Effect of Carbon Nanostructures and Fatty Acid Treatment on the Mechanical and Thermal Performances of Flax/Polypropylene Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Treatment of Flax Fabrics with Carbon Nanostructures
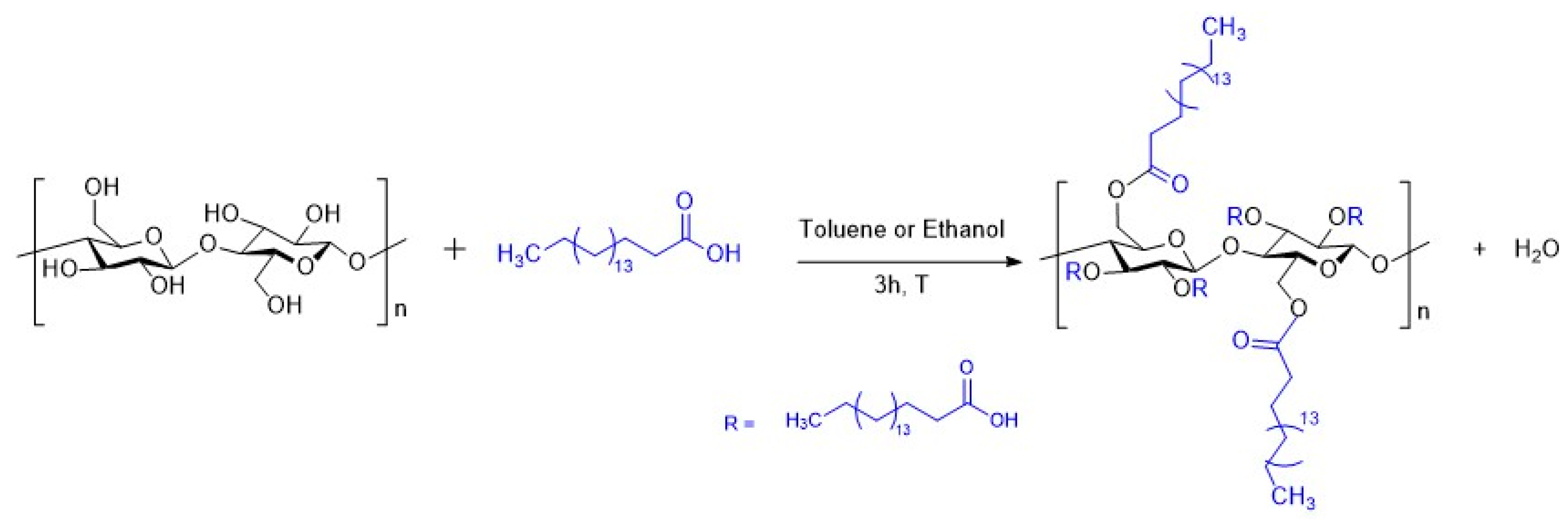
2.3. Surface Treatment of Flax Fabrics with Stearic Acid
2.4. Composite Manufacturing
2.5. Characterization Techniques
3. Results and Discussion
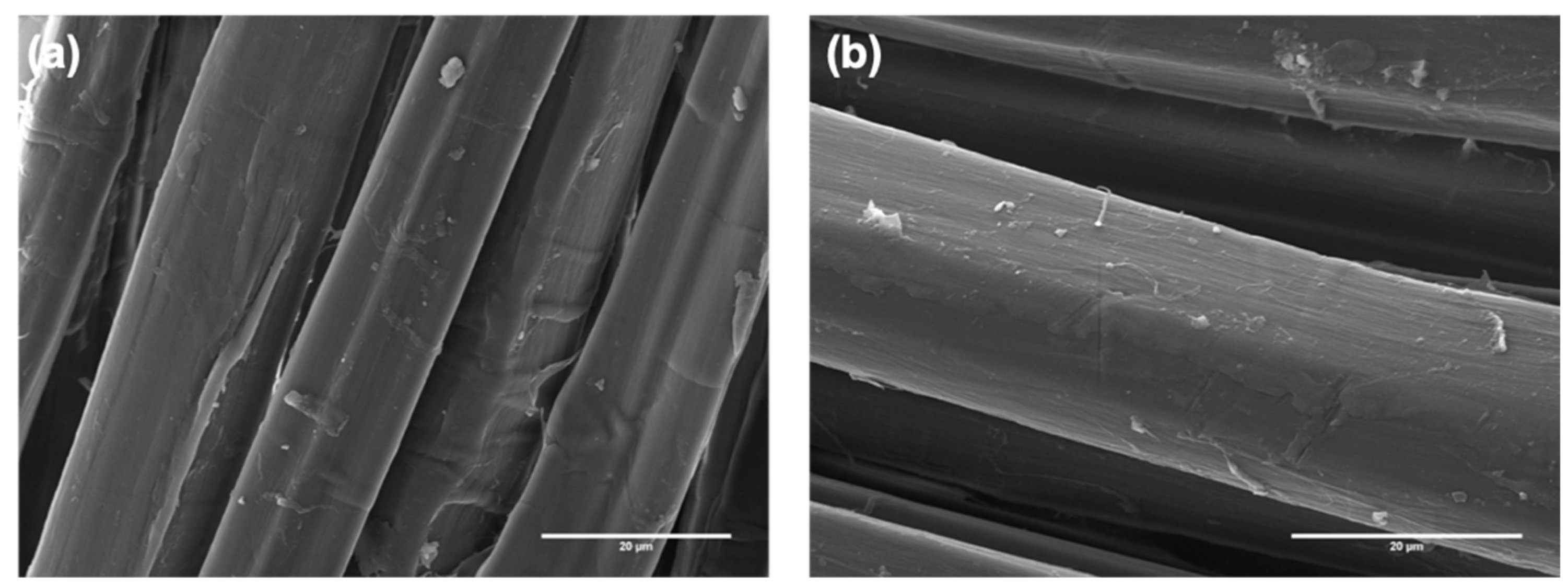
3.1. Characterization of Composites Reinforced with Flax Fabrics Decorated with Carbon Nanostructures
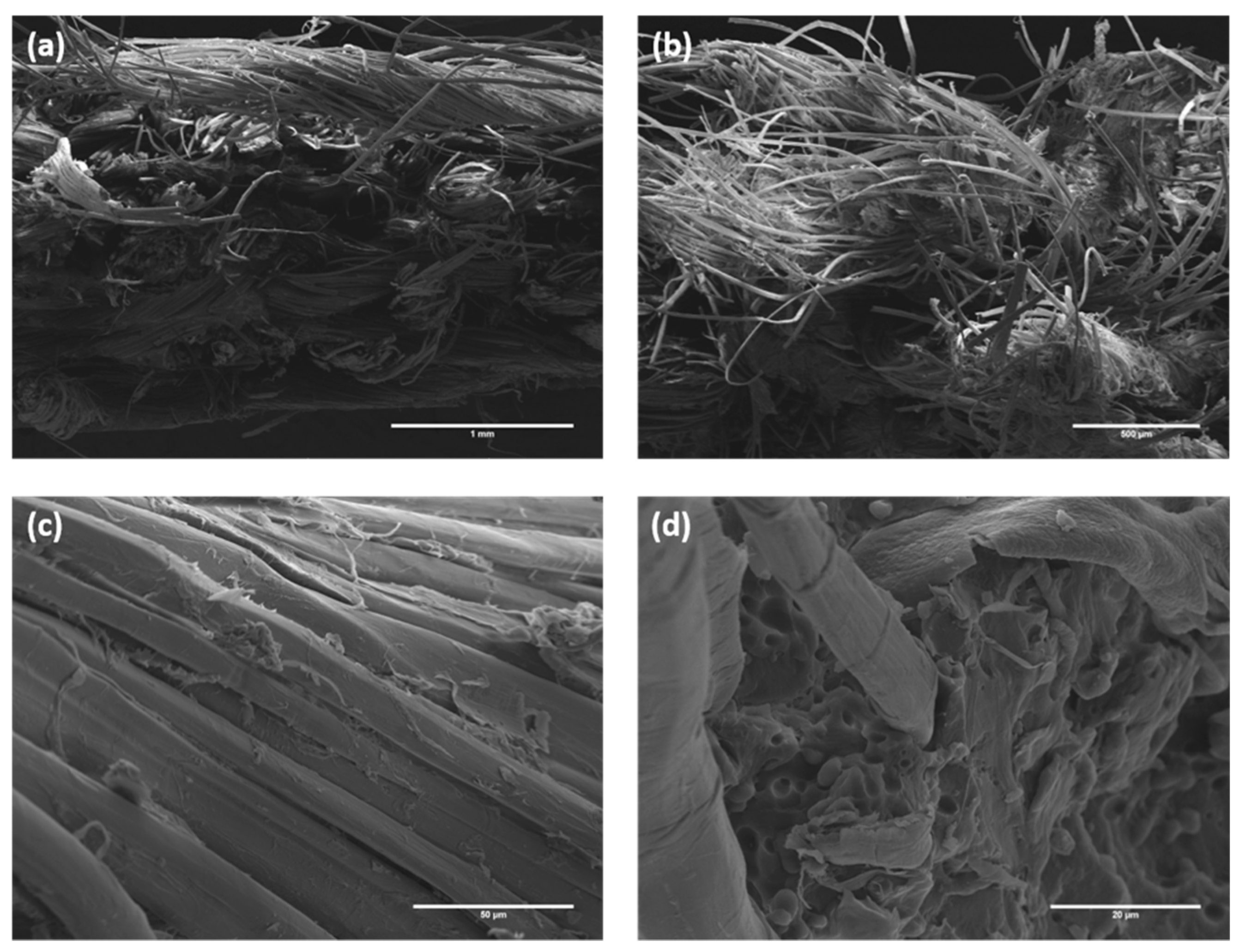
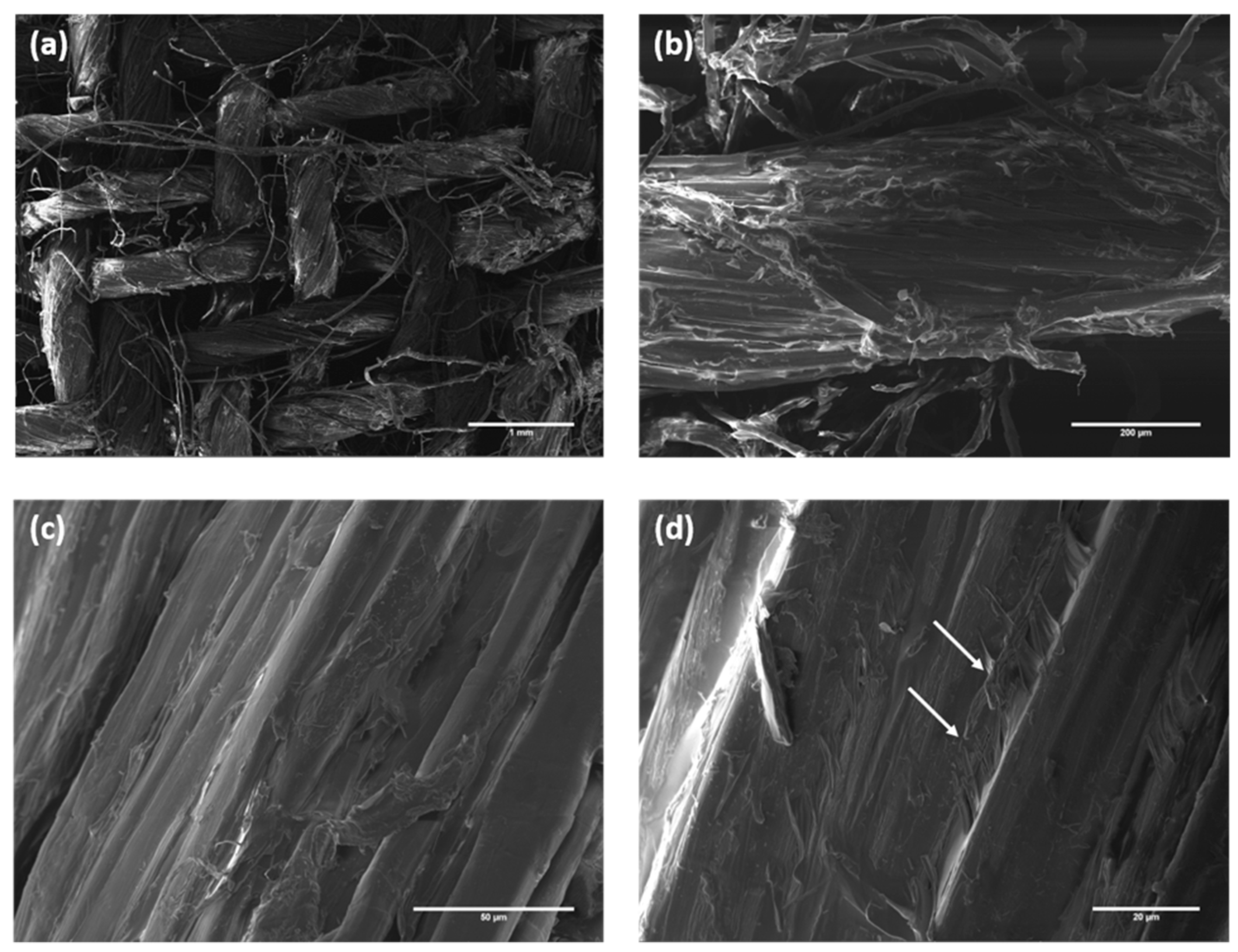
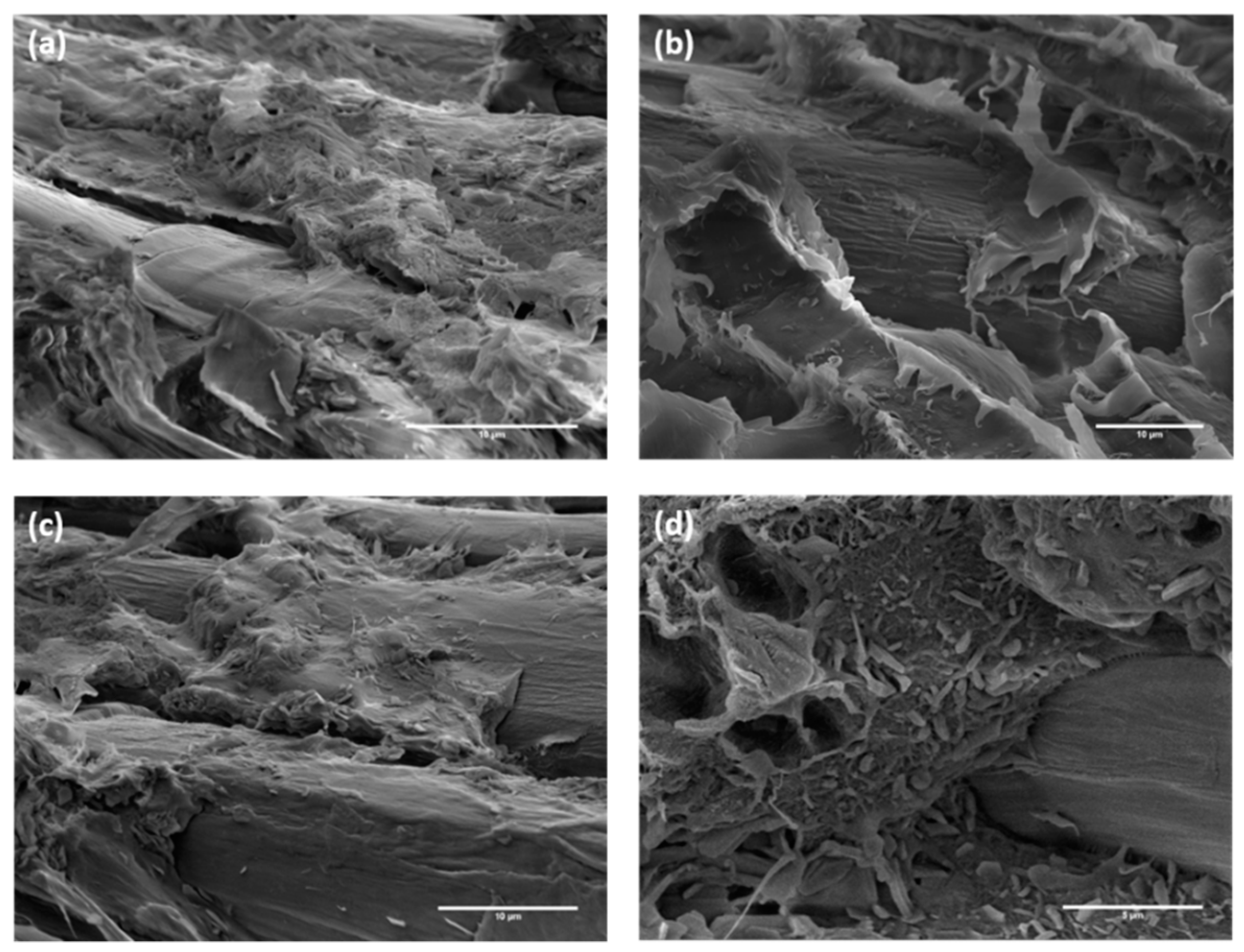
3.2. Characterization of Composites Reinforced with Flax Fabrics Treated with Stearic Acid
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koronis, G.; Silva, A.; Fontul, M. Green composites: A review of adequate materials for automotive applications. Compos. Part B Eng. 2013, 44, 120–127. [Google Scholar] [CrossRef]
- Pickering, K.L.; Aruan Efendy, M.G.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Choi, H.S.; Park, M.K. A Review: Natural Fiber Composites Selection in View of Mechanical, Light Weight, and Economic Properties. Macromol. Mater. Eng. 2015, 300, 10–24. [Google Scholar] [CrossRef]
- Akampumuza, O.; Wambua, P.M.; Ahmed, A.; Li, W.; Qin, X. Review of the applications of biocomposites in the automotive industry. Polym. Compos. 2017, 38, 2553–2569. [Google Scholar] [CrossRef]
- Shah, D.U. Developing plant fibre composites for structural applications by optimising composite parameters: A critical review. J. Mater. Sci. 2013, 48, 6083–6107. [Google Scholar] [CrossRef]
- Baley, C.; Gomina, M.; Breard, J.; Bourmaud, A.; Davies, P. Variability of mechanical properties of flax fibres for composite reinforcement. A review. Ind. Crops Prod. 2019, 111984. [Google Scholar] [CrossRef]
- Goudenhooft, C.; Bourmaud, A.; Baley, C. Varietal selection of flax over time: Evolution of plant architecture related to influence on the mechanical properties of fibers. Ind. Crops Prod. 2017, 97, 56–64. [Google Scholar] [CrossRef]
- Ausias, G.; Bourmaud, A.; Coroller, G.; Baley, C. Study of the fibre morphology stability in polypropylene-flax composites. Polym. Degrad. Stab. 2013, 98, 1216–1224. [Google Scholar] [CrossRef]
