CD Oxyanions as a Tool for Synthesis of Highly Anionic Cyclodextrin Polymers †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
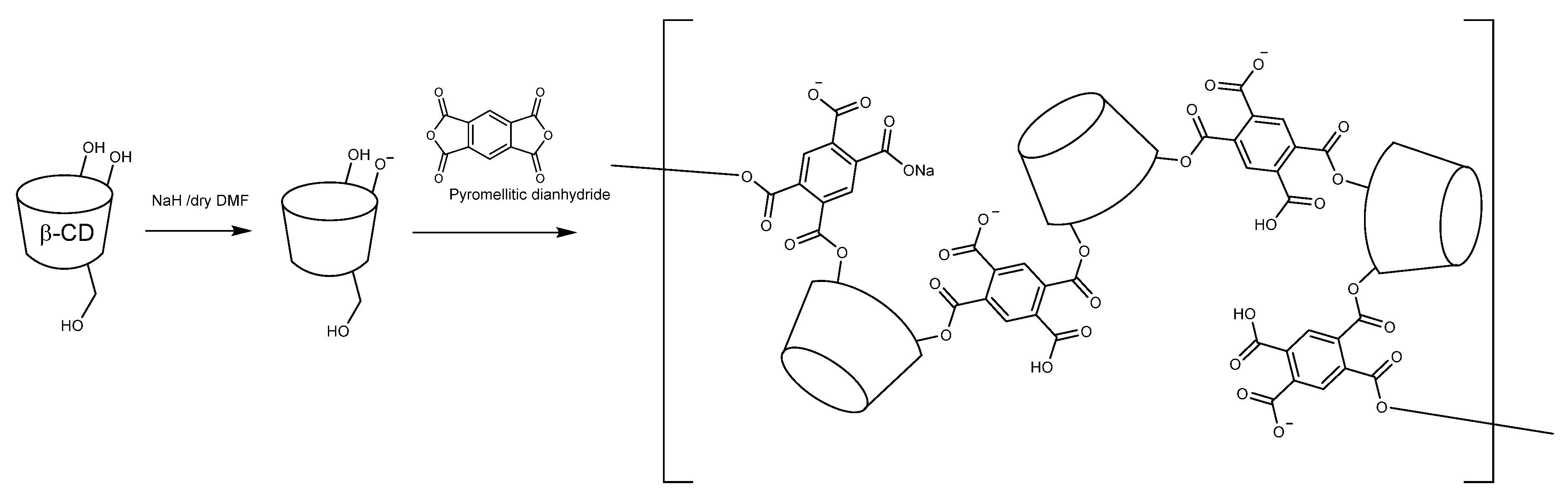
2.2. Synthesis of the CD Polymer
2.3. Separation of the Polymer due to Particle Size
2.4. NMR Measurement
2.5. FTIR Measurement
2.6. DSC and TG Measurements
2.7. Scaning Electron Microscopy (SEM) Measurement
2.8. Molecular Mass Analysis
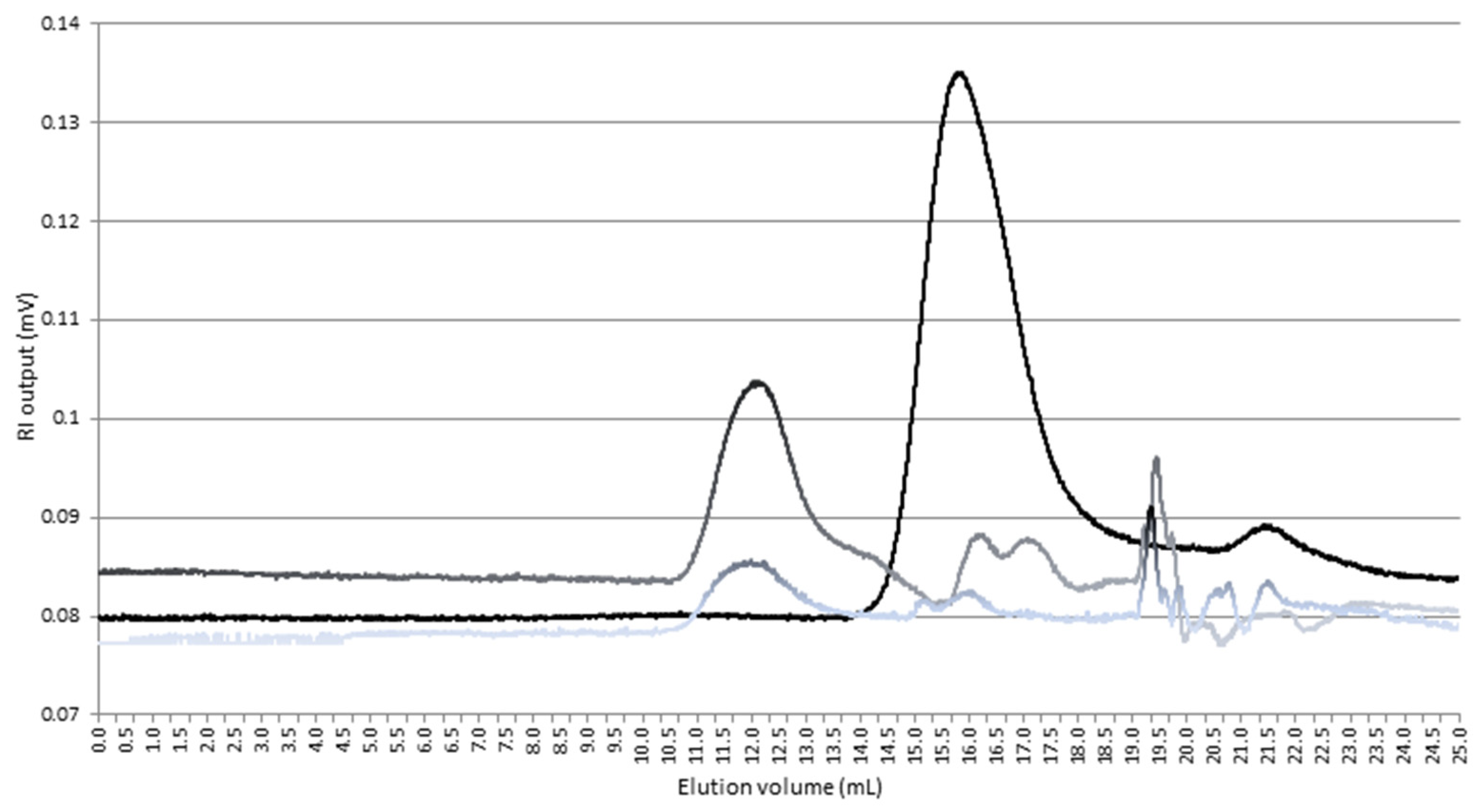
2.8.1. HPSEC-MALLS-RI Measurement
2.8.2. MALDI-TOF MS Experiment
2.9. Potentiometric and pH Experiment
2.10. Solubility Experiment
3. Results and Discussion
3.1. Synthesis of β-CDP (CDPA)
3.2. Molecular Weight Characterization
3.2.1. HPSEC-MALLS-RI Analysis
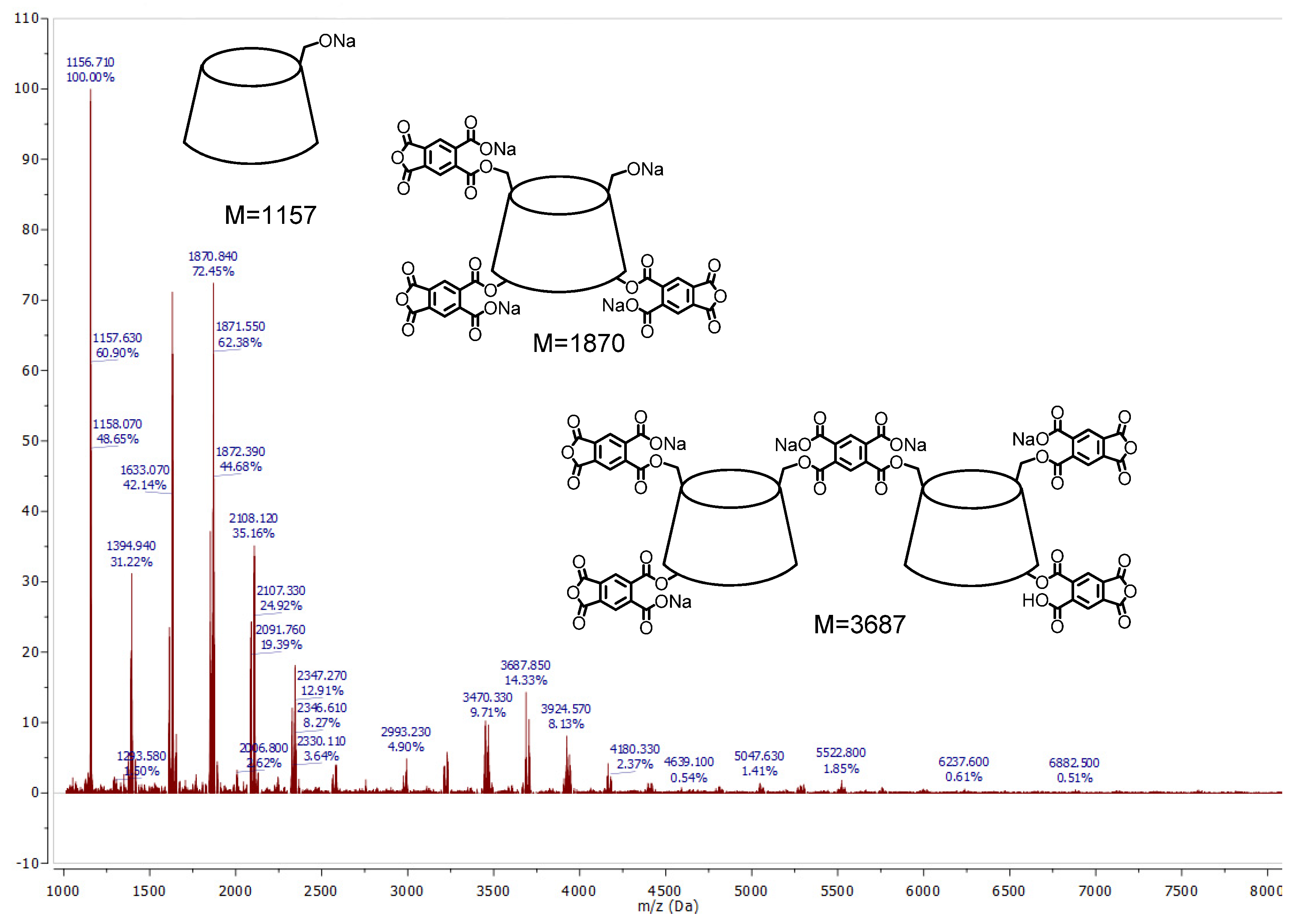
3.2.2. MALDI-TOF Experiment
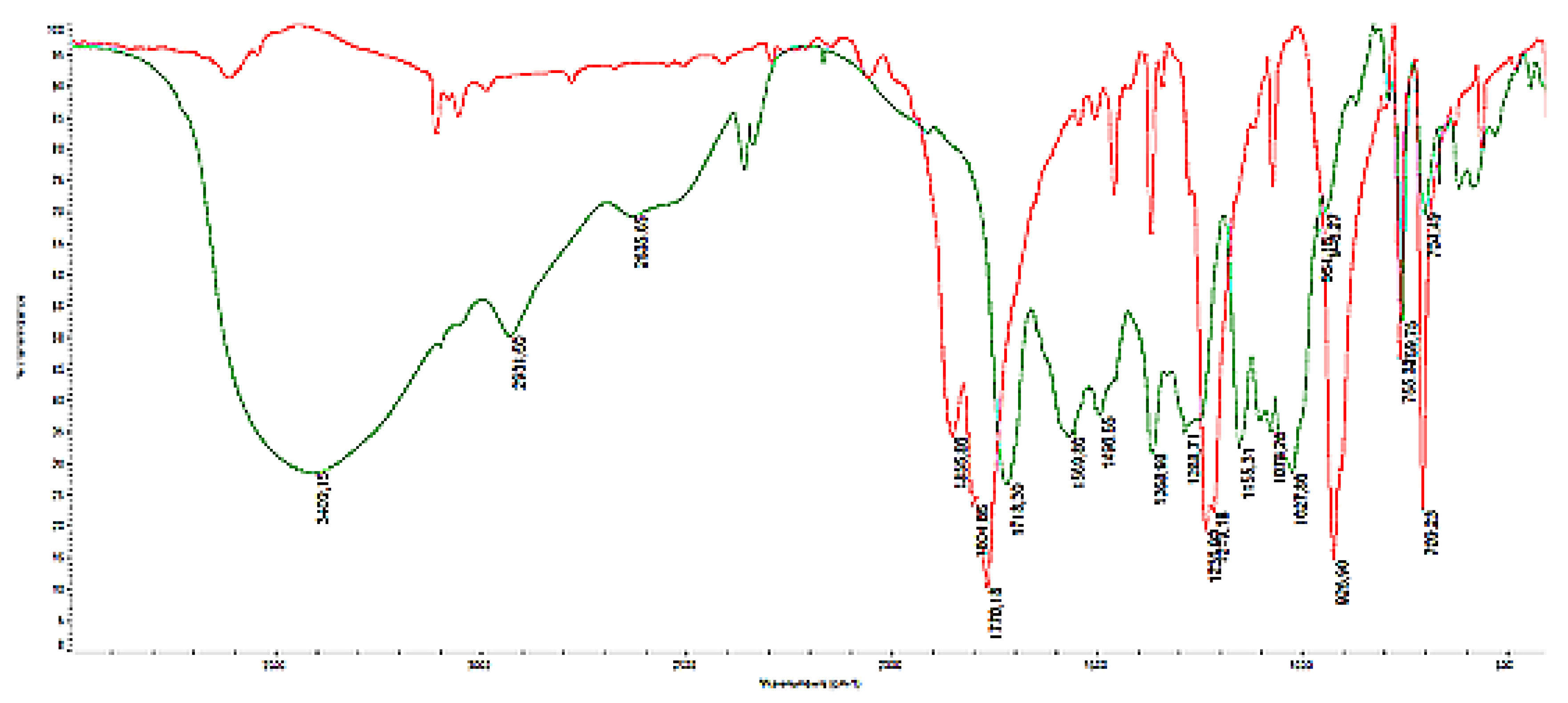
3.3. FTIR Characterization
3.4. NMR Investigation
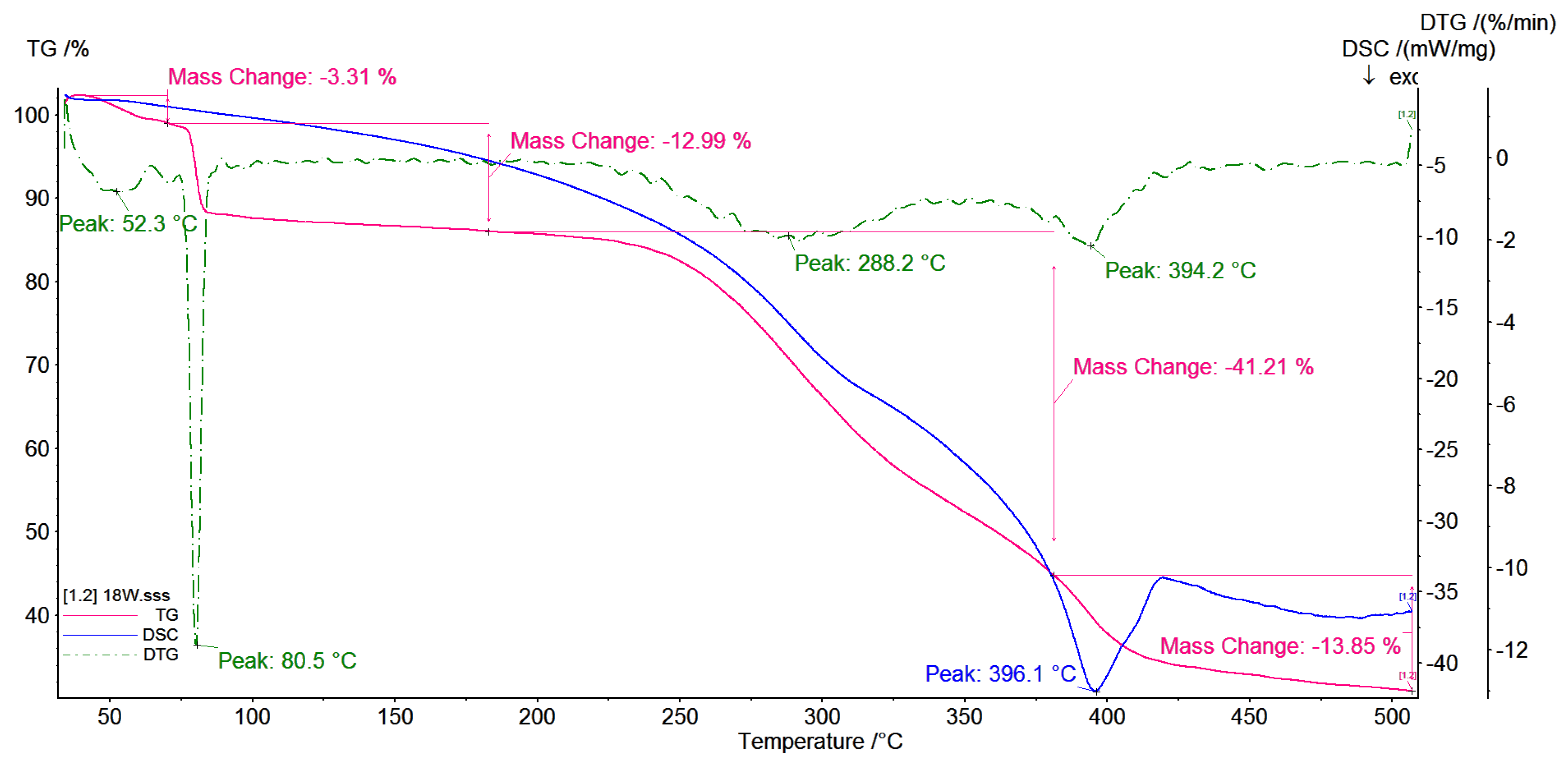
3.5. TG/DSC Investigations
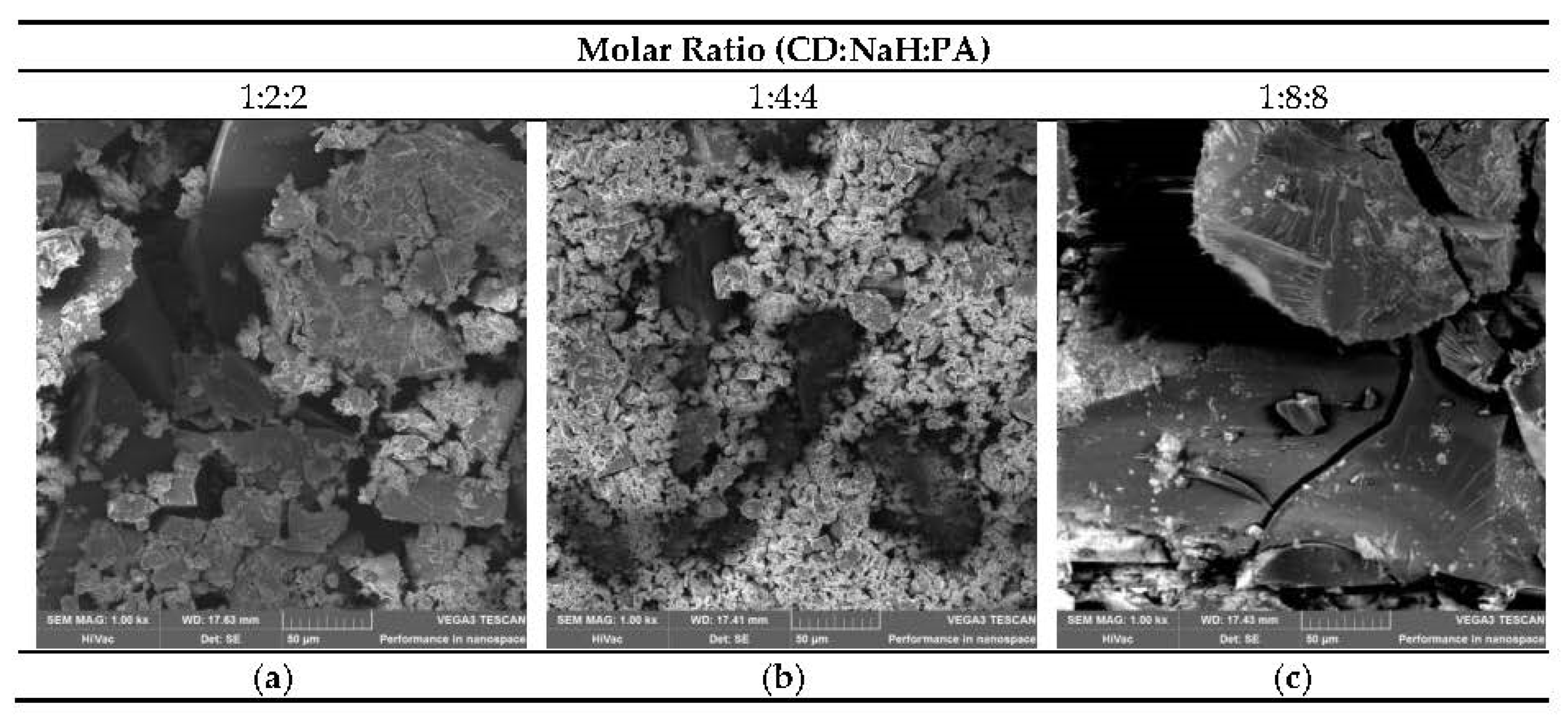
3.6. SEM Investigation
3.7. Solubility in Water
3.8. Potentiometric and pH Experiment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sliwa, W.; Girek, T. Cyclodextrins Properties and Applications; Wiley-VCH Verlag: Weinheim, Germany, 2017; p. 336. [Google Scholar]
- Furue, M.; Harada, A.; Nozakura, S.-I. Preparation of cyclodextrin-containing polymers and their catalysis in ester-hydrolysis. J. Polym. Sci. Polym. Lett. Ed. 1975, 13, 357–360. [Google Scholar] [CrossRef]
