Competition between Structural Relaxation and Crystallization in the Glass Transition Range of Random Copolymers †
Abstract
:1. Introduction
2. Materials and Methods
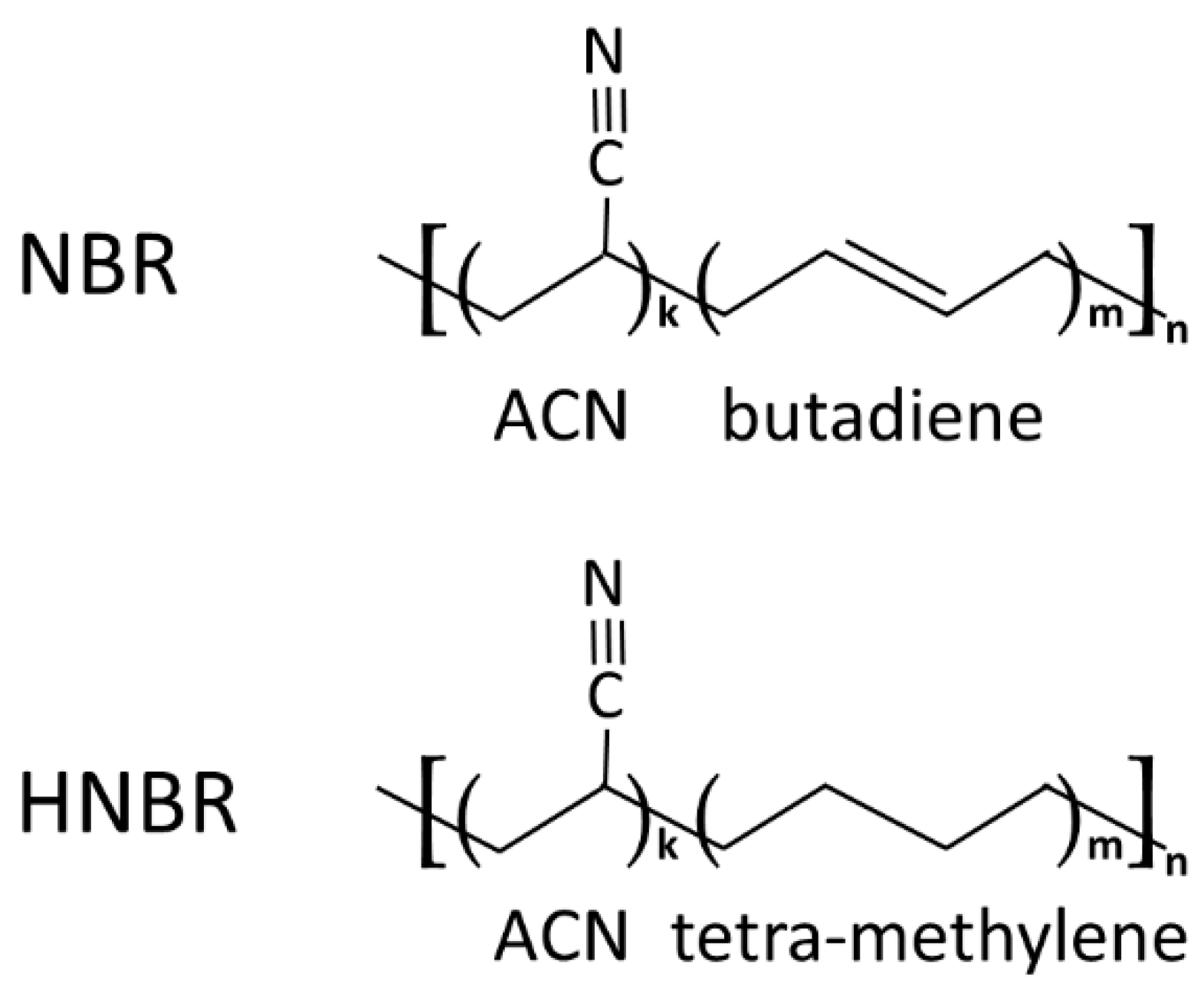
2.1. Materials
2.2. Conventional Differntial Scanning Calorimete (DSC)
2.3. Fast Differential Scanning Calorimetry (FDSC)
2.4. Sample Preparation for FDSC
3. Results and Discussion
3.1. NBR and HNBR by Conventional DSC
3.1.1. Characterization of the Materials
3.1.2. The Rigid Amorphous Fraction in HNBR
3.2. FDSC Measurements and Data Evaluation
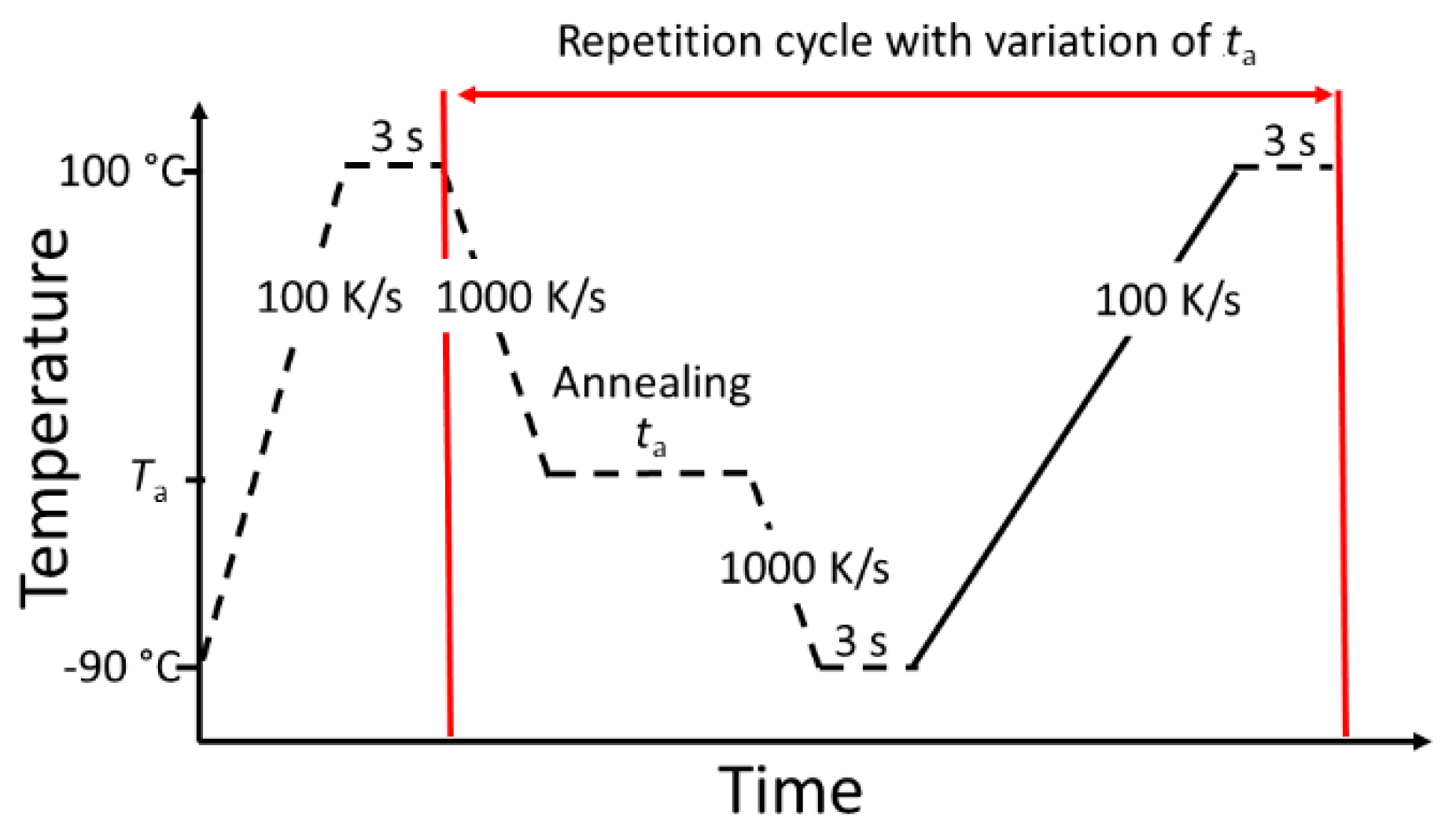
3.2.1. The Temperature Program of the FDSC Measurements
3.2.2. Enthalpy Determination
3.2.3. Kinetic Evaluation
3.2.4. The Avrami Exponent and the Model for Relaxation and Overall Crystallization
3.2.5. The Crystallization Half Time
3.2.6. Determination of RAF from FDSC Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. General Comment about the Evaluation of the Glass Transition by Conventional DSC
Appendix A.2. Determination of Δcp and Δhm for HNBR

Appendix A.3. Validation of the evaluation method using poly(ethylene terephthalate) as an example

| Crystallinity [%] | Δcp [J/(g K)] measured | Δc1/2 [mJ/K] | Δcp [J/(g K)] Calculated |
|---|---|---|---|
| 0.0 | 0.350 | 2.97 | - |
| 4.3 | 0.303 | 2.55 | 0.300 |
| 16.5 | 0.223 | 1.85 | 0.218 |
| 27.0 | 0.157 | 1.20 | 0.141 |
References
- Keller, A.; Cheng, S.Z.D. The role of metastability in polymer phase transitions. Polymer 1998, 39, 4461–4487. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Zhu, L.; Li, C.Y.; Honigfort, P.S.; Keller, A. Size effect of metastable states on semicrystalline polymer structures and morphologies. Thermochim. Acta 1999, 332, 105–113. [Google Scholar] [CrossRef]
