Tailoring Intrinsic Properties of Polyaniline by Functionalization with Phosphonic Groups
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Chemical Synthesis
2.3. Copolymer Characterization
2.3.1. Spectroscopic Analysis
2.3.2. Copolymer Composition
2.3.3. Solubility Testing
2.3.4. Electrochemical Characterization
2.3.5. Electrochemical Impedance Spectroscopy
2.3.6. UV-Vis Analysis
2.3.7. Thermogravimetric Analysis
2.3.8. Computational Calculations
3. Results and Discussion
3.1. Copolymer Composition
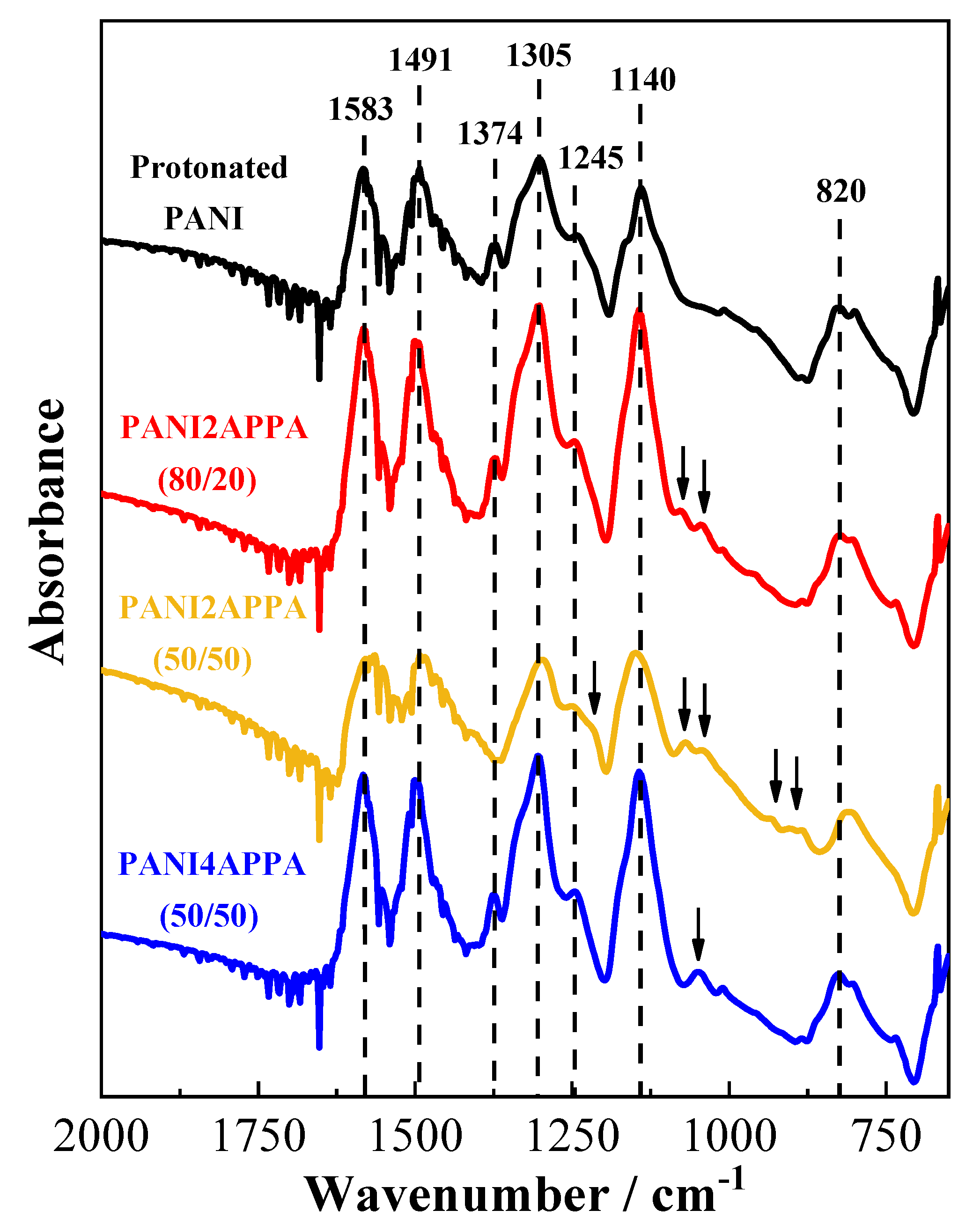
3.2. FTIR Spectroscopy
3.3. Solubility
3.4. Electrochemical Results
3.5. Electrochemical Impedance Spectroscopy
3.6. UV-Vis Spectroscopy
3.7. Thermal Stability
3.8. Computational Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, W.S.; Humphrey, B.D.; MacDiarmid, A.G. Polyaniline, a Novel Conducting Polymer. Morphology and Chemistry of Its Oxidation and Reduction in Aqueous Electrolytes. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 2385–2400. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G. Recent Advances in Polyaniline Research: Polymerization Mechanisms, Structural Aspects, Properties and Applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Hong, X.; Liu, Y.; Li, Y.; Wang, X.; Fu, J.; Wang, X. Application Progress of Polyaniline, Polypyrrole and Polythiophene in Lithium-Sulfur Batteries. Polymers 2020, 12, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Xu, L.; Li, W.; Waje, M.; Yan, Y. Polyaniline Nanofibre Supported Platinum Nanoelectrocatalysts for Direct Methanol Fuel Cells. Nanotechnology 2006, 17, 5254–5259. [Google Scholar] [CrossRef]
- Zhai, D.D.; Fang, Z.; Jin, H.; Hui, M.; Kirubaharan, C.J.; Yu, Y.Y.; Yong, Y.-C. Vertical Alignment of Polyaniline Nanofibers on Electrode Surface for High-Performance Microbial Fuel Cells. Bioresour. Technol. 2019, 288, 121499. [Google Scholar] [CrossRef] [PubMed]
- Shoaie, N.; Daneshpour, M.; Azimzadeh, M.; Mahshid, S.; Khoshfetrat, S.M.; Jahanpeyma, F.; Gholaminejad, A.; Omidfar, K.; Foruzandeh, M. Electrochemical Sensors and Biosensors Based on the Use of Polyaniline and Its Nanocomposites: A Review on Recent Advances. Microchim. Acta 2019, 186, 465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, B.; Li, X.; Xu, G.; Dou, S.; Zhang, X.; Chen, X.; Zhao, J.; Zhang, K.; Li, Y. Further Understanding of the Mechanisms of Electrochromic Devices with Variable Infrared Emissivity Based on Polyaniline Conducting Polymers. J. Mater. Chem. C. 2019, 7, 9878–9891. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Oxygen-Reduction Catalysis of N-Doped Carbons Prepared: Via Heat Treatment of Polyaniline at over 1100 °C. Chem. Commun. 2018, 54, 4441–4444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ćirić-Marjanović, G.; Pašti, I.; Gavrilov, N.; Janošević, A.; Mentus, S. Carbonised Polyaniline and Polypyrrole: Towards Advanced Nitrogen-Containing Carbon Materials. Chem. Pap. 2013, 67, 781–813. [Google Scholar] [CrossRef]