- Lebaupin, Y.; Chauvin, M.; Hoang, T.-Q.T.; Touchard, F.; Beigbeder, A. Influence of constituents and process parameters on mechanical properties of flax fibre-reinforced polyamide 11 composite. J. Thermoplast. Compos. Mater. 2017, 30, 1503–1521. [Google Scholar] [CrossRef]
- Dobah, Y.; Zampetakis, I.; Ward, C.; Scarpa, F. Thermoformability characterisation of Flax reinforced polypropylene composite materials. Compos. Part B Eng. 2020, 184, 107727. [Google Scholar] [CrossRef]
- Bourmaud, A.; Beaugrand, J.; Shaf, D.U.; Placet, V.; Baley, C. Towards the design of high-performance plant fibre composites. Prog. Mater. Sci. 2018, 97, 347–408. [Google Scholar] [CrossRef]
- Bulut, Y.; Aksit, A. A comparative study on chemical treatment of jute fiber: Potassium dichromate, potassium permanganate and sodium perborate trihydrate. Cellulose 2013, 20, 3155–3164. [Google Scholar] [CrossRef]
- Amiri, A.; Ulven, C.; Huo, S. Effect of Chemical Treatment of Flax Fiber and Resin Manipulation on Service Life of Their Composites Using Time-Temperature Superposition. Polymers 2015, 7, 1965–1978. [Google Scholar] [CrossRef] [Green Version]
- Van de Weyenberg, I.; Chi Truong, T.; Vangrimde, B.; Verpoest, I. Improving the properties of UD flax fibre reinforced composites by applying an alkaline fibre treatment. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1368–1376. [Google Scholar] [CrossRef]
- Doan, T.T.L.; Brodowsky, H.; Mäder, E. Jute fibre/epoxy composites: Surface properties and interfacial adhesion. Compos. Sci. Technol. 2012, 72, 1160–1166. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Khalil, H.P.S.A.; Hale, M.D. A study of the potential of acetylation to improve the properties of plant fibres. Ind. Crops Prod. 1998, 8, 53–63. [Google Scholar] [CrossRef]
- Bozaci, E.; Sever, K.; Sarikanat, M.; Seki, Y.; Demir, A.; Ozdogan, E.; Tavman, I. Effects of the atmospheric plasma treatments on surface and mechanical properties of flax fiber and adhesion between fiber-matrix for composite materials. Compos. Part B Eng. 2013, 45, 565–572. [Google Scholar] [CrossRef]
- Yuan, X.; Jayaraman, K.; Bhattacharyya, D. Plasma treatment of sisal fibres and its effects on tensile strength and interfacial bonding. J. Adhes. Sci. Technol. 2002, 16, 703–727. [Google Scholar] [CrossRef]