- Toda, A.; Androsch, R.; Schick, C. Insights into polymer crystallization and melting from fast scanning chip calorimetry. Polymer 2016, 91, 239–263. [Google Scholar] [CrossRef]
- Schick, C.; Donth, E. Characteristic length of glass transition: Experimental evidence. Phys. Scr. 1991, 43, 423–429. [Google Scholar] [CrossRef]
- Men, Y.; Rieger, J.; Strobl, G. Role of the entangled amorphous network in tensile deformation of semicrystalline polymers. Phys. Rev. Lett. 2003, 095502. [Google Scholar] [CrossRef] [PubMed]
- Menczel, J.; Wunderlich, B. Heat capacity hysteresis of semicrystalline macromolecular glasses. J. Polym. Sci. Polym. Lett. 1981, 19, 261–264. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Cao, M.Y.; Wunderlich, B. Glass transition and melting behavior of poly(oxy-1,4-phenyleneoxy-1,4-phenylenecarbonyl-1,4-phenylene) (PEEK). Macromolecules 1986, 19, 1868–1876. [Google Scholar] [CrossRef]
- Schick, C.; Wurm, A.; Mohammed, A. Formation and disappearance of the rigid amorphous fraction in semicrystalline polymers revealed from frequency dependent heat capacity. Thermochim. Acta 2003, 396, 119–132. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Rigahetti, M.C.; Cocca, M.; Wunderlich, B. Coupling between crystal melting and rigid amorphous fraction mobilization in Poly(ethylene terephthalate). Macromolecules 2010, 43, 7689–7694. [Google Scholar] [CrossRef]
- Martin, J.; Stingelin, N.; Cangialosi, D. Direct calorimetric observation of the rigid amorphous fraction in a semiconducting polymer. J. Phys. Chem. Lett. 2018, 9, 990–995. [Google Scholar] [CrossRef]
