Identification and Quantitation Studies of Migrants from BPA Alternative Food-Contact Metal Can Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Food Packaging Samples
2.2. Reagents, Reference Standards, and Materials
2.3. Migration Testing of Can and Lidstock Samples
2.4. Extract Workup Procedure
2.5. GC-MS Analysis Methodology
2.6. TMS-Derivatization
2.7. GC-MS Method Validation
3. Results and Discussion
3.1. Identification and Semi-Quantitation of Coating-Borne Migrants
3.2. Quantitation of Bisphenol Monomer Migrants
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simal-Gándara, J.; Paz-Abuín, S.; Ahrné, L. A critical review of the quality and safety of BADGE-based epoxy coatings for cans: Implications for legislation on epoxy coatings for food contact. Crit. Rev. Food Sci. Nutr. 1998, 38, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Fenichel, P.; Chevalier, N.; Brucker-Davis, F. Bisphenol A: An endocrine and metabolic disruptor. Ann. d’Endocrinol. 2013, 74, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Berger, U.; Oehme, M. Identification of derivatives of bisphenol A diglycidyl ether and novolac glycidyl ether in can coatings by liquid chromatography/ion trap mass spectrometry. J. AOAC Int. 2000, 83, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Soto, A.M.; Schaeberle, C.; Maier, M.S.; Sonnenschein, C.; Maffini, M.V. Evidence of absence: Estrogenicity assessment of a new food-contact coating and the bisphenol used in its synthesis. Environ. Sci. Technol. 2017, 51, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Errico, S.; Bianco, M.; Mita, L.; Migliaccio, M.; Rossi, S.; Nicolucci, C.; Menale, C.; Portaccio, M.; Gallo, P.; Mita, D.G. Migration of bisphenol A into canned tomatoes produced in Italy: Dependence on temperature and storage conditions. Food Chem. 2014, 160, 157–164. [Google Scholar] [CrossRef]