- Harada, A.; Furue, M.; Nozakura, S.-I. Cyclodextrin-Containing Polymers. 1. Preparation of Polymers. Macromolecules 1976, 9, 701–704. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Yu, S.; Wu, W.; Jiang, X. Synthesis and Self-Assembly of a Nanoscaled Multiarm Polymer Terminated by β-Cyclodextrin. ACS Macro Lett. 2013, 2, 82–85. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Harada, A. Redox-Responsive Macroscopic Gel Assembly Based on Discrete Dual Interactions. Angew. Chem. Int. Ed. 2014, 53, 3617–3621. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Hashidzume, A.; Harada, A. Redox-Generated Mechanical Motion of a Supramolecular Polymeric Actuator Based on Host–Guest Interactions. Angew. Chem. Int. Ed. 2013, 52, 5731–5735. [Google Scholar] [CrossRef]
- MNSimoes, S.; Veiga, F.; JTorres-Labandeira, J.; Ribeiro, C.F.; Concheiro, A.; Alvarez-Lorenzo, C. Syringeable Self-Assembled Cyclodextrin Gels for Drug Delivery. Curr. Top. Med. Chem. 2014, 14, 494–509. [Google Scholar] [CrossRef]
- Cserhati, T.; Fenyvesi, E.; Szejtli, J. Interaction of nonylphenyl and tributylphenyl ethylene oxide ionic surfactants with highly soluble cyclodextrin derivatives. J. Incl. Phenom. Mol. Recognit. Chem. 1992, 14, 181–188. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Shao, Y.; Martel, B.; Morcellet, M.; Weltrowski, M.; Crini, G. Sorption of textile dyes on β-cyclodextrin-epichlorhydrin gels. J. Incl. Phenom. Mol. Recognit. Chem. 1996, 25, 209–212. [Google Scholar] [CrossRef]