- Monnier, X.; Chevalier, L.; Esposito, A.; Fernandez-Ballester, L.; Saiter, A.; Dargent, E. Local and segmental motions of the mobile amorphous fraction in semi-crystalline polylactide crystallized under quiescent and flow-induced. Polymer 2017, 126, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Cavallo, D.; Zhang, L.; Portale, G.; Alfonso, G.C.; Janani, H. Unusual crystallization behavior of isotactic polypropylene and propene/1-alkene copolymers at large undercoolings. Polymer 2014, 55, 3234–3241. [Google Scholar] [CrossRef]
- Du, Z.-X.; Yang, Y.; Xu, J.-T.; Fan, Z.-Q. Effect of molecular weight on spherulitic growth rate of poly(ε-caprolactone) and poly(ε-caprolactone)-b-poly(ethylene glycol). J. Appl. Polym. Sci. 2007, 104, 2986–2991. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Janimak, J.J.; Zhang, A.; Hsieh, E.T. Isotacticity effects on crystallization and melting in polypropylene fractions: 1. Crystalline structure and thermodynamic property changes. Polymer 1991, 32, 648–655. [Google Scholar] [CrossRef]
- Stolte, I.; Androsch, R.; Di Lorenzo, M.L. Spherulite growth rate and fold surface free energy of the form II mesophase in isotactic polybutene-1 and random butene-1/ethylene copolymers. Colloid Polym. Sci. 2014, 292, 1479–1485. [Google Scholar] [CrossRef]
- Schawe, J.E.K. Identification of three groups of polymers regarding their non-isothermal crystallization kinetics. Polymer 2019, 167, 167–175. [Google Scholar] [CrossRef]
- Corradini, P.; Guerra, G. Polymorphism in polymers. Adv. Polym. Sci. 1992, 100, 183–217. [Google Scholar]
- Androsch, R.; Di Lorenzo, M.L.; Schick, C.; Wunderlich, B. Mesophases in polyethylene, polypropylene, and poly(1-butene). Polymer 2010, 51, 4639–4662. [Google Scholar] [CrossRef] [Green Version]