- Seghini, M.C.; Touchard, F.; Sarasini, F.; Chocinski-Arnault, L.; Tirillò, J.; Bracciale, M.P.; Zvonek, M.; Cech, V. Effects of oxygen and tetravinylsilane plasma treatments on mechanical and interfacial properties of flax yarns in thermoset matrix composites. Cellulose 2020, 27, 511–530. [Google Scholar] [CrossRef]
- Seki, Y.; Sarikanat, M.; Sever, K.; Erden, S.; Gulec, H.A. Effect of the low and radio frequency oxygen plasma treatment of jute fiber on mechanical properties of jute fiber/polyester composite. Fibers Polym. 2010, 11, 1159–1164. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Jin, X.; Wang, C.; Wang, D.; Ge, H. Modifying glass fibers with graphene oxide: Towards high-performance polymer composites. Compos. Sci. Technol. 2014, 97, 41–45. [Google Scholar] [CrossRef]
- Park, B.; Lee, W.; Lee, E.; Min, S.H.; Kim, B.-S. Highly Tunable Interfacial Adhesion of Glass Fiber by Hybrid Multilayers of Graphene Oxide and Aramid Nanofiber. ACS Appl. Mater. Interfaces 2015, 7, 3329–3334. [Google Scholar] [CrossRef]
- An, Q.; Rider, A.N.; Thostenson, E.T. Hierarchical Composite Structures Prepared by Electrophoretic Deposition of Carbon Nanotubes onto Glass Fibers. ACS Appl. Mater. Interfaces 2013, 5, 2022–2032. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, Z.; Tang, X.Z.; Yue, C.Y.; Yang, J. Enhanced interphase between epoxy matrix and carbon fiber with carbon nanotube-modified silane coating. Compos. Sci. Technol. 2014, 99, 131–140. [Google Scholar] [CrossRef]
- Hung, P.Y.; Lau, K.T.; Fox, B.; Hameed, N.; Lee, J.H.; Hui, D. Surface modification of carbon fibre using graphene–related materials for multifunctional composites. Compos. Part B Eng. 2018, 133, 240–257. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.B.; Hao, L.F.; Jiao, W.C.; Yang, F.; Wang, R.G. Preparation of carbon nanotube/carbon fiber hybrid fiber by combining electrophoretic deposition and sizing process for enhancing interfacial strength in carbon fiber composites. Compos. Sci. Technol. 2013, 88, 120–125. [Google Scholar] [CrossRef]
- Wang, H.; Xian, G.; Li, H. Grafting of nano-TiO2 onto flax fibers and the enhancement of the mechanical properties of the flax fiber and flax fiber/epoxy composite. Compos. Part A Appl. Sci. Manuf. 2015, 76, 172–180. [Google Scholar] [CrossRef]
- Sherief, Z.; Xian, G.; Thomas, S.; Ajith, A. Effects of surface grafting of copper nanoparticles on the tensile and bonding properties of flax fibers. Sci. Eng. Compos. Mater. 2017, 24, 651–660. [Google Scholar] [CrossRef]
