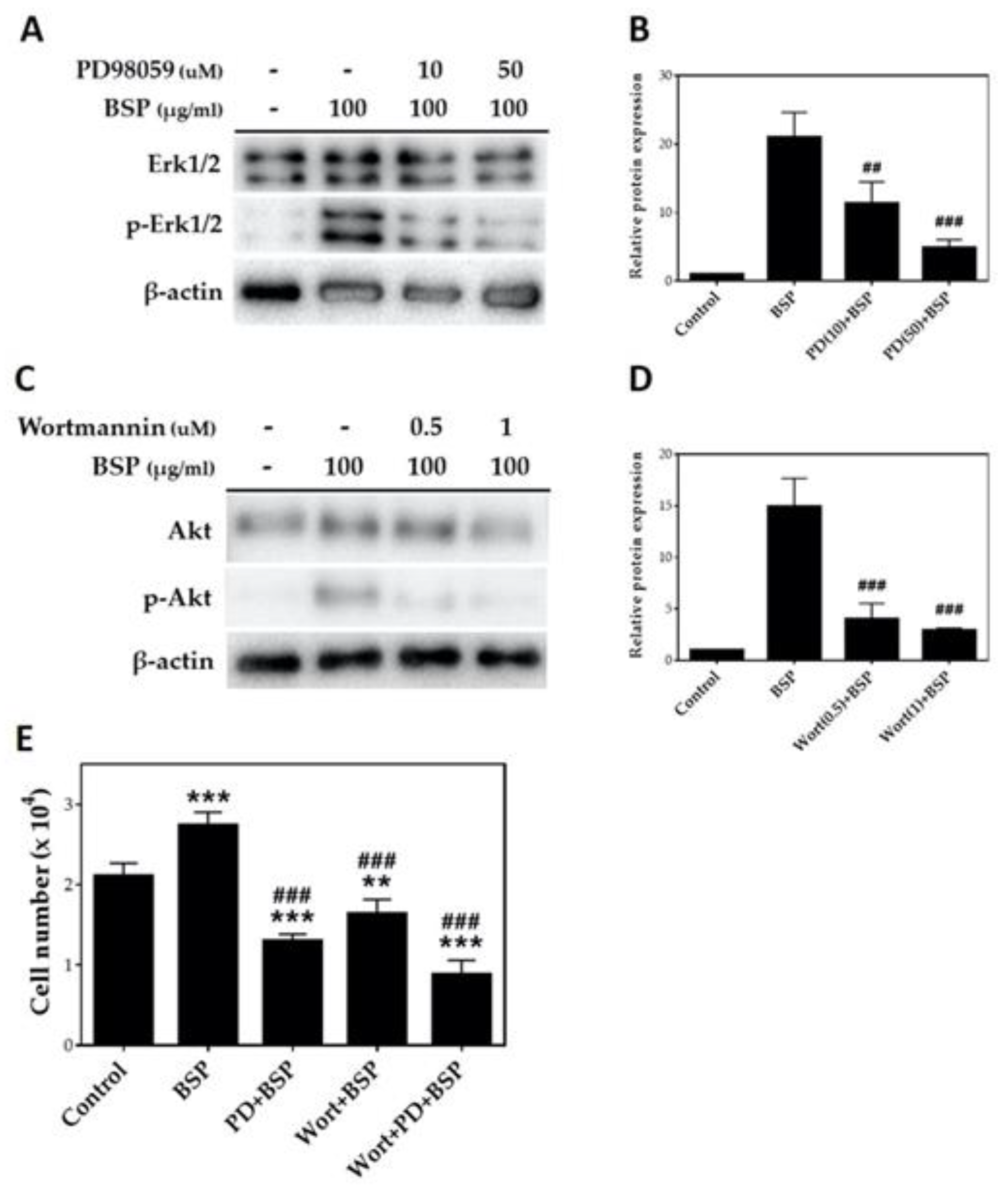
3.4. HT Migration Test
3.5. HT ECM Synthesis Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon healing: Repair and regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Tendon injury and tendinopathy: Healing and repair. J. Bone Joint Surg. Am. 2005, 87, 187–202. [Google Scholar] [PubMed] [Green Version]
- Riley, G.P. Gene expression and matrix turnover in overused and damaged tendons. Scand J. Med. Sci. Sports 2005, 15, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshita, T.; Tobita, M.; Tajima, S.; Mizuno, H. Adipose-derived stem cells improve collagenase-induced tendinopathy in a rat model. Am. J. Sports Med. 2016, 44, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Scott, R.; Anderson, I. Hand problems in an accident and emergency department. J. Hand Surg. Br. Eur. Vol. 1985, 10, 297–299. [Google Scholar] [CrossRef]
- de Jong, J.P.; Nguyen, J.T.; Sonnema, A.J.; Nguyen, E.C.; Amadio, P.C.; Moran, S.L. The incidence of acute traumatic tendon injuries in the hand and wrist: A 10-year population-based study. Clin. Orthop. Surg. 2014, 6, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, Y.C.; Rui, Y.F.; Xu, H.L.; Chen, H.; Wang, C.; Teng, G.J. Therapeutic roles of tendon stem/progenitor cells in tendinopathy. Stem. Cells Int. 2016, 2016, 4076578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffulli, N.; Wong, J.; Almekinders, L.C. Types and epidemiology of tendinopathy. Clin. Sports Med. 2003, 22, 675–692. [Google Scholar] [CrossRef]
- Walden, G.; Liao, X.; Donell, S.; Raxworthy, M.J.; Riley, G.P.; Saeed, A. A Clinical, Biological, and Biomaterials Perspective into Tendon Injuries and Regeneration. Tissue Eng. Part B Rev. 2017, 23, 44–58. [Google Scholar] [CrossRef] [Green Version]
- James, R.; Kesturu, G.; Balian, G.; Chhabra, A.B. Tendon: Biology, biomechanics, repair, growth factors, and evolving treatment options. J. Hand Surg. Am. 2008, 33, 102–112. [Google Scholar] [CrossRef]
- Chien, C.H.; Huang, J.F.; Chen, L.L.; Tsai, W.C.; Pang, J.H.S.; Liao, Y.H.; Yu, T.Y. The effects of hyaluronic acid and glucosamine on the migration and proliferation of tenocytes. Taiwan J. Phys. Med. Rehabil. 2013, 41, 13–19. [Google Scholar]
- Schneider, M.; Angele, P.; Jarvinen, T.A.H.; Docheva, D. Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv. Drug Deliv. Rev. 2018, 129, 352–375. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Yeh, W.L.; Chao, C.L.; Hsu, Y.H.; Yu, Y.H.; Chen, J.K.; Liu, S.J. Enhancement of tendon-bone healing via the combination of biodegradable collagen-loaded nanofibrous membranes and a three-dimensional printed bone-anchoring bolt. Int. J. Nanomed. 2016, 11, 4173–4186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evrova, O.; Houska, J.; Welti, M.; Bonavoglia, E.; Calcagni, M.; Giovanoli, P.; Vogel, V.; Buschmann, J. Bioactive, elastic, and biodegradable emulsion electrospun DegraPol tube delivering PDGF-BB for tendon rupture repair. Macromol. Biosci. 2016, 16, 1048–1063. [Google Scholar] [CrossRef]