- Appel, E.A.; del Barrio, J.; Loh, X.J.; Scherman, O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012, 41, 6195–6214. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X. Binary Crystallized Supramolecular Aerogels Derived from Host–Guest Inclusion Complexes. ACS Nano 2015, 9, 11389–11397. [Google Scholar] [CrossRef] [PubMed]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Swaminathan, S.; Pastero, L.; Serpe, L.; Trotta, F.; Vavia, P.; Aquilano, D.; Trotta, M.; Zara, G.; Cavalli, R. Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization, stability and cytotoxicity. Eur. J. Pharm. Biopharm. 2010, 74, 193–201. [Google Scholar] [CrossRef]
- Shende, P.; Deshmukh, K.; Trotta, F.; Caldera, F. Novel cyclodextrin nanosponges for delivery of calcium in hyperphosphatemia. Int. J. Pharm. 2013, 456, 95–100. [Google Scholar] [CrossRef]
- Shende, P.; Trotta, F.; Gaud, R.S.; Deshmukh, K.; Cavalli, R.; Biasizzo, M. Influence of different techniques on formulation and comparative characterization of inclusion complexes of ASA with β-cyclodextrin and inclusion complexes of ASA with PMDA cross-linked β-cyclodextrin nanosponges. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 447–454. [Google Scholar] [CrossRef]