- Huang, X.; Yin, X.; Yu, X.; Tian, J.; Wu, W. Preparation of Nitrogen-Doped Carbon Materials Based on Polyaniline Fiber and Their Oxygen Reduction Properties. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 163–170. [Google Scholar] [CrossRef]
- Gabe, A.; Mostazo-López, M.J.; Salinas-Torres, D.; Morallón, E.; Cazorla-Amorós, D. Synthesis of Conducting Polymer/Carbon Material Composites and Their Application in Electrical Energy Storage. In Hybrid Polymer Composite Materials; Thakur, V., Thakur, M., Gupta, R., Eds.; Woodhead Publishing: Sawston/Cambridge, UK, 2017; pp. 173–209. [Google Scholar] [CrossRef]
- Rauhala, T.; Davodi, F.; Sainio, J.; Sorsa, O.; Kallio, T. On the Stability of Polyaniline/Carbon Nanotube Composites as Binder-Free Positive Electrodes for Electrochemical Energy Storage. Electrochim. Acta 2020, 336, 135735. [Google Scholar] [CrossRef]
- Ratlam, C.; Phanichphant, S.; Sriwichai, S. Development of Dopamine Biosensor Based on Polyaniline/Carbon Quantum Dots Composite. J. Polym. Res. 2020, 27, 183. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Rawool, C.R.; Karna, S.P.; Srivastava, A.K. Nitrogen-Doped Graphene/Palladium Nanoparticles/Porous Polyaniline Ternary Composite as an Efficient Electrode Material for High Performance Supercapacitor. Mater. Sci. Energy Technol. 2019, 2, 246–257. [Google Scholar] [CrossRef]
- Liao, G.; Li, Q.; Xu, Z. The Chemical Modification of Polyaniline with Enhanced Properties: A Review. Prog. Org. Coat. 2019, 126, 35–43. [Google Scholar] [CrossRef]
- Jaymand, M. Recent Progress in Chemical Modification of Polyaniline. Prog. Polym. Sci. 2013, 38, 1287–1306. [Google Scholar] [CrossRef]
- Malinauskas, A. Self-Doped Polyanilines. J. Power Sources 2004, 126, 214–220. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Strakhova, A.K.; Yatsimirsky, A.K. Self-Doped Polyanilines Electrochemically Active in Neutral and Basic Aqueous Solutions.: Electropolymerization of Substituted Anilines. J. Electroanal. Chem. 1994, 371, 259–265. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Acevedo, D.F.; Miras, M.C.; Motheo, A.J.; Barbero, C.A. Comparative Study of 2-Amino and 3-Aminobenzoic Acid Copolymerization with Aniline Synthesis and Copolymer Properties. J. Polym. Sci. Part. A Polym. Chem. 2004, 42, 5587–5599. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Kasai, P.; Miller, J.L.; Diaz, A.F. Synthesis and Properties of Novel Water-Soluble Conducting Polyaniline Copolymers. Macromolecules 1994, 27, 3625–3631. [Google Scholar] [CrossRef]
- Yue, J.; Epstein, A.J.; Macdiarmid, A.G. Sulfonic Acid Ring-Substituted Polyaniline, A Self-Doped Conducting Polymer. Mol. Cryst. Liq. Cryst. Inc. Nonlinear Opt. 1990, 189, 255–261. [Google Scholar] [CrossRef]