- Li, Z.; Shen, X.; Cao, L.; Yuan, Z.; Chen, S.; Zheng, X.; Tang, M.; Lee, K.K.; Cai, D. Bone morphogenetic protein 2 improves patellar tendon healing by promoting migration and proliferation of tenocytes. Chin. Sci. Bull. 2011, 56, 1361–1369. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Qiu, Y.; Triffitt, J.; Carr, A.; Xia, Z.; Sabokbar, A. Proliferation and differentiation of human tenocytes in response to platelet rich plasma: An in vitro and in vivo study. J. Orthop. Res. 2012, 30, 982–990. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, Q.; Zhuang, W.; Zhou, J.; Zhang, B.; Xu, P.; Ju, Y.; Morita, Y.; Luo, Q.; Song, G. Tenocyte proliferation and migration promoted by rat bone marrow mesenchymal stem cell-derived conditioned medium. Biotechnol. Lett. 2018, 40, 215–224. [Google Scholar] [CrossRef]
- Hasan, N.; Al Sorkhy, M. Herbs that promote cell proliferation. Int. J. Herbal Med. 2014, 1, 18–21. [Google Scholar]
- Vidya Udagama, P.; Udalamaththa, V. Application of herbal medicine as proliferation and differentiation effectors of human stem cells. In Herbal Medicine; IntechOpen: London, UK, 2019. [Google Scholar]
- Si, Y.C.; Li, Q.; Xie, C.E.; Niu, X.; Xia, X.H.; Yu, C.Y. Chinese herbs and their active ingredients for activating xue (blood) promote the proliferation and differentiation of neural stem cells and mesenchymal stem cells. Chin. Med. 2014, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Rastegar, H.; Ashtiani, H.A.; Aghaei, M.; Barikbin, B.; Ehsani, A. Herbal extracts induce dermal papilla cell proliferation of human hair follicles. Ann. Dermatol. 2015, 27, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Sun, J.; Luo, Y.; Xue, W.; Diao, H.; Dong, L.; Chen, J.; Zhang, J. A polysaccharide isolated from the medicinal herb Bletilla striata induces endothelial cells proliferation and vascular endothelial growth factor expression in vitro. Biotechnol. Lett. 2006, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.G.; Xin, M.; Chen, H.; Yang, L.N.; Jiang, H.R. Novel mucoadhesive polysaccharide isolated from Bletilla striata improves the intraocular penetration and efficacy of levofloxacin in the topical treatment of experimental bacterial keratitis. J. Pharm. Pharmacol. 2010, 62, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Li, M.; Xue, F.; Liu, H. Structure and immunobiological activity of a new polysaccharide from Bletilla striata. Carbohydr. Polym. 2014, 107, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Diao, H.; Xia, S.; Dong, L.; Chen, J.; Zhang, J. A physiologically active polysaccharide hydrogel promotes wound healing. J. Biomed. Mater. Res. A 2010, 94, 193–204. [Google Scholar] [CrossRef]
- Jiang, F.; Li, W.; Huang, Y.; Chen, Y.; Jin, B.; Chen, N.; Ding, Z.; Ding, X. Antioxidant, antityrosinase and antitumor activity comparison: The potential utilization of fibrous root part of Bletilla striata (Thunb.) Reichb.f. PLoS ONE 2013, 8, e58004. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Chen, S.; Wang, Y.; Jiang, H.; Yin, H. A new glucomannan from Bletilla striata: Structural and anti-fibrosis effects. Fitoterapia 2014, 92, 72–78. [Google Scholar] [CrossRef]