- Li, D.; Ma, M. Nanosponges for water purification. Clean Prod. Process. 2000, 2, 112–116. [Google Scholar] [CrossRef]
- Varan, G.; Varan, C.; Erdogar, N.; Hincal, A.A.; Bilensoy, E. Amphiphilic cyclodextrin nanoparticles. Int. J. Pharm. 2017, 531, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Caldera, F.; Cavalli, R.; Mele, A.; Punta, C.; Melone, L.; Castiglione, F.; Rossi, B.; Ferro, M.; Crupi, V.; et al. Synthesis and characterization of a hyper-branched water-soluble -cyclodextrin polymer. Beilstein J. Org. Chem. 2014, 10, 2586–2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for Selective Modifications of Cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Rong, D.; D’Souza, V.T. A convenient method for functionalization of the 2-position of cyclodextrins. Tetrahedron Lett. 1990, 31, 4275–4278. [Google Scholar] [CrossRef]
- Khan, A.R.; Barton, L.; D’Souza, V.T. Epoxides of the Secondary Side of Cyclodextrins. J. Org. Chem. 1996, 61, 8301–8303. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Girek, T.; Shin, D.H.; Lim, S.T. Structural and physical characterization of octenylsuccinyl β-cyclodextrin. Carbohydr. Polym. 2002, 49. [Google Scholar] [CrossRef]
- Girek, T.; Ciesielski, W. Polymerization of β-cyclodextrin with maleic anhydride along with thermogravimetric study of polymers. J. Incl. Phenom. Macrocycl. Chem. 2011, 69. [Google Scholar] [CrossRef]