- Cataldo, F.; Maltese, P. Synthesis of Alkyl and N-Alkyl-Substituted Polyanilines: A Study on Their Spectral Properties and Thermal Stability. Eur. Polym. J. 2002, 38, 1791–1803. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Arias, J.; Garcés, P.; Morallón, E.; Barbero, C.; Vázquez, J.L. Spectroelectrochemical Study of the Oxidation of Aminophenols on Platinum Electrode in Acid Medium. J. Electroanal. Chem. 2004, 565, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Benyoucef, A.; Huerta, F.; Vázquez, J.L.; Morallon, E. Synthesis and in Situ FTIRS Characterization of Conducting Polymers Obtained from Aminobenzoic Acid Isomers at Platinum Electrodes. Eur. Polym. J. 2005, 41, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Sanchís, C.; Salavagione, H.J.; Arias-Pardilla, J.; Morallón, E. Tuning the Electroactivity of Conductive Polymer at Physiological pH. Electrochim. Acta 2007, 52, 2978–2986. [Google Scholar] [CrossRef]
- Arias-Pardilla, J.; Salavagione, H.J.; Barbero, C.; Morallón, E.; Vázquez, J.L. Study of the Chemical Copolymerization of 2-Aminoterephthalic Acid and Aniline.: Synthesis and Copolymer Properties. Eur. Polym. J. 2006, 42, 1521–1532. [Google Scholar] [CrossRef]
- Chan, H.S.O.; Ng, S.C.; Ho, P.K.H. Polyanilines Doped with Phosphonic Acids: Their Preparation and Characterization. Macromolecules 1994, 27, 2159–2164. [Google Scholar] [CrossRef]
- Ghil, L.J.; Youn, T.Y.; Park, N.R.; Rhee, H.W. Proton Conductive Nano-Channel Membranes Based on Polyaniline with Phosphonic Acid Moieties for Low Relative Humidity. J. Nanosci. Nanotechnol. 2013, 13, 7912–7915. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Ghisolfi, A.; Grau-Marín, D.; San-Fabián, E.; Morallón, E.; Cazorla-Amorós, D. Post-Synthetic Efficient Functionalization of Polyaniline with Phosphorus-Containing Groups. Effect of Phosphorus on Electrochemical Properties. Eur. Polym. J. 2019, 119, 272–280. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.J.; Guo, L.R.; Li, J.; Xia, X.H.; Zheng, L.M. Direct Electrochemistry and Electrocatalysis of Hemoglobin at Three-Dimensional Gold Film Electrode Modified with Self-Assembled Monolayers of 3-Mercaptopropylphosphonic Acid. Anal. Chim. Acta 2009, 644, 83–89. [Google Scholar] [CrossRef]
- Panella, L.; Broos, J.; Jin, J.; Fraaije, M.W.; Janssen, D.B.; Jeronimus-Stratingh, M.; Feringa, B.L.; Minnaard, A.J.; De Vries, J.G. Merging Homogeneous Catalysis with Biocatalysis; Papain as Hydrogenation Catalyst. Chem. Commun. 2005, 23, 5656–5658. [Google Scholar] [CrossRef]
- Quintero-Jaime, A.F.; Cazorla-Amorós, D.; Morallón, E. Electrochemical Functionalization of Carbon Nanomaterials and Their Application in Immobilization of Enzymes. In Nanomaterials for Bio-Catalysis; Castro, G., Kurma, A., Nguyen, Y., Qi, X., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands.