- Ajith, A.; Xian, G.; Li, H.; Sherief, Z.; Thomas, S. Surface grafting of flax fibres with hydrous zirconia nanoparticles and the effects on the tensile and bonding properties. J. Compos. Mater. 2016, 50, 627–635. [Google Scholar] [CrossRef]
- Lakshmanan, A.; Chakraborty, S. Coating of silver nanoparticles on jute fibre by in situ synthesis. Cellulose 2017, 24, 1563–1577. [Google Scholar] [CrossRef]
- Wang, W.; Xian, G.; Li, H. Surface modification of ramie fibers with silanized CNTs through a simple spray-coating method. Cellulose 2019, 26, 8165–8178. [Google Scholar] [CrossRef]
- Sarker, F.; Karim, N.; Afroj, S.; Koncherry, V.; Novoselov, K.S.; Potluri, P. High-Performance Graphene-Based Natural Fiber Composites. ACS Appl. Mater. Interfaces 2018, 10, 34502–34512. [Google Scholar] [CrossRef]
- Sarker, F.; Potluri, P.; Afroj, S.; Koncherry, V.; Novoselov, K.S.; Karim, N. Ultrahigh Performance of Nanoengineered Graphene-Based Natural Jute Fiber Composites. ACS Appl. Mater. Interfaces 2019, 11, 21166–21176. [Google Scholar] [CrossRef]
- Zhuang, R.C.; Doan, T.T.L.; Liu, J.W.; Zhang, J.; Gao, S.L.; Mäder, E. Multi-functional multi-walled carbon nanotube-jute fibres and composites. Carbon 2011, 49, 2683–2692. [Google Scholar] [CrossRef]
- Souri, H.; Bhattacharyya, D. Wearable strain sensors based on electrically conductive natural fiber yarns. Mater. Des. 2018, 154, 217–227. [Google Scholar] [CrossRef]
- Joly, C.; Gauthier, R.; Chabert, B. Physical chemistry of the interface in polypropylene/cellulosic-fibre composites. Compos. Sci. Technol. 1996, 56, 761–765. [Google Scholar] [CrossRef]
- Sojoudiasli, H.; Heuzey, M.C.; Carreau, P.J. Rheological, morphological and mechanical properties of flax fiber polypropylene composites: Influence of compatibilizers. Cellulose 2014, 21, 3797–3812. [Google Scholar] [CrossRef]
- George, G.; Tomlal Jose, E.; Jayanarayanan, K.; Nagarajan, E.R.; Skrifvars, M.; Joseph, K. Novel bio-commingled composites based on jute/polypropylene yarns: Effect of chemical treatments on the mechanical properties. Compos. Part A Appl. Sci. Manuf. 2012, 43, 219–230. [Google Scholar] [CrossRef]
- Dányádi, L.; Móczó, J.; Pukánszky, B. Effect of various surface modifications of wood flour on the properties of PP/wood composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 199–206. [Google Scholar] [CrossRef]
