Filler-Modified Castor Oil-Based Polyurethane Foam for the Removal of Aqueous Heavy Metals Detected Using Laser-Induced Breakdown Spectroscopy (LIBS) Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
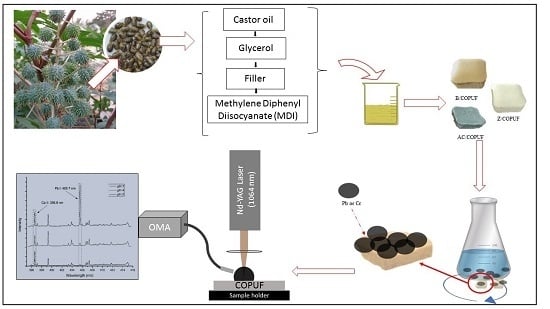
2.2. Synthesis of Modified PUF
2.3. Characterization
2.4. Batch Adsorption
2.5. LIBS Analysis
3. Results and Discussion
3.1. Reaction of Modified PUF and Functional Groups’ Analysis
3.2. Scanning Electron Microscopy (SEM)
3.3. Swelling Degree Profile
3.4. Thermal Degradation Profile
3.5. Adsorption of Pb2+ Ions on Various COPUFs
3.6. Effect of Contact Time
3.7. Effect of pH
3.8. Comparison with the Commercial Super-Hydrophilic Polyurethane Foam (CPUF)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Li, Z.; Wang, D.; Li, J.; Zou, B.; Tao, Y.; Lei, L.; Qiao, F.; Huang, J. Assessment of residents’ total environmental exposure to heavy metals in China. Sci. Rep. 2019, 9, 16386. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.F.; Miranda, L.P.; Ramirez, A.M.; Quintero, K.P.; Bernard, F.L.; Einloft, S.; Díaz, L.A.C. New biocomposites based on castor oil polyurethane foams and ionic liquids for CO2 capture. Fluid Phase Equilibria 2017, 452, 103–112. [Google Scholar] [CrossRef]
- Wang, M.; Xie, R.; Chen, Y.; Pu, X.; Jiang, W.; Yao, L. A novel mesoporous zeolite-activated carbon composite as an effective adsorbent for removal of ammonia-nitrogen and methylene blue from aqueous solution. Bioresour. Technol. 2018, 268, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Abdulmadjid, S.N.; Idris, N.; Pardede, M.; Jobiliong, E.; Hedwig, R.; Lie, Z.S.; Suyanto, H.; Tjia, M.O.; Kurniawan, K.H.; Kagawa, K. Sensitive analysis of carbon, chromium and silicon in steel using picosecond laser induced low pressure helium plasma. Spectrochim. Acta Part B At. Spectrosc. 2015, 114, 1–6. [Google Scholar] [CrossRef]
- Iqbal, J.; Pardede, M.; Jobiliong, E.; Hedwig, R.; Ramli, M.; Khumaeni, A.; Budi, W.S.; Idris, N.; Abdulmadjid, S.N.; Lahna, K.; et al. Shock wave plasma generation in low pressure ambient gas from powder sample using subtarget supported micro mesh as a sample holder and its potential applications for sensitive analysis of powder samples. Microchem. J. 2018, 142, 108–116. [Google Scholar] [CrossRef]
- Santos, K.M.; Cortez, J.; Raimundo, I.M.; Pasquini, C.; Morte, E.S.B.; Korn, M.G.A. An assessment of the applicability of the use of a plasticised PVC membrane containing pyrochatecol violet complexing reagent for the determination of Cu2+ ions in aqueous solutions by LIBS. Microchem. J. 2013, 110, 435–438. [Google Scholar] [CrossRef]
- Rezk, R.A.; Galmed, A.H.; Abdelkreem, M.; Ghany, N.A.A.; Harith, M.A. Detachment of Cu (II) and Co (II) ions from synthetic wastewater via adsorption on Lates niloticus fish bones using LIBS and XRF. J. Adv. Res. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Zhu, H.; Han, J. Dual pulse laser induced breakdown spectroscopy on Cu concentration in CuSO4 solution with liquid jet. Proc. SPIE 2017, 10461, 1046105. [Google Scholar]
- Attar, K.; Demey, H.; Bouazza, D.; Sastre, A. Sorption and Desorption Studies of Pb(II) and Ni(II) from Aqueous Solutions by a New Composite Based on Alginate and Magadiite Materials. Polymers 2019, 11, 340. [Google Scholar] [CrossRef] [Green Version]
- Pedrazzo, R.; Smarra, A.; Caldera, F.; Musso, G.; Dhakar, N.K.; Cecone, C.; Hamedi, A.; Corsi, I.; Trotta, F.; Pedrazzo, A.R. Eco-Friendly β-cyclodextrin and Linecaps Polymers for the Removal of Heavy Metals. Polymers 2019, 11, 1658. [Google Scholar] [CrossRef] [Green Version]
- Ślusarczyk, C.; Fryczkowska, B. Structure–Property Relationships of Pure Cellulose and GO/CEL Membranes Regenerated from Ionic Liquid Solutions. Polymers 2019, 11, 1178. [Google Scholar] [CrossRef] [Green Version]
- Kalaivani, S.S.; Muthukrishnaraj, A.; Sivanesan, S.; Ravikumar, L. Novel hyperbranched polyurethane resins for the removal of heavy metal ions from aqueous solution. Process. Saf. Environ. Prot. 2016, 104, 11–23. [Google Scholar] [CrossRef]