- El Moussawi, S.N.; Ouaini, R.; Matta, J.; Chébib, H.; Cladière, M.; Camel, V. Simultaneous migration of bisphenol compounds and trace metals in canned vegetable food. Food Chem. 2019, 288, 228–238. [Google Scholar] [CrossRef]
- Cacho, J.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Stir bar sorptive extraction coupled to gas chromatography–mass spectrometry for the determination of bisphenols in canned beverages and filling liquids of canned vegetables. J. Chromatogr. A 2012, 1247, 146–153. [Google Scholar] [CrossRef]
- Míguez, J.; Herrero, C.; Quintás, I.; Rodríguez, C.; Gigosos, P.; Mariz, O. A LC–MS/MS method for the determination of BADGE-related and BFDGE-related compounds in canned fish food samples based on the formation of [M+ NH4]+ aducts. Food Chem. 2012, 135, 1310–1315. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, X.-H.; Pan, S.-D.; Wang, J.-L.; Zheng, Y.-B.; Xu, J.-J.; Zhao, Y.-G.; Cai, Z.-X.; Jin, M.-C. Contamination status of bisphenol A and its analogues (bisphenol S, F and B) in foodstuffs and the implications for dietary exposure on adult residents in Zhejiang Province. Food Chem. 2019, 294, 160–170. [Google Scholar] [CrossRef]
- Cwiek-Ludwicka, K. Bisphenol A (BPA) in food contact materials-new scientific opinion from EFSA regarding public health risk. Roczniki Państwowego Zakładu Higieny 2015, 66, 299–307. [Google Scholar]
- Welshons, W.V.; Nagel, S.C.; vom Saal, F.S. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 2006, 147, s56–s69. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Sonnenschein, C. Environmental causes of cancer: Endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 2010, 6, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konieczna, A.; Rutkowska, A.; Rachon, D. Health risk of exposure to Bisphenol A (BPA). Roczniki Państwowego Zakładu Higieny 2015, 66, 5–11. [Google Scholar] [PubMed]