- Gonzalez, L.; Lafleur, P.; Lozano, T.; Morales, A.B.; Garcia, R.; Angeles, M.; Rodriguez, F.; Sanchez, S. Mechanical and thermal properties of polypropylene/montmorillonite nanocomposites using stearic acid as both an interface and a clay surface modifier. Polym. Compos. 2014, 35, 1–9. [Google Scholar] [CrossRef]
- Kumar, V.; Dev, A.; Gupta, A.P. Studies of poly(lactic acid) based calcium carbonate nanocomposites. Compos. Part B Eng. 2014, 56, 184–188. [Google Scholar] [CrossRef]
- Xie, L.; Shan, B.; Sun, X.; Tian, Y.; Xie, H.; He, M.; Xiong, Y.; Zheng, Q. Natural Fiber-Anchored Few-Layer Graphene Oxide Nanosheets for Ultrastrong Interfaces in Poly(lactic acid). ACS Sustain. Chem. Eng. 2017, 5, 3279–3289. [Google Scholar] [CrossRef]
- Salem, I.A.S.; Rozyanty, A.R.; Betar, B.O.; Adam, T.; Mohammed, M.; Mohammed, A.M. Study of the effect of surface treatment of kenaf fiber on chemical structure and water absorption of kenaf filled unsaturated polyester composite. J. Phys. Conf. Ser. 2017, 908, 012001. [Google Scholar] [CrossRef]
- Jin, S.H.; Kang, C.H.; Yoon, K.H.; Bang, D.S.; Park, Y.-B. Effect of compatibilizer on morphology, thermal, and rheological properties of polypropylene/functionalized multi-walled carbon nanotubes composite. J. Appl. Polym. Sci. 2008. [Google Scholar] [CrossRef]
- Ezat, G.; Kelly, A.; Mitchell, S.; Youseffi, M.; Coates, P.D. Influence of maleic anhydride compatibiliser on properties of polpropylene/multiwalled carbon nanotube composites. Plast. Rubber Compos. 2011, 40, 438–448. [Google Scholar] [CrossRef]
- Ambrogi, V.; Gentile, G.; Ducati, C.; Oliva, M.C.; Carfagna, C. Multiwalled carbon nanotubes functionalized with maleated poly(propylene) by a dry mechano-chemical process. Polymer 2012, 53, 291–299. [Google Scholar] [CrossRef]
- Lee, G.W.; Jagannathan, S.; Chae, H.G.; Minus, M.L.; Kumar, S. Carbon nanotube dispersion and exfoliation in polypropylene and structure and properties of the resulting composites. Polymer 2008, 49, 1831–1840. [Google Scholar] [CrossRef]
- Roh, J.U.; Ma, S.W.; Lee, W., II; Thomas Hahn, H.; Lee, D.W. Electrical and mechanical properties of graphite/maleic anhydride grafted polypropylene nanocomposites. Compos. Part B Eng. 2013, 45, 1548–1553. [Google Scholar] [CrossRef]