- Girek, T.; Ciesielski, W. Polymerization of β-cyclodextrin with succinic anhydride and thermogravimetric study of the polymers. J. Incl. Phenom. Macrocycl. Chem. 2011, 69. [Google Scholar] [CrossRef]
- Girek, T.; Kozlowski, C.A.; Koziol, J.J.; Walkowiak, W.; Korus, I. Polymerisation of β-cyclodextrin with succinic anhydride. Synthesis, characterisation, and ion flotation of transition metals. Carbohydr. Polym. 2005, 59. [Google Scholar] [CrossRef]
- Girek, T.; Shin, D.H.; Lim, S.T. Polymerization of β-cyclodextrin with maleic anhydride and structural characterization of the polymers. Carbohydr. Polym. 2000, 42. [Google Scholar] [CrossRef]
- Ju, J.-F.; Syu, M.-J.; Teng, H.-S.; Chou, S.-K.; Chang, Y.-S. Preparation and identification of β-cyclodextrin polymer thin film for quartz crystal microbalance sensing of benzene, toluene, and p-xylene. Sens. Actuators B Chem. 2008, 132, 319–326. [Google Scholar] [CrossRef]
- Raeisi, A.; Shabanian, M.; Faghihi, K. Preparation of β-cyclodextrin-ester network and new organo-modified LDH as dual additives of PVA: Thermal, dynamic-mechanical and migration study. Prog. Org. Coat. 2017, 111, 402–415. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W.; Girek, T. Modified cyclodextrin polymers as selective ion carriers for Pb(II) separation across plasticized membranes. J. Membr. Sci. 2008, 310, 312–320. [Google Scholar] [CrossRef]