- Summerfield, W.; Goodson, A.; Cooper, I. Survey of bisphenol a diglycidyl ether (BADGE) in canned foods. Food Addit. Contam. 1998, 15, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Hammarling, L.; Gustavsson, H.; Svensson, K.; Oskarsson, A. Migration of bisphenol-A diglycidyl ether (BADGE) and its reaction products in canned foods. Food Addit. Contam. 2000, 17, 937–943. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Zhang, W.; Kannan, K. Widespread occurrence and distribution of bisphenol A diglycidyl ether (BADGE) and its derivatives in human urine from the United States and China. Environ. Sci. Technol. 2012, 46, 12968–12976. [Google Scholar] [CrossRef]
- Buka, I.; Osornio-Vargas, A.; Walker, R. Canada declares bisphenol A a ‘dangerous substance’: Questioning the safety of plastics. Paediatr. Child Health 2009, 14, 11–13. [Google Scholar] [CrossRef] [Green Version]
- Resnik, D.B.; Elliott, K.C. Bisphenol A and risk management ethics. Bioethics 2015, 29, 182–189. [Google Scholar] [CrossRef]
- Commission, E. European Commission. (EU) No 2011/8 of 28 January 2011 amending Directive 2002/72/EC as regards the restriction of use of Bisphenol A in plastic infant feeding bottles. Off. J. Eur. Union 2011, L 26, 11–14. [Google Scholar]
- Code of Federal Regulations, 21CFR175.300 Indirect Food Additives: Adhesives and Components of Coatings. Subpart C—Substances for Use as Components of Coatings, Resinous and Polymeric Coatings; Food and Drug Administration, Washington, DC, USA: 2019.
- Commission, E. Commission Regulation (EU) 2018/213 of 12 February 2018 on the use of bisphenol A in varnishes and coatings intended to come into contact with food and amending Regulation (EU) No 10/2011 as regards the use of that substance in plastic food contact materials. Off. J. Eur. Union 2018, L 41, 6–11. [Google Scholar]
- USFDA. Update on Bisphenol A for Use in Food Contact Applications; Food and Drug Administration: Washington, DC, USA, 2010. [Google Scholar]