- Papadimitriou, K.D.; Andreopoulou, A.K.; Kallitsis, J.K. Phosphonated Fully Aromatic Polyethers for PEMFCs Applications. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2817–2827. [Google Scholar] [CrossRef]
- Chan, H.S.O.; Ho, P.K.H.; Ng, S.C.; Tan, B.T.G.; Tan, K.L. A New Water-Soluble, Self-Doping Conducting Polyaniline from Poly(o-Aminobenzylphosphonic Acid) and Its Sodium Salts: Synthesis and Characterization. J. Am. Chem. Soc. 1995, 117, 8517–8523. [Google Scholar] [CrossRef]
- Amaya, T.; Abe, Y.; Inada, Y.; Hirao, T. Synthesis of Self-Doped Conducting Polyaniline Bearing Phosphonic Acid. Tetrahedron Lett. 2014, 55, 3976–3978. [Google Scholar] [CrossRef]
- Amaya, T.; Kurata, I.; Inada, Y.; Hatai, T.; Hirao, T. Synthesis of Phosphonic Acid Ring-Substituted Polyanilines via Direct Phosphonation to Polymer Main Chains. RSC Adv. 2017, 7, 39306–39313. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Lucht, B.L. Poly-p-Phenylene Phosphine/Polyaniline Alternating Copolymers: Electronic Delocalization through Phosphorus. J. Am. Chem. Soc. 2005, 127, 5586–5595. [Google Scholar] [CrossRef] [PubMed]
- Bober, P.; Trchová, M.; Morávková, Z.; Kovářová, J.; Vulić, I.; Gavrilov, N.; Pašti, I.A.; Stejskal, J. Phosphorus and Nitrogen-Containing Carbons Obtained by the Carbonization of Conducting Polyaniline Complex with Phosphites. Electrochim. Acta 2017, 246, 443–450. [Google Scholar] [CrossRef]
- Martínez-Sánchez, B.; Quintero-Jaime, A.F.; Huerta, F.; Cazorla-Amorós, D.; Morallón, E. Synthesis of Phosphorus-Containing Polyanilines by Electrochemical Copolymerization. Polymers 2020, 12, 1029. [Google Scholar] [CrossRef] [PubMed]
- Odian, G. Chain Copolymerization. In Principles of Polymerization, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NY, USA, 1991; pp. 464–543. [Google Scholar] [CrossRef]
- Ramachandran, K.I.; Deepa, G.; Namboori, K. Computational Chemistry and Molecular Modeling; Springer: Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian Development Version, Revision I. 13; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. The Intrinsic Redox States in Polypyrrole and Polyaniline: A Comparative Study by XPS. Surf. Interface Anal. 1992, 19, 33–37. [Google Scholar] [CrossRef]
- Botelho do Rego, A.M.; Ferraria, A.M.; El Beghdadi, J.; Debontridder, F.; Brogueira, P.; Naaman, R.; Rei Vilar, M. Adsorption of Phenylphosphonic Acid on GaAs (100) Surfaces. Langmuir 2005, 21, 8765–8773. [Google Scholar] [CrossRef]
- Blanchard, P.E.R.; Grosvenor, A.P.; Cavell, R.G.; Mar, A. X-Ray Photoelectron and Absorption Spectroscopy of Metal-Rich Phosphides M2P and M3P (M = Cr−Ni). Chem. Mater. 2008, 20, 7081–7088. [Google Scholar] [CrossRef]
- Quintero-Jaime, A.F.; Cazorla-Amorós, D.; Morallón, E. Electrochemical Functionalization of Single Wall Carbon Nanotubes with Phosphorus and Nitrogen Species. Electrochim. Acta 2020, 340, 135935. [Google Scholar] [CrossRef]
- Brožová, L.; Holler, P.; Kovářová, J.; Stejskal, J.; Trchová, M. The Stability of Polyaniline in Strongly Alkaline or Acidic Aqueous Media. Polym. Degrad. Stab. 2008, 93, 592–600. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Hampton, C.; Demoin, D.; Glaser, R. Vibrational Spectroscopy Tutorial: Sulfur and Phosphorus, Vib. Spectrosc. 2010. Available online: http://faculty.missouri.edu/~glaserr/8160f10/A03_Silver.pdf (accessed on 25 August 2020).