- Lai, Y.-L.; Lin, Y.-Y.; Sadhasivam, S.; Kuan, C.-Y.; Chi, C.-Y.; Dong, G.-C.; Lin, F.-H. Efficacy of Bletilla striata polysaccharide on hydrogen peroxide-induced apoptosis of osteoarthritic chondrocytes. J. Polym. Res. 2018, 25, 49. [Google Scholar] [CrossRef]
- Wang, C.; Luo, W.; Li, P.; Li, S.; Yang, Z.; Hu, Z.; Liu, Y.; Ao, N. Preparation and evaluation of chitosan/alginate porous microspheres/Bletilla striata polysaccharide composite hemostatic sponges. Carbohydr. Polym. 2017, 174, 432–442. [Google Scholar] [CrossRef]
- Ding, L.; Shan, X.; Zhao, X.; Zha, H.; Chen, X.; Wang, J.; Cai, C.; Wang, X.; Li, G.; Hao, J.; et al. Spongy bilayer dressing composed of chitosan-Ag nanoparticles and chitosan-Bletilla striata polysaccharide for wound healing applications. Carbohydr. Polym. 2017, 157, 1538–1547. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wang, M.C.; Miyagawa, T.; Chen, Z.Y.; Lin, F.H.; Chen, K.H.; Liu, G.S.; Tseng, C.L. Preparation of arginine-glycine-aspartic acid-modified biopolymeric nanoparticles containing epigalloccatechin-3-gallate for targeting vascular endothelial cells to inhibit corneal neovascularization. Int. J. Nanomed. 2017, 12, 279–294. [Google Scholar] [CrossRef] [Green Version]
- Nichols, A.E.C.; Settlage, R.E.; Werre, S.R.; Dahlgren, L.A. Novel roles for scleraxis in regulating adult tenocyte function. BMC Cell Biol. 2018, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Kacuráková, M. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Li, Q.; Yu, N.; Wang, Y.; Sun, Y.; Lu, K.; Guan, W. Extraction optimization of Bruguiera gymnorrhiza polysaccharides with radical scavenging activities. Carbohydr. Polym. 2013, 96, 148–155. [Google Scholar] [CrossRef]
- Lake, S.P.; Miller, K.S.; Elliott, D.M.; Soslowsky, L.J. Tensile properties and fiber alignment of human supraspinatus tendon in the transverse direction demonstrate inhomogeneity, nonlinearity, and regional isotropy. J. Biomech. 2010, 43, 727–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Wang, Y.; Streubel, P.N.; Duan, B. Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation. Acta Biomater. 2017, 62, 102–115. [Google Scholar] [CrossRef]
- Margaret, A.; Lawlor, D.R.A. PKB/Akt: A key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 2001, 114, 2903–2910. [Google Scholar]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Liu, S.Q.; Chen, Q.; Li, H.H.; Ding, W.J.; Deng, M. Carboxymethylated chitosan stimulates proliferation of Schwann cells in vitro via the activation of the ERK and Akt signaling pathways. Eur. J. Pharmacol. 2011, 667, 195–201. [Google Scholar] [CrossRef]
- Wipf, P.; Halter, R.J. Chemistry and biology of wortmannin. Org. Biomol. Chem. 2005, 3, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, J.; Fukui, K.; Okada, M.; Yamawaki, H. T3 peptide, a fragment of tumstatin, stimulates proliferation and migration of cardiac fibroblasts through activation of Akt signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 1135–1144. [Google Scholar] [CrossRef]
- Sugiyama, A.; Hirano, Y.; Okada, M.; Yamawaki, H. Endostatin Stimulates Proliferation and Migration of Myofibroblasts Isolated from Myocardial Infarction Model Rats. Int. J. Mol. Sci. 2018, 19, 741. [Google Scholar] [CrossRef] [Green Version]