- Pedrazzoli, D.; Pegoretti, A.; Kalaitzidou, K. Synergistic effect of graphite nanoplatelets and glass fibers in polypropylene composites. J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- Kannan, T.G.; Wu, C.M.; Cheng, K.B.; Wang, C.Y. Effect of reinforcement on the mechanical and thermal properties of flax/polypropylene interwoven fabric composites. J. Ind. Text. 2013, 42, 417–433. [Google Scholar] [CrossRef]
- Huang, G.; Liu, L. Research on properties of thermoplastic composites reinforced by flax fabrics. Mater. Des. 2008, 29, 1075–1079. [Google Scholar] [CrossRef]
- Bar, M.; Alagirusamy, R.; Das, A. Properties of flax-polypropylene composites made through hybrid yarn and film stacking methods. Compos. Struct. 2018, 197, 63–71. [Google Scholar] [CrossRef]
- Van de Velde, K.; Baetens, E. Thermal and Mechanical Properties of Flax Fibres as Potential Composite Reinforcement. Macromol. Mater. Eng. 2001, 286, 342–349. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Y.; Wang, J.; Lai, M.; Li, J.; Liu, J.; Tong, X.; Cheng, H. Morphology, thermal stability, and dynamic mechanical properties of atactic polypropylene/carbon nanotube composites. J. Appl. Polym. Sci. 2005, 98, 1087–1091. [Google Scholar] [CrossRef]
- Liauw, C.M. Filler surface modification with organic acids. Plast. Addit. Compd. 2000, 2, 26–29. [Google Scholar]
- Zafeiropoulos, N.E.; Williams, D.R.; Baillie, C.A.; Matthews, F.L. Engineering and characterisation of the interface in flax fibre/polypropylene composite materials. Part, I. Development and investigation of surface treatments. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1083–1093. [Google Scholar] [CrossRef]
- Spoljaric, S.; Genovese, A.; Shanks, R.A. Polypropylene-microcrystalline cellulose composites with enhanced compatibility and properties. Compos. Part A Appl. Sci. Manuf. 2009, 40, 791–799. [Google Scholar] [CrossRef]
- Kiattipanich, N.; Kreua-ongarjnukool, N.; Pongpayoon, T.; Phalakornkule, C. Properties of polypropylene composites reinforced with stearic acid treated sugarcane fiber. J. Polym. Eng. 2007, 27, 411–428. [Google Scholar] [CrossRef]
- Fan, H.; Wang, X.; Wang, Y. Rapid determination on cellulose content of wood by using FTIR spectrometry. Wood Proc. Mach. 2014, 4, 33–37. [Google Scholar]
- Webber, J.; Zorzi, J.E.; Perottoni, C.A.; Moura e Silva, S.; Cruz, R.C.D. Identification of α-Al2O3 surface sites and their role in the adsorption of stearic acid. J. Mater. Sci. 2016, 51, 5170–5184. [Google Scholar] [CrossRef]