- Shao, W.; Jamal, R.; Xu, F.; Ubul, A.; Abdiryim, T. The Effect of a Small Amount of Water on the Structure and Electrochemical Properties of Solid-State Synthesized Polyaniline. Materials 2012, 5, 1811–1825. [Google Scholar] [CrossRef] [Green Version]
- Duić, L.; Mandić, Z.; Kovač, S. Polymer-Dimer Distribution in the Electrochemical Synthesis of Polyaniline. Electrochim. Acta 1995, 40, 1681–1688. [Google Scholar] [CrossRef]
- Berenguer, R.; Ruiz-Rosas, R.; Gallardo, A.; Cazorla-Amorós, D.; Morallón, E.; Nishihara, H.; Kyotani, T.; Rodríguez-Mirasol, J.; Cordero, T. Enhanced Electro-Oxidation Resistance of Carbon Electrodes Induced by Phosphorus Surface Groups. Carbon 2015, 95, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Glarum, S.H.; Marshall, J.H. Electron Delocalization in Poly(Aniline). J. Phys. Chem. 1988, 92, 4210–4217. [Google Scholar] [CrossRef]
- MacDiarmind, A.G.; Epstein, A.J. Polyaniline: Synthesis, Chemistry, and Processing. In New Aspects of Organic Chemistry II; Kodansha: Tokyo, Japan; VCH: Weinheim, Germany, 1992; p. 271. [Google Scholar]
- Wei, Y.; Hsueh, K.F. Thermal Analysis of Chemically Synthesized Polyaniline and Effects of Thermal Aging on Conductivity. J. Polym. Sci. Part. A Polym. Chem. 1989, 27, 4351–4363. [Google Scholar] [CrossRef]
- Lafitte, B.; Jannasch, P. On the Prospects for Phosphonated Polymers as Proton-Exchange Fuel Cell Membranes. In Advances in Fuel Cells; Zhao, T.S., Kreuer, K.-D., Nguyen, T.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 119–185. [Google Scholar]


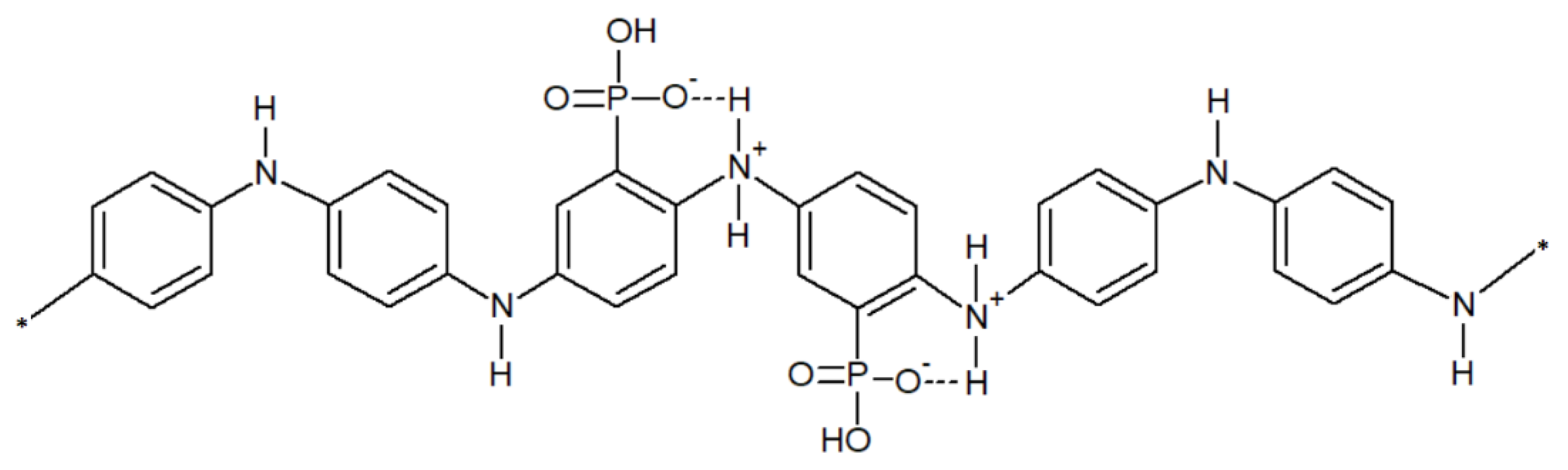




| Sample | Atomic Formula * | N/at% | C/at% | O/at% | P/at% | P/N | O/P ** | N+/N/% |
|---|---|---|---|---|---|---|---|---|
| PANI | C7.4HxN1.0O0.3 | 11.4 | 84.0 | 2.9 | 0.0 | - | - | 7 |
| PANI2APPA (80/20) | C7.1HxN1.0P0.2O0.9 | 10.8 | 76.5 | 9.9 | 2.3 | 0.21 | 3.0 | 18 |
| PANI2APPA (50/50) | C6.8HxN1.0P0.5O2.0 | 9.6 | 65.0 | 19.1 | 5.1 | 0.53 | 3.2 | 30 |
| PANI4APPA (50/50) | C7.6HxN1.0P0.2O0.7 | 10.4 | 79.5 | 6.9 | 1.7 | 0.16 | 2.4 | 5 |