- Hong, H.-J.; Lim, J.S.; Hwang, J.Y.; Kim, M.; Jeong, H.S.; Park, M.S. Carboxymethlyated cellulose nanofibrils(CMCNFs) embedded in polyurethane foam as a modular adsorbent of heavy metal ions. Carbohydr. Polym. 2018, 195, 136–142. [Google Scholar] [CrossRef]
- Carriço, C.S.; Fraga, T.; Pasa, V.M.D. Production and characterization of polyurethane foams from a simple mixture of castor oil, crude glycerol and untreated lignin as bio-based polyols. Eur. Polym. J. 2016, 85, 53–61. [Google Scholar] [CrossRef]
- Carriço, C.S.; Fraga, T.; Carvalho, V.E.; Pasa, V.M.D. Polyurethane foams for thermal insulation uses produced from castor oil and crude glycerol biopolyols. Molecules 2017, 22, 1091. [Google Scholar] [CrossRef]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. Synthesis and mechanical properties of bio-sourced polyurethane adhesives obtained from castor oil and MDI-modified cellulose acetate: Influence of cellulose acetate modification. Int. J. Adhes. Adhes. 2019, 95, 102404. [Google Scholar] [CrossRef]
- Lubczak, R.; Szczęch, D.; Lubczak, J. From starch to oligoetherols and polyurethane foams. Polym. Bull. 2019. [Google Scholar] [CrossRef]
- Bernardini, J.; Licursi, D.; Anguillesi, I.; Cinelli, P.; Coltelli, M.-B.; Antonetti, C.; Galletti, A.M.R.; Lazzeri, A. Exploitation of Arundo donax L. Hydrolysis Residue for the Green Synthesis of Flexible Polyurethane Foams. BioResources 2017, 12, 3630–3655. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Mandal, B.; Katiyar, V. Environment-friendly synthesis of sustainable chitosan-based nonisocyanate polyurethane: A biobased polymeric film. J. Appl. Polym. Sci. 2020, 137, 49050. [Google Scholar] [CrossRef]
- Bortoletto-Santos, R.; Guimarães, G.G.F.; Junior, V.R.; da Cruz, D.F.; Polito, W.L.; Ribeiro, C. Biodegradable oil-based polymeric coatings on urea fertilizer: N release kinetic transformations of urea in soil. Sci. Agric. 2020, 77, 1–9. [Google Scholar] [CrossRef]
- Darmadi, D.; Irfan, M.; Iqhramullah, M.; Marlina, M.; Mirna, R.L. Synthesis of Chitosan Modified Polyurethane Foam for Adsorption of Mercury (II) Ions. J. Bahan Alam Terbarukan 2018, 7, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Yu, Q.; Cui, Y.; Xie, F.; Li, W.; Li, Y.; Chen, M. Adsorption properties of activated carbon from reed with a high adsorption capacity. Ecol. Eng. 2017, 102, 443–450. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Al-Odayni, A.-B.; Saeed, W.S.; Al-Kahtani, A.; Alharthi, F.A.; Aouak, T. Efficient Adsorption of Lead (II) from Aqueous Phase Solutions Using Polypyrrole-Based Activated Carbon. Materials 2019, 12, 2020. [Google Scholar] [CrossRef] [Green Version]
- Horňáčková, M.; Plavčan, J.; Horňáček, M.; Hudec, P.; Veis, P. Heavy Metals Detection in Zeolites Using the LIBS Method. Atoms 2019, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, X.; Lei, H.; Ye, W.; Cui, Y. Adsorption Property of Pb(II) by the Laterite-Bentonite Mixture Used as Waste Landfill Liner. Adv. Civ. Eng. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Patel, V.R.; Dumancas, G.G.; Viswanath, L.C.K.; Maples, R.; Subong, B.J.J. Castor Oil: Properties, Uses, and Optimization of Processing Parameters in Commercial Production. Lipid Insights 2016, 9, LPI.S40233. [Google Scholar] [CrossRef] [Green Version]
- Chun, B.C.; Chong, M.H.; Chung, Y.-C. Effect of glycerol cross-linking and hard segment content on the shape memory property of polyurethane block copolymer. J. Mater. Sci. 2007, 42, 6524–6531. [Google Scholar] [CrossRef]
- Motawie, A.; Madani, M.; Esmail, E.; Dacrorry, A.; Othman, H.; Badr, M.; Abulyazied, D. Electrophysical characteristics of polyurethane/organo-bentonite nanocomposites. Egypt. J. Pet. 2014, 23, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Shaaban, A.; Se, S.-M.; Ibrahim, I.M.; Ahsan, Q. Preparation of rubber wood sawdust-based activated carbon and its use as a filler of polyurethane matrix composites for microwave absorption. New Carbon Mater. 2015, 30, 167–175. [Google Scholar] [CrossRef]