- Zhou, X.; Kramer, J.P.; Calafat, A.M.; Ye, X. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J. Chromatogr. B 2014, 944, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Szafran, A.T.; Stossi, F.; Mancini, M.G.; Walker, C.L.; Mancini, M.A. Characterizing properties of non-estrogenic substituted bisphenol analogs using high throughput microscopy and image analysis. PLoS ONE 2017, 12, e0180141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffini, M.V.; Canatsey, R.D. An expanded toxicological profile of tetramethyl bisphenol F (TMBPF), a precursor for a new food-contact metal packaging coating. Food Chem. Toxicol. 2020, 135, 110889. [Google Scholar] [CrossRef]
- Schaefer, A.; Ohm, V.; Simat, T. Migration from can coatings: Part 2. Identification and quantification of migrating cyclic oligoesters below 1000 Da. Food Addit. Contam. 2004, 21, 377–389. [Google Scholar] [CrossRef]
- Zhang, N.; Kenion, G.; Bankmann, D.; Mezouari, S.; Hartman, T.G. Migration studies and chemical characterization of low molecular weight cyclic polyester oligomers from food packaging lamination adhesives. Packag. Technol. Sci. 2018, 31, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Isella, F.; Canellas, E.; Bosetti, O.; Nerin, C. Migration of non intentionally added substances from adhesives by UPLC–Q-TOF/MS and the role of EVOH to avoid migration in multilayer packaging materials. J. Mass Spectrom. 2013, 48, 430–437. [Google Scholar] [CrossRef]
- Paseiro-Cerrato, R.; Noonan, G.O.; Begley, T.H. Evaluation of Long-Term Migration Testing from Can Coatings into Food Simulants: Polyester Coatings. J. Agric. Food Chem. 2016, 64, 2377–2385. [Google Scholar] [CrossRef]
- Paseiro-Cerrato, R.; DeJager, L.; Begley, T. Determining the migration of nadic acid, terephthalic acid, isophthalic acid and two oligomers from polyester food cans into food in the US market. Food Control 2019, 101, 69–76. [Google Scholar] [CrossRef]
- Eckardt, M.; Hetzel, L.; Brenz, F.; Simat, T.J. Release and migration of cyclic polyester oligomers from bisphenol A non-intent polyester–phenol-coatings into food simulants and infant food–a comprehensive study. Food Addit. Contam. Part A 2020, 37, 681–703. [Google Scholar] [CrossRef]