- Duan, B.; Hockaday, L.A.; Das, S.; Xu, C.; Butcher, J.T. Comparison of mesenchymal stem cell source differentiation toward human pediatric aortic valve interstitial cells within 3D engineered matrices. Tissue Eng. Part C Methods 2015, 21, 795–807. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Cheng, L.; He, Y.; Wei, X. Extraction, characterization, utilization as wound dressing and drug delivery of Bletilla striata polysaccharide: A review. Int. J. Biol. Macromol. 2018, 120, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Nagata, Y.; Wada, E.; Zammit, P.S.; Shiozuka, M.; Matsuda, R. Zinc promotes proliferation and activation of myogenic cells via the PI3K/Akt and ERK signaling cascade. Exp. Cell Res. 2015, 333, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Deters, A.M.; Schroder, K.R.; Hensel, A. Kiwi fruit (Actinidia chinensis L.) polysaccharides exert stimulating effects on cell proliferation via enhanced growth factor receptors, energy production, and collagen synthesis of human keratinocytes, fibroblasts, and skin equivalents. J. Cell Physiol. 2005, 202, 717–722. [Google Scholar] [CrossRef]
- Shen, L.; Du, G. Lycium barbarum polysaccharide stimulates proliferation of MCF-7 cells by the ERK pathway. Life Sci. 2012, 91, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, T.; Cramer, L. Actin-based cell motility and cell locomotion. Cell 1996, 84, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar]
- Zubilewicz, A.; Hecquet, C.; Jeanny, J.; Soubrane, G.; Courtois, Y.; Mascarelli, F. Proliferation of CECs requires dual signaling through both MAPK/ERK and PI 3-K/Akt pathways. Invest. Ophthalmol. Vis. Sci. 2001, 42, 488–496. [Google Scholar] [PubMed]
- Tanimura, S.; Takeda, K. ERK signalling as a regulator of cell motility. J. Biochem. 2017, 162, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; He, Y.; Chen, Z.; Shi, J.; Qu, Y.; Zhang, J. Effect of Polysaccharides from Bletilla striata on the healing of dermal wounds in mice. Evid. Based Complement. Alternat. Med. 2019, 2019, 9212314. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Yield (%) | Total Sugar Content (%) | Molecular Weight (kDA) | |
|---|---|---|---|
| Previous BSP extraction method | 17.1 ± 0.2 | 68.5 ± 4.3 | 198 |
| Modified BSP extraction method | * 23.3 ± 0.1 | 73.9 ± 1.9 | 165 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.-Y.; Chen, S.-H.; Chen, C.-H.; Chou, P.-Y.; Yang, C.-C.; Lin, F.-H. Polysaccharide Extracted from Bletilla striata Promotes Proliferation and Migration of Human Tenocytes. Polymers 2020, 12, 2567. https://doi.org/10.3390/polym12112567
Chen Z-Y, Chen S-H, Chen C-H, Chou P-Y, Yang C-C, Lin F-H. Polysaccharide Extracted from Bletilla striata Promotes Proliferation and Migration of Human Tenocytes. Polymers. 2020; 12(11):2567. https://doi.org/10.3390/polym12112567
Chicago/Turabian StyleChen, Zhi-Yu, Shih-Heng Chen, Chih-Hao Chen, Pang-Yun Chou, Chun-Chen Yang, and Feng-Huei Lin. 2020. "Polysaccharide Extracted from Bletilla striata Promotes Proliferation and Migration of Human Tenocytes" Polymers 12, no. 11: 2567. https://doi.org/10.3390/polym12112567




_周(Chou).jpg)