- Arata, S.; Kurosu, H.; Kuroki, S.; Ando, I. Structural characterization of stearic acid in the crystalline state by the cross polarization in solid state 13C NMR. J. Mol. Struct. 1999, 513, 133–138. [Google Scholar] [CrossRef]
- Ribeiro, G.A.C.; Silva, D.S.A.; Dos Santos, C.C.; Vieira, A.P.; Bezerra, C.W.B.; Tanaka, A.A.; Santana, S.A.A. Removal of Remazol brilliant violet textile dye by adsorption using rice hulls. Polimeros 2017, 27, 16–26. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Xu, M.; Zhang, L. Controllable Stearic Acid Crystal Induced High Hydrophobicity on Cellulose Film Surface. ACS Appl. Mater. Interfaces 2013, 5, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.; Speier, A. Darstellung der Ester. Ber. Dtsch. Chem. Ges. 1895, 28, 3252–3258. [Google Scholar] [CrossRef]
- Bouguerra Neji, S.; Trabelsi, M.; Frikha, M. Esterification of Fatty Acids with Short-Chain Alcohols over Commercial Acid Clays in a Semi-Continuous Reactor. Energies 2009, 2, 1107–1117. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Malta, V.; Celotti, G.; Zannetti, R.; Martelli, A.F. Crystal structure of the C form of stearic acid. J. Chem. Soc. B Phys. Org. 1971, 548–553. [Google Scholar] [CrossRef]
- Mazian, B.; Bergeret, A.; Benezet, J.C.; Malhautier, L. Influence of field retting duration on the biochemical, microstructural, thermal and mechanical properties of hemp fibres harvested at the beginning of flowering. Ind. Crops Prod. 2018, 116, 170–181. [Google Scholar] [CrossRef]
- Fu, Z.; Dai, L.; Yi, Y.; Luo, J.; Li, B. Structure and thermal properties of stearic acid/silica composites as form-stable phase change materials. J. Sol-Gel Sci. Technol. 2018, 87, 419–426. [Google Scholar] [CrossRef]
- Zafeiropoulos, N.E.; Baillie, C.A.; Hodgkinson, J.M. Engineering and characterisation of the interface in flax fibre/polypropylene composite materials. Part II. The effect of surface treatments on the interface. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1185–1190. [Google Scholar] [CrossRef]
- Zafeiropoulos, N.E.; Baillie, C.A.; Matthews, F.L. Study of transcrystallinity and its effect on the interface in flax fibre reinforced composite materials. Compos. Part A Appl. Sci. Manuf. 2001, 32, 525–543. [Google Scholar] [CrossRef]



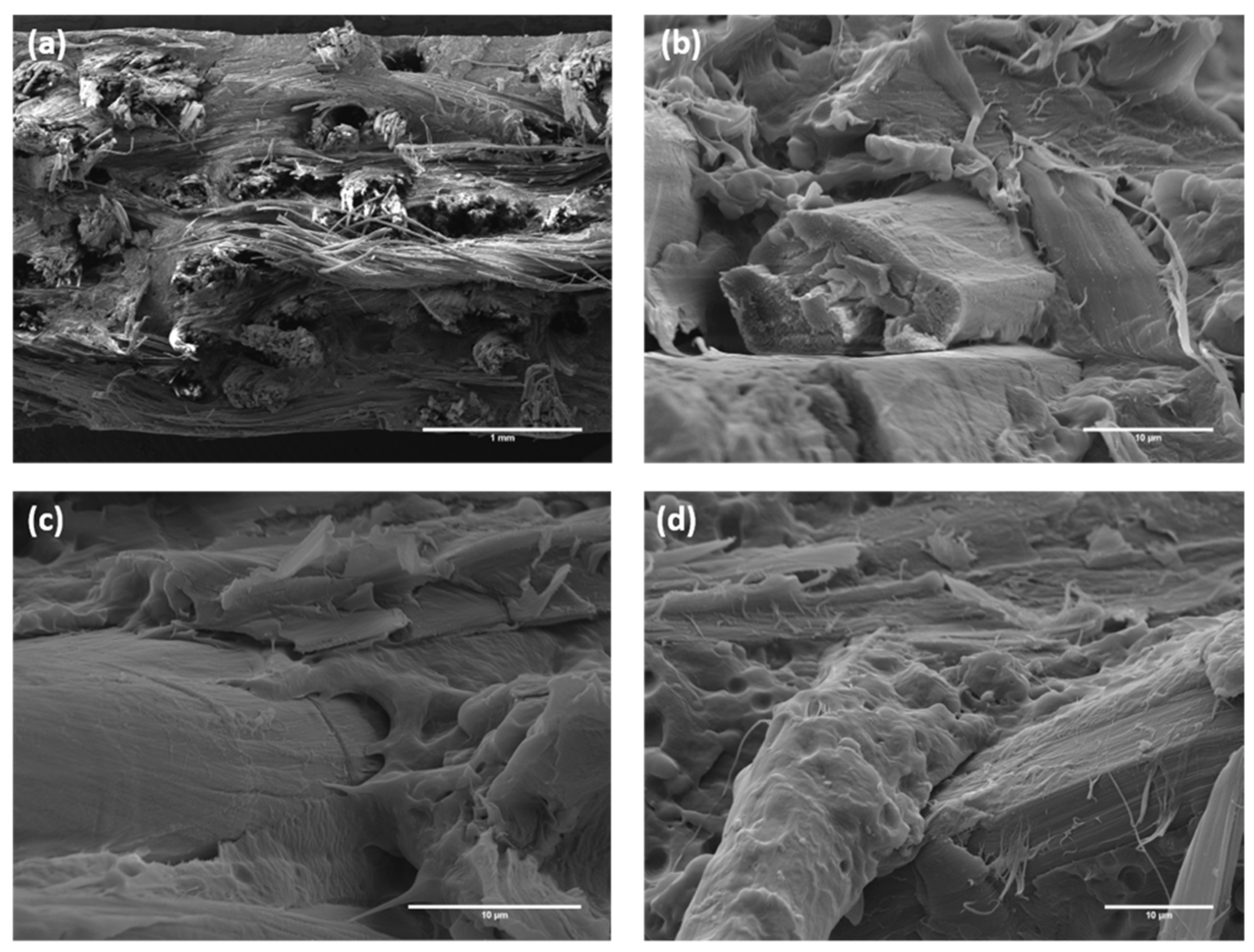