- Paseiro-Cerrato, R.; MacMahon, S.; Ridge, C.D.; Noonan, G.O.; Begley, T.H. Identification of unknown compounds from polyester cans coatings that may potentially migrate into food or food simulants. J. Chromatogr. A 2016, 1444, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koster, S.; Bani-Estivals, M.; Bonuomo, M.; Bradley, E.; Chagnon, M.-C.; Garcia, M.; Godts, F.; Gude, T.; Helling, R.; Paseiro-Losada, P. Guidance on best practices on the risk assessment of non intentionally added substances (NIAS) in food contact materials and articles. In ILSI Europe Report Series; ILSI Europe: Brussels, Belgium, 2015; pp. 1–70. [Google Scholar]
- Scarsella, J.B.; Zhang, N.; Hartman, T.G. Identification and migration studies of photolytic decomposition products of UV-photoinitiators in food packaging. Molecules 2019, 24, 3592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, G.V.; Hartman, T.G. Migration studies of 3-chloro-1, 2-propanediol (3-MCPD) in polyethylene extrusion-coated paperboard food packaging. Food Addit. Contam. 2010, 27, 884–891. [Google Scholar] [CrossRef] [PubMed]
- USFDA. Guidance for Industry: Preparation of Premarket Submissions for Food Contact Substances (Chemistry Recommendations); Food and Drug Administration: Washington, DC, USA, 2007. [Google Scholar]
- Han, S.; Kim, W.G.; Yoon, H.G.; Moon, T.J. Curing reaction of biphenyl epoxy resin with different phenolic functional hardeners. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 773–783. [Google Scholar] [CrossRef]
- Wong, C. Polymers for Electronic & Photonic Application; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Wagner, J.; Castle, L.; Oldring, P.K.; Moschakis, T.; Wedzicha, B.L. Factors affecting migration kinetics from a generic epoxy-phenolic food can coating system. Food Res. Int. 2018, 106, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Bott, J.; Störmer, A.; Albers, P. Investigation into the release of nanomaterials from can coatings into food. Food Packag. Shelf Life 2018, 16, 112–121. [Google Scholar] [CrossRef]