- Gultom, F.; Wirjosentono, B.; Thamrin, T.; Nainggolan, H.; Eddiyanto, E. Preparation and Characterization of North Sumatera Natural Zeolite Polyurethane Nanocomposite Foams for Light-weight Engineering Materials. Procedia Chem. 2016, 19, 1007–1013. [Google Scholar] [CrossRef]
- Abdeen, Z. Enhanced Recovery of Pb2+ Ions from Aquatic Media by Using Polyurethane Composite as Adsorbent. Environ. Process. 2015, 2, 189–203. [Google Scholar] [CrossRef]
- Massalha, N.; Brenner, A.; Sheindorf, C.; Haimov, Y.; Sabbah, I. Enriching composite hydrophilic polyurethane foams with PAC to enhance adsorption of phenol from aqueous solutions. Chem. Eng. J. 2015, 280, 283–292. [Google Scholar] [CrossRef]
- Adak, B.; Joshi, M.; Butola, B.S. Polyurethane/clay nanocomposites with improved helium gas barrier and mechanical properties: Direct versus master-batch melt mixing route. J. Appl. Polym. Sci. 2018, 135, 46422. [Google Scholar] [CrossRef]
- Alaa, M.A.; Yusoh, K.; Hasany, S.F. Synthesis and characterization of polyurethane–organoclay nanocomposites based on renewable castor oil polyols. Polym. Bull. 2015, 72, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Ye, L.; Peng, J.; Song, K.; Shen, T.; Zhang, C.; He, Y. Fast Detection of Copper Content in Rice by Laser-Induced Breakdown Spectroscopy with Uni- and Multivariate Analysis. Sensors 2018, 18, 705. [Google Scholar] [CrossRef] [Green Version]
- Anah, L.; Astrini, N. Influence of pH on Cr(VI) ions removal from aqueous solutions using carboxymethyl cellulose-based hydrogel as adsorbent. J. Phys. Conf. Ser. 2016, 755, 011001. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wu, X.; Hu, Y.; Dai, C.; Peng, Q.; Liang, D. Effects of Cu(II) on the Adsorption Behaviors of Cr(III) and Cr(VI) onto Kaolin. J. Chem. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Zhang, T.; Dong, J.; Sheng, L.; Tang, H.; Yang, X.; Li, H. Quantitative determination of Cr in ink by laser-induced breakdown spectroscopy (LIBS) using ZnO as adsorbent. Chem. Res. Chin. Univ. 2015, 31, 909–913. [Google Scholar] [CrossRef]
- Marlina, M.; Iqhrammullah, M.; Darmadi, D.; Mustafa, I.; Rahmi, R. The Application of Chitosan Modified Polyurethane Foam Adsorbent. Rasayan J. Chem. 2019, 12, 494–501. [Google Scholar] [CrossRef]












| Sample | Filler | Castor Oil (g) | MDI (g) | Glycerol (g) | Deionized Water (g) |
|---|---|---|---|---|---|
| COPUF | - | 10 | 5 | 4 | 2 |
| Z/COPUF | Zeolite (0.1 g) | 10 | 5 | 4 | 2 |
| B/COPUF | Bentonite (0.1 g) | 10 | 5 | 4 | 2 |
| AC/COPUF | Activated Carbon (0.1 g) | 10 | 5 | 4 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqhrammullah, M.; Marlina; Hedwig, R.; Karnadi, I.; Kurniawan, K.H.; Olaiya, N.G.; Mohamad Haafiz, M.K.; Abdul Khalil, H.P.S.; Abdulmadjid, S.N. Filler-Modified Castor Oil-Based Polyurethane Foam for the Removal of Aqueous Heavy Metals Detected Using Laser-Induced Breakdown Spectroscopy (LIBS) Technique. Polymers 2020, 12, 903. https://doi.org/10.3390/polym12040903
Iqhrammullah M, Marlina, Hedwig R, Karnadi I, Kurniawan KH, Olaiya NG, Mohamad Haafiz MK, Abdul Khalil HPS, Abdulmadjid SN. Filler-Modified Castor Oil-Based Polyurethane Foam for the Removal of Aqueous Heavy Metals Detected Using Laser-Induced Breakdown Spectroscopy (LIBS) Technique. Polymers. 2020; 12(4):903. https://doi.org/10.3390/polym12040903
Chicago/Turabian StyleIqhrammullah, M., Marlina, R. Hedwig, I. Karnadi, K. H. Kurniawan, N. G. Olaiya, M. K. Mohamad Haafiz, H. P. S. Abdul Khalil, and S. N. Abdulmadjid. 2020. "Filler-Modified Castor Oil-Based Polyurethane Foam for the Removal of Aqueous Heavy Metals Detected Using Laser-Induced Breakdown Spectroscopy (LIBS) Technique" Polymers 12, no. 4: 903. https://doi.org/10.3390/polym12040903