- Takashima, Y.; Osaki, M.; Harada, A. Cyclodextrin-Initiated Polymerization of Cyclic Esters in Bulk: Formation of Polyester-Tethered Cyclodextrins. J. Am. Chem. Soc. 2004, 126, 13588–13589. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.L.; Farahani, M.; Dickens, S.H.; Guttman, C.M. MALDI-TOF MS analysis of a library of polymerizable cyclodextrin derivatives. J. Dent. Res. 2000, 79, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Hoonor, R.; Jin, H.-S.; Kim, J. Synthesis and characterization of cationic and anionic cyclodextrin oligomers and their use in layer-by-layer film formation. Bull. Korean Chem. Soc. 2013, 34, 2016–2022. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, F.; Noguchi, S.; Takahashi, J.; Takada, M. Retrogradation of Gelatinized Potato Starch Studied by Differential Scanning Calorimetry AU—Nakazawa, Fumiko. Agric. Biol. Chem. 1985, 49, 953–957. [Google Scholar] [CrossRef]





| Molar Ratio (CD:NaH:PA) | Cut-Off | Weight | % of Whole Sample |
|---|---|---|---|
| 1:1:1 | Whole sample | 3.00 g | 100.00% |
| <5000 Da | 2.63 g | 87.00% | |
| >5000 Da | 0.26 g | 8.67% | |
| 1:2:2 | Whole sample | 4.00 g | 100.00% |
| <5000 Da | 3.04 g | 76.00% | |
| >5000 Da | 0.59 g | 14.75% | |
| 1:4:4 | Whole sample | 4.00 g | 100.00% |
| <5000 Da | 2.07 g | 51.75% | |
| >5000 Da | 0.99 g | 24.75% | |
| 1:8:8 | Whole sample | 4.00 g | 100.00% |