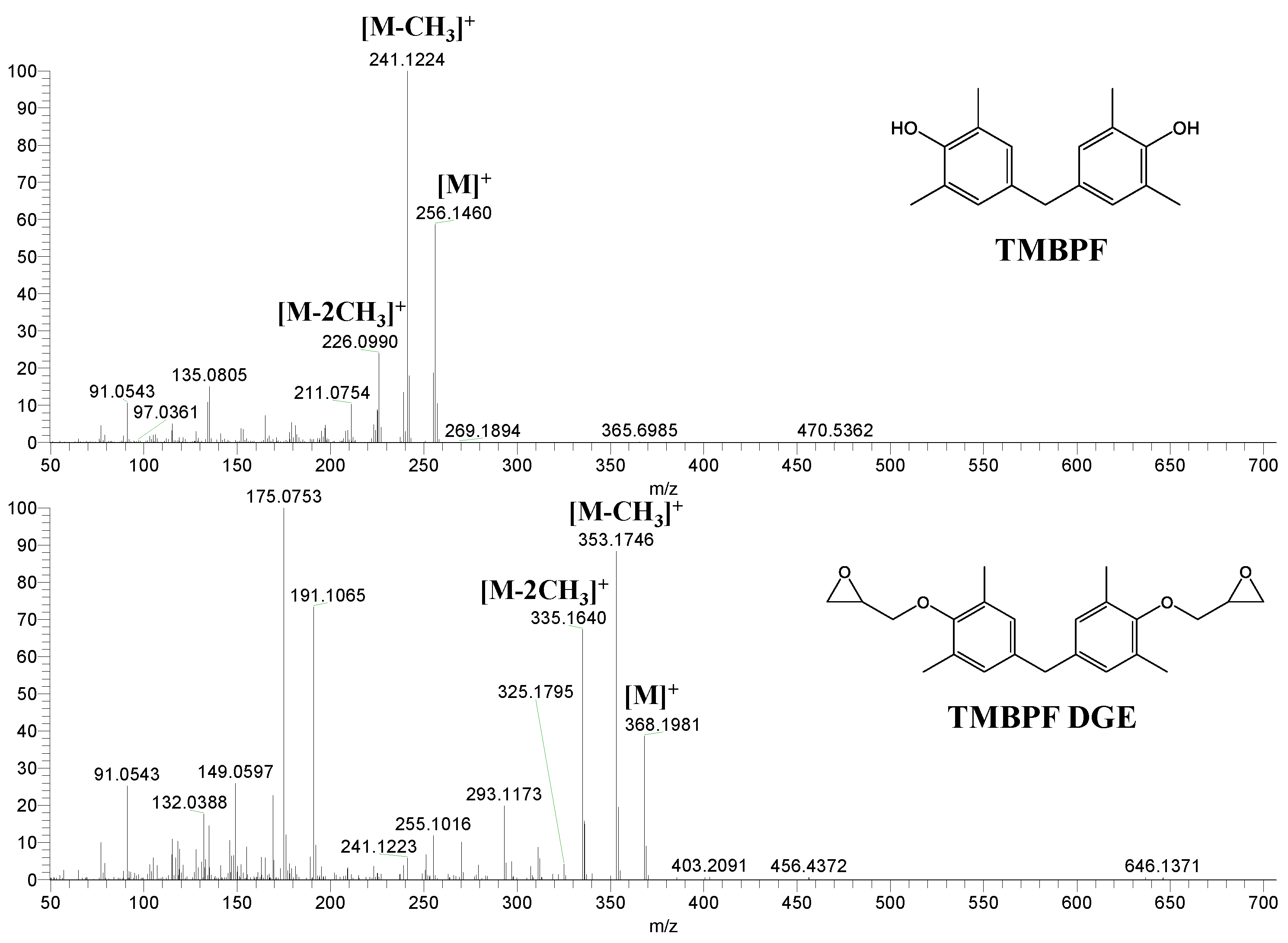
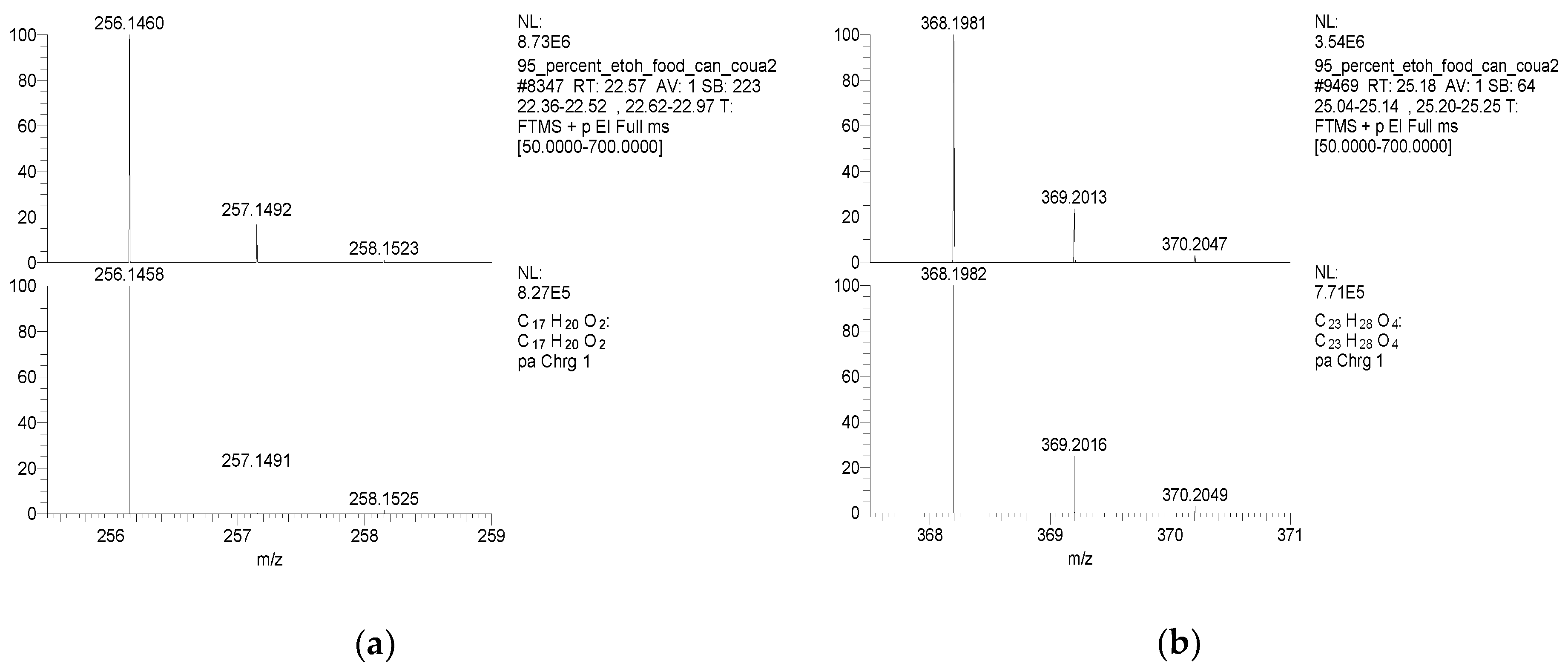
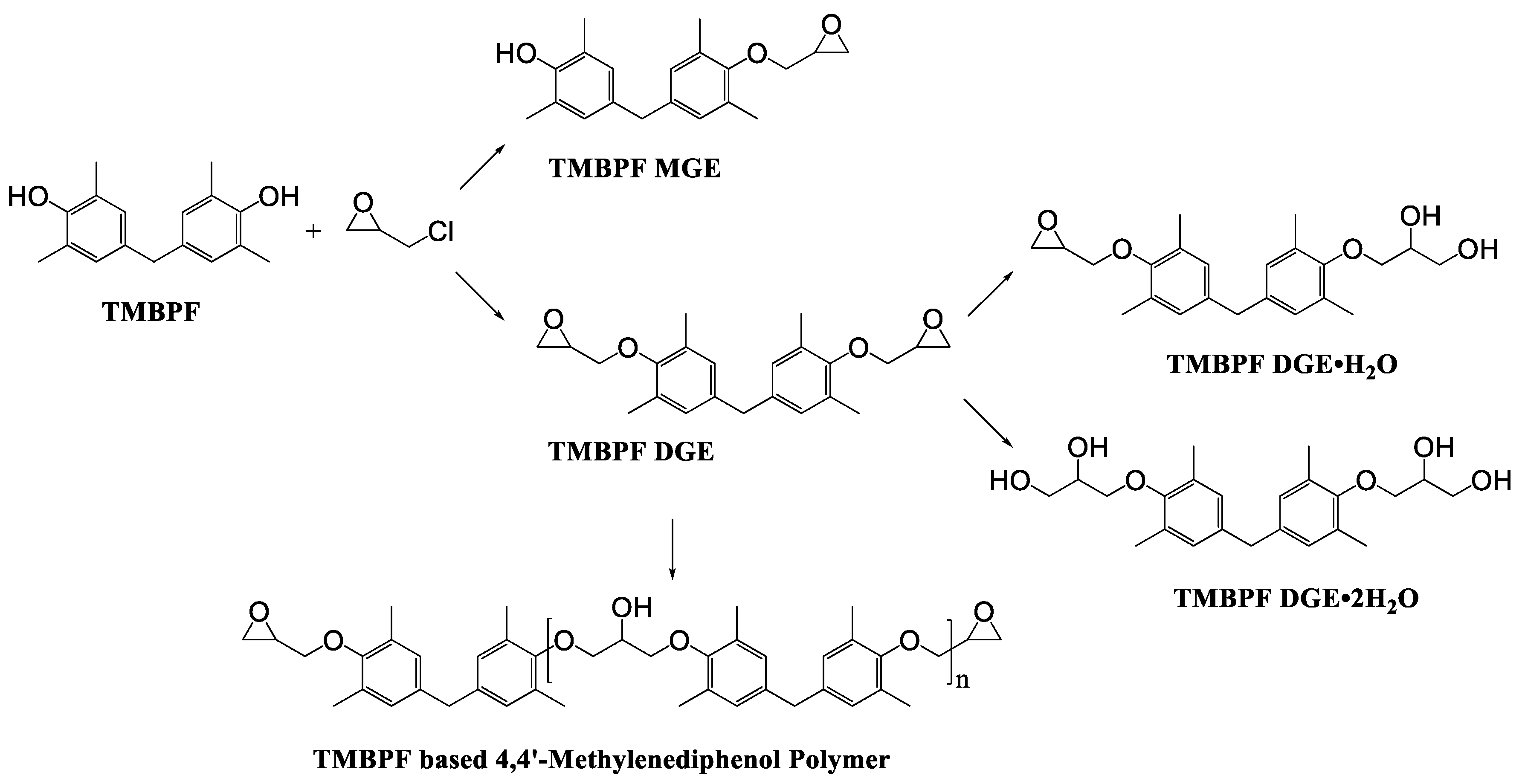
| Samples | Number of Detected Migrants | Estimated Level of Total Migrants (μg/dm2) | ||||
|---|---|---|---|---|---|---|
| 3% AA | 10% ETOH | 95% ETOH | 3% AA | 10% ETOH | 95% ETOH | |
| Food can set | 38 | 42 | 41 | 34.13 | 51.65 | 140.03 |
| Food can lid alone | 26 | 25 | 26 | 3.37 | 3.16 | 41.42 |
| Beverage can 1 | 4 | 2 | - | 0.44 | 0.08 | - |
| Beverage can 2 | 7 | 2 | - | 0.80 | 0.08 | - |
| Beverage can 3 | 6 | 6 | - | 0.58 | 0.23 | - |
| Beverage can lid 1 | 26 | 12 | - | 42.73 | 50.02 | - |
| Beverage can lid 2 | 15 | 10 | - | 1.36 | 3.81 | - |
| Beverage can lid 3 | 15 | 8 | - | 1.49 | 0.47 | - |
| Name 1 | Molecular Formula and Structure | Mass | 5 Major EI Fragments (% Abundance) | Estimated Level (μg/dm2) | Source |
|---|---|---|---|---|---|
| TMBPF | C17H20O2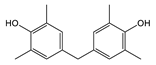 | 256 | 241(100.00) 256(59.39) 226(24.63) 255(18.67) 242(17.99) | 0.04–0.78 | Food can set Food can lid alone |
| TMBPF MGE | C20H24O3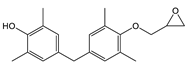 | 312 | 312(100.00) 239(45.30) 254(41.26) 209(30.63) 135(27.34) | 0.03–1.33 | Food can lid alone |
| TMBPF DGE | C23H28O4 | 368 | 175(100.00) 353(86.00) 191(66.51) 335(63.68) 368(35.86) | 0.04–5.10 | Food can set Food can lid alone |
| TMBPF DGE•H2O | C23H30O5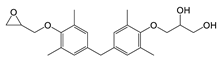 | 386 | 241(100.00) 256(58.60) 135(28.19) 75(19.47) 386(16.59) | 0.04–0.40 | Food can set Food can lid alone |
| TPPO | C18H15OP | 278 | 277(100.00) 278(58.99) 77(27.03) 201(25.00) 199(15.50) | 0.05–2.91 | Food can set Food can lid alone |
| BPA | C15H16O2 | 228 | 213(100.00) 228(24.99) 119(20.01) 214(17.26) 91(10.02) | 0.04–0.06 | Beverage can lid 1 and lid 3 |
| BPC | C17H20O2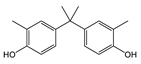 | 256 | 241(100.00) 133(22.57) 256(19.37) 242(18.21) 77(8.76) | 0.01–0.03 | Beverage can lid 1 and lid 3 |