| Sample | fANI | FANI | Reactivity | Reactivity Ratio (Rr = rANI/rAPPA) | Average Length Segment | ||
|---|---|---|---|---|---|---|---|
| rANI | rAPPA | NANI | NAPPA | ||||
| PANI2APPA | 0.8 | 0.79 | 1.0 | 1.3 | 0.8 | 5.0 | 1.3 |
| 0.5 | 0.47 | 2.0 | 2.3 | ||||
| PANI4APPA | 0.5 | 0.84 | 5.6 | 0.3 | 18.9 | 6.6 | 1.3 |
| Wavenumber/cm−1 | Assignments | References | |
|---|---|---|---|
| Experimental | Theoretical | ||
| 1583 | 1550 | C–C quinoid ring stretching | [47,48] |
| 1491 | 1509 | C–C benzenoid ring stretching | [47,48] |
| 1374 | - | C–N= in the neighborhoud of a quinoid ring | [47,50] |
| 1305 | 1327–1340 | C-H stretching, C–N–C stretching, or p-electron delocalization | [25,47,50] |
| 1245 | - | C–N+ stretching of secondary aromatic amine in the polaron structure | [25,50] |
| 1214 * | 1221–1257 * | P=O stretching | [27,48] |
| 1140 | 1146 | C–H aromatic bending in-plane | [47] |
| 1075 * | 1074 * | P–O–C out-of-plane stretching, P–Ar stretching | [48,49] |
| 1040 * | 1054 * | P–O stretching in O=P–OH with a single neighboring –OH group | [34,48,49] |
| 900–930 * | 930 * | P–O stretching in O=P–OH with a single neighboring –OH group | [34,48,49] |
| 820 | 834–850 | C–H aromatic bending out-of-plane | [50] |
| Samples | Rs/Ω |
|---|---|
| PANI | 6.7 |
| PANI2APPA (80/20) | 10.5 |
| PANI2APPA (50/50) | 35.6 |
| PANI4APPA (50/50) | 11.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Sánchez, B.; Cazorla-Amorós, D.; Morallón, E. Tailoring Intrinsic Properties of Polyaniline by Functionalization with Phosphonic Groups. Polymers 2020, 12, 2820. https://doi.org/10.3390/polym12122820
Martínez-Sánchez B, Cazorla-Amorós D, Morallón E. Tailoring Intrinsic Properties of Polyaniline by Functionalization with Phosphonic Groups. Polymers. 2020; 12(12):2820. https://doi.org/10.3390/polym12122820
Chicago/Turabian StyleMartínez-Sánchez, Beatriz, Diego Cazorla-Amorós, and Emilia Morallón. 2020. "Tailoring Intrinsic Properties of Polyaniline by Functionalization with Phosphonic Groups" Polymers 12, no. 12: 2820. https://doi.org/10.3390/polym12122820