| <5000 Da | 1.84 g | 46.00% | |
| >5000 Da | 1.60 g | 40.00% |
| Molar Ratio (β-CD:NaH:PA) | Weight-Average Molecular Weight (MW) | Polydispersity (Mw/Mn) | |
|---|---|---|---|
| 1:1:1 | (Whole sample) | 6.83 × 104 | 1.08 |
| 8.62 × 103 | 1.12 | ||
| (<5000 Da) | 3.87 × 103 | 7.93 | |
| (>5000 Da) | 1.12 × 104 | 1.07 | |
| 5.13 × 103 | 3.39 | ||
| 1:2:2 | (Whole sample) | 2.84 × 104 | 1.32 |
| 2.13 × 104 | 2.20 | ||
| (<5000 Da) | 1.011 × 104 | 12.3 | |
| (>5000 Da) | 8.06 × 104 | 1.19 | |
| 3.38 × 103 | 1.89 | ||
| 1:4:4 | (Whole sample) | 8.84 × 104 | 1.34 |
| 2.77 × 103 | 1.27 | ||
| (<5000 Da) | 9.89 × 103 | 8.34 | |
| (>5000 Da) | 8.91 × 104 | 1.17 | |
| 2.30 × 104 | 1.03 | ||
| 1:8:8 | (Whole sample) | 6.65 × 105 | 1.13 |
| 8.22 × 104 | 1.70 | ||
| 1.23 × 104 | 1.39 | ||
| (<5000 Da) | 5.49 × 103 | 5.07 | |
| (>5000 Da) | 6.22 × 105 | 1.18 | |
| 5.96 × 103 | 1.03 |
| Structure of Anionic Polymer Fragment | Calculated Molecular Masses | Observed Molecular Masses |
|---|---|---|
| CD+Na | 1157 | 1157 |
| CD + M + 2Na | 1397 | 1395 |
| CD + 2M + 3Na | 1637 | 1632 |
| CD + 3M + 4Na | 1876 | 1870 |
| 2CD + L + M + 2Na | 2749 | 2756 |
| 2CD + L + 2M + 3Na | 2989 | 2994 |
| 2CD + L + 3M + 4Na | 3229 | 3232 |
| 2CD + L + 5M + 5Na | 3687 | 3687 |
| 3CD + 2L + 2M + 2Na | 4348 | 4355 |
| 3CD + 2L + 3M + 4Na | 4589 | 4594 |
| 3CD + 2L + 4M + 4Na | 4829 | 4832 |