| BPF | C13H12O2 | 200 | 200(100.00) 104(77.47) 199(46.37) 183(21.16) 94(14.20) | 0.04 | Beverage can lid 1 |
| Cyclic DEG-AA | C10H16O5 | 216 | 173(100.00) 55(78.00) 99(35.86) 84(32.54) 56(25.04) | 0.02–0.04 | Beverage Can 1,2 and 3 |
| Cyclic DEG-PA | C12H12O5 | 236 | 149(100.00) 193(98.57) 104(33.37) 76(24.76) 148(17.20) | 0.10 | Beverage Can 2 |
| Cyclic EG-AA-EG-AA | C16H24O8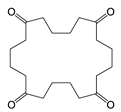 | 344 | 173(100.00) 99(61.02) 55(41.78) 113(32.68) 111(24.45) | 0.05 | Beverage can lid 1 |
| Cyclic EG-SeA-EG-PA | C22H28O8 | 420 | 237(100.00) 149(81.43) 193(64.50) 104(62.39) 148(37.35) | 5.05–9.77 | Beverage can lid 1 |
| Target Analyte | Linearity (R2) | LOD (µg/mL) | LOQ (µg/mL) | Precision (%) | Spiking and Recovery (Recovery%, n = 3) | ||
|---|---|---|---|---|---|---|---|
| 10% ETOH | 95% ETOH | 3% AA | |||||
| BPA | 0.9913 | 0.501 | 1.001 | 9.01 | 96.12 ± 8.35 | 99.69 ± 8.71 | 76.31 ± 5.92 |
| BPC | 0.9957 | 0.499 | 0.998 | 10.24 | 81.45 ± 7.42 | 96.64 ± 6.16 | 94.21 ± 7.99 |
| BPF | 0.9904 | 0.501 | 1.002 | 8.67 | 74.60 ± 5.45 | 93.38 ± 8.07 | 86.05 ± 9.41 |
| TMBPF | 0.9915 | 0.100 | 0.500 | 9.63 | 122.78 ± 9.38 | 103.99 ± 9.02 | 77.41 ± 4.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Scarsella, J.B.; Hartman, T.G. Identification and Quantitation Studies of Migrants from BPA Alternative Food-Contact Metal Can Coatings. Polymers 2020, 12, 2846. https://doi.org/10.3390/polym12122846
Zhang N, Scarsella JB, Hartman TG. Identification and Quantitation Studies of Migrants from BPA Alternative Food-Contact Metal Can Coatings. Polymers. 2020; 12(12):2846. https://doi.org/10.3390/polym12122846
Chicago/Turabian StyleZhang, Nan, Joseph B. Scarsella, and Thomas G. Hartman. 2020. "Identification and Quantitation Studies of Migrants from BPA Alternative Food-Contact Metal Can Coatings" Polymers 12, no. 12: 2846. https://doi.org/10.3390/polym12122846