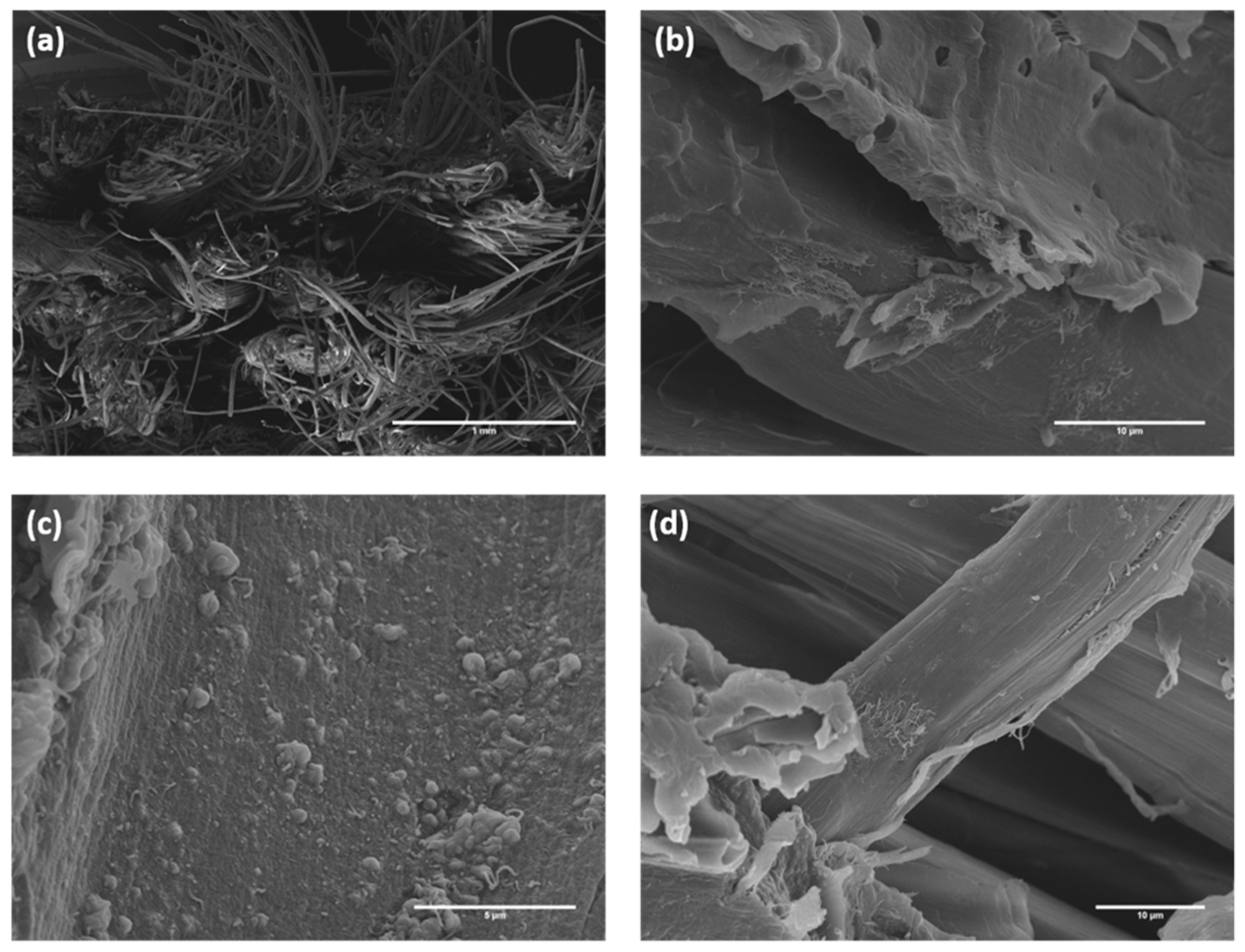
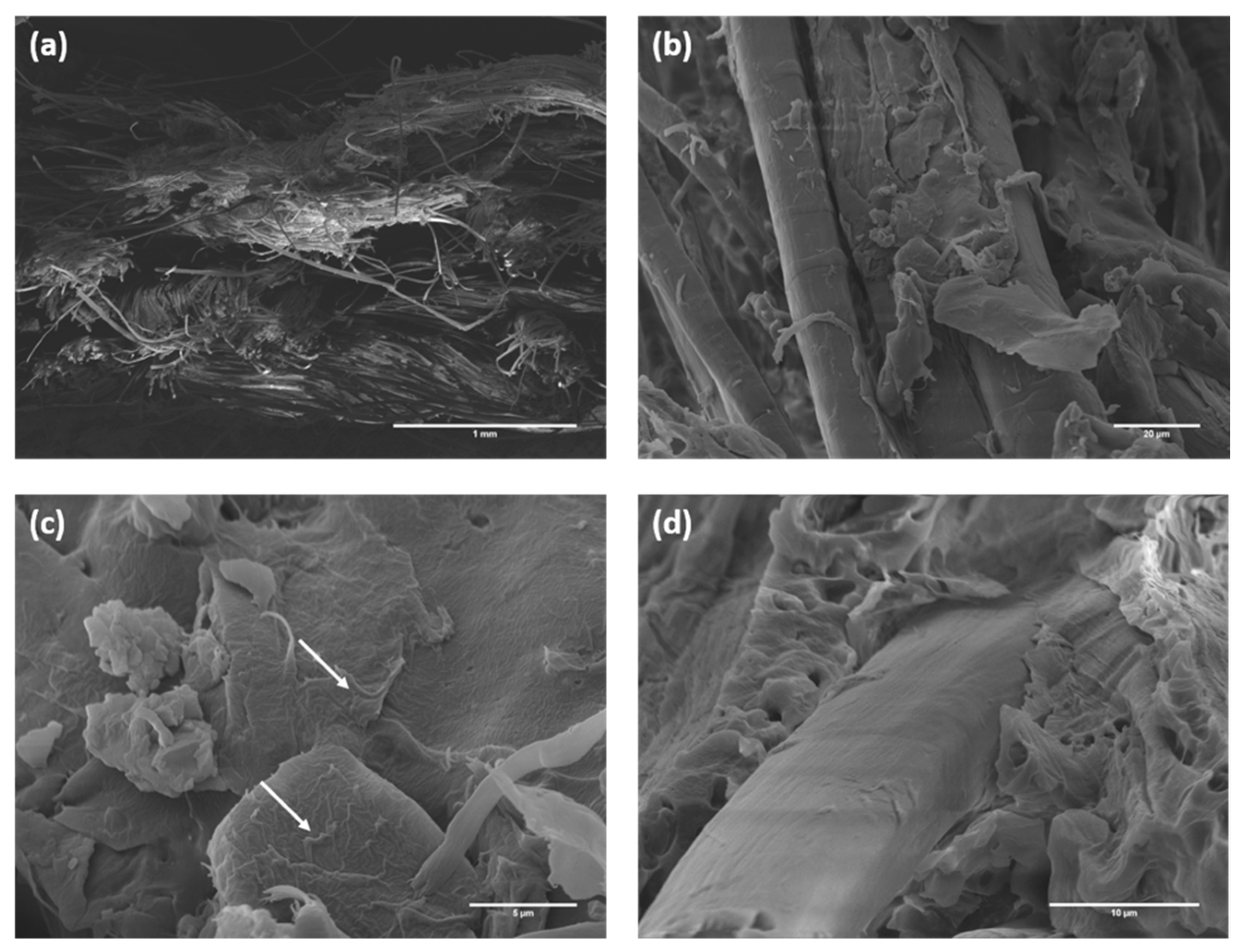


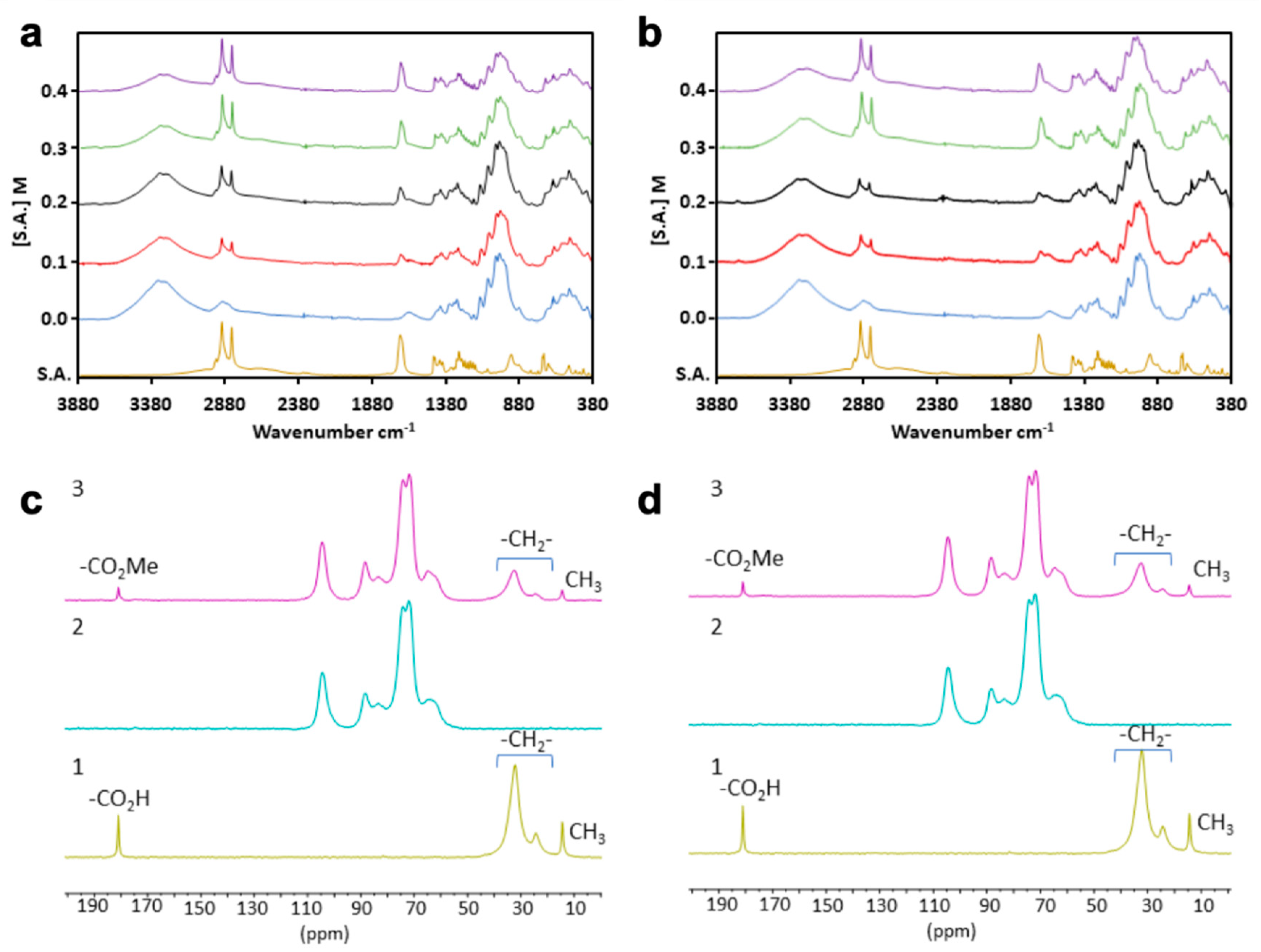




| Specimen ID | YM (GPa) | TS (MPa) | Percentage Variation, YM (%) | Percentage Variation, TS (%) | FM (GPa) | FS (MPa) | Percentage Variation, FM (%) | Percentage Variation, FS (%) |
|---|---|---|---|---|---|---|---|---|
| PP/Flax | 6.75 ± 0.50 | 66.09 ± 4.83 | - | - | 5.42 ± 0.96 | 49.47 ± 6.77 | - | - |
| PPC/Flax | 9.98 ± 0.78 | 89.46 ± 2.33 | +47.85 | +35.36 | 7.80 ± 0.43 | 94.34 ± 3.68 | +43.91 | +90.70 |
| PP/Flax_GNP | 6.18 ± 0.17 | 59.54 ± 1.01 | −8.44 | −9.91 | 4.29 ± 0.25 | 51.25 ± 1.32 | −20.85 | +3.60 |
| PPC/Flax_GNP | 10.46 ± 0.29 | 94.15 ± 1.70 | +54.96 | +42.46 | 7.98 ± 0.32 | 106.36 ± 0.63 | +47.23 | +114.99 |
| PP/Flax_CNT | 5.17 ± 0.17 | 59.72 ± 3.01 | −23.41 | −9.64 | 3.69 ± 0.23 | 43.23 ± 2.38 | −31.92 | −12.61 |
| PP/Flax_SA | 13.70 ± 0.04 | 68.33 ± 3.73 | +102.96 | +3.39 | 8.77 ± 0.72 | 56.38 ± 1.11 | +61.81 | +13.97 |
| PPC/Flax_SA | 13.41 ± 1.69 | 73.28 ± 1.71 | +98.67 | +10.88 | 10.88 ± 0.88 | 87.44 ± 3.31 | +100.74 | +76.87 |
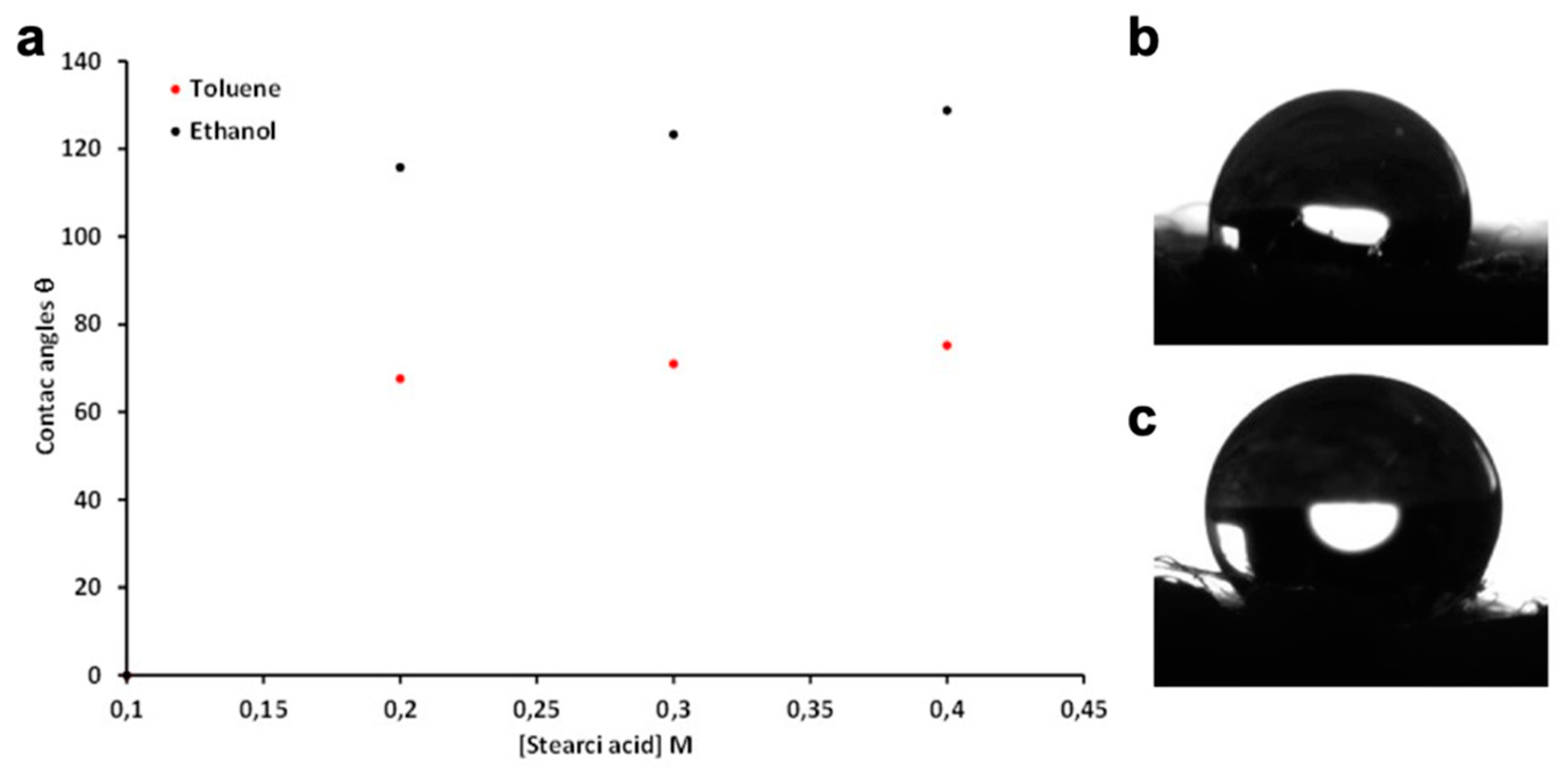
| Contact angle, θ [°] | ||
|---|---|---|
| Stearic Acid [M] | Solvent | |
| Toluene | Ethanol | |
| 0.1 | - | - |
| 0.2 | 67.6 ± 0.05 | 115.8 ± 0.27 |
| 0.3 | 71 ± 0.32 | 123.3 ± 0.31 |
| 0.4 | 75.2 ± 0.02 | 128.8 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, P.; Vitiello, L.; Sbardella, F.; Santos, J.I.; Tirillò, J.; Bracciale, M.P.; Rivilla, I.; Sarasini, F. Effect of Carbon Nanostructures and Fatty Acid Treatment on the Mechanical and Thermal Performances of Flax/Polypropylene Composites. Polymers 2020, 12, 438. https://doi.org/10.3390/polym12020438
Russo P, Vitiello L, Sbardella F, Santos JI, Tirillò J, Bracciale MP, Rivilla I, Sarasini F. Effect of Carbon Nanostructures and Fatty Acid Treatment on the Mechanical and Thermal Performances of Flax/Polypropylene Composites. Polymers. 2020; 12(2):438. https://doi.org/10.3390/polym12020438
Chicago/Turabian StyleRusso, Pietro, Libera Vitiello, Francesca Sbardella, Jose I. Santos, Jacopo Tirillò, Maria Paola Bracciale, Iván Rivilla, and Fabrizio Sarasini. 2020. "Effect of Carbon Nanostructures and Fatty Acid Treatment on the Mechanical and Thermal Performances of Flax/Polypropylene Composites" Polymers 12, no. 2: 438. https://doi.org/10.3390/polym12020438