| 4CD + 3L + 4M + 4Na | 6195 | 6191 |
| 5CD + 4L + 5M +4 Na | 7780 | 7783 |
| Molar Ratio (CD:NaH:PA) | Whole Sample [mg/mL] | <5000 Da [mg/mL] | >5000 Da [mg/mL] |
|---|---|---|---|
| 1:1:1 | 28.7 | 20.4 | 91.1 |
| 1:2:2 | 83.1 | 79.4 | 157.1 |
| 1:4:4 | 85.4 | 62.9 | 171.3 |
| 1:8:8 | 75.6 | 42.8 | 172.1 |
| Metal Ion | pH of Salt Solution | pH of Salt + Polymer Solution | Comments |
|---|---|---|---|
| Fe2+ | 3.85 | 3.40 | Precipitation |
| Cd2+ | 6.84 | 3.24 | |
| Cu2+ | 4.28 | 3.04 | |
| Ca2+ | 7.81 | 3.21 | |
| Ba2+ | 6.58 | 3.24 | |
| Hg2+ | 4.06 | 3.48 | |
| Ni2+ | 7.30 | 3.21 | |
| Ag+ | 10.51 | 9.25 | Precipitation |
| Pb2+ | 7.01 | 5.64 | Precipitation |
| Fe3+ | 2.29 | 2.35 | Precipitation |
| Sr2+ | 6.84 | 3.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girek, T.; Koziel, K.; Girek, B.; Ciesielski, W. CD Oxyanions as a Tool for Synthesis of Highly Anionic Cyclodextrin Polymers. Polymers 2020, 12, 2845. https://doi.org/10.3390/polym12122845
Girek T, Koziel K, Girek B, Ciesielski W. CD Oxyanions as a Tool for Synthesis of Highly Anionic Cyclodextrin Polymers. Polymers. 2020; 12(12):2845. https://doi.org/10.3390/polym12122845
Chicago/Turabian StyleGirek, Tomasz, Kinga Koziel, Beata Girek, and Wojciech Ciesielski. 2020. "CD Oxyanions as a Tool for Synthesis of Highly Anionic Cyclodextrin Polymers" Polymers 12, no. 12: 2845. https://doi.org/10.3390/polym12122845