- Schawe, J.E.K. Influence of processing conditions on polymer crystallization measured by fast scanning DSC. J. Thermal. Anal. Calorim. 2014, 116, 1165–1173. [Google Scholar] [CrossRef]
- Schawe, J.E.K. Analysis of non-isothermal crystallization during cooling and reorganization during heating of isotactic polypropylene by fast scanning DSC. Thermochim. Acta 2015, 603, 85–93. [Google Scholar] [CrossRef]
- Schawe, J.E.K. Cooling rate dependence of the crystallinity at nonisothermal crystallization of polymers: A phenomenological model. J. Appl. Polymer Sci. 2016, 133, 42977. [Google Scholar] [CrossRef]
- Al-Hussein, M.; Strobl, G. The melting line, the crystallization line, and the equilibrium melting temperature of isotactic polystyrene. Macromolecules 2002, 35, 1672–1676. [Google Scholar] [CrossRef]
- Strobl, G. Crystallization and melting of bulk polymers: New observations, conclusions and a thermodynamic scheme. Prog. Polym. Sci. 2006, 31, 398–442. [Google Scholar] [CrossRef]
- Luo, C.; Sommer, J.-U. Coexistence of melting and growth during heating of a semicrystalline polymer. Phys. Rev. Lett. 2009, 102, 147801. [Google Scholar] [CrossRef] [PubMed]
- Mileva, D.; Androsch, R.; Zuravlev, E.; Schick, C.; Wunderlich, B. Isotropization: Perfection and reorganization of the mesophase of isotactic polypropylene. Thermochim. Acta 2011, 522, 100–109. [Google Scholar] [CrossRef]
- Furushima, Y.; Toda, A.; Rousseaux, V.; Bailly, C.; Zhuravlev, E.; Schick, C. Quantitative understanding of two distinct melting kinetics of an isothermally crystallized poly(ether ether ketone). Polymer 2016, 99, 97–104. [Google Scholar] [CrossRef]
- Furushima, Y.; Kumazawa, S.; Umetsu, H.; Toda, A.; Zhuravlev, E.; Schick, C. Melting and recrystallization kinetics of poly(butylene terephthalate). Polymer 2017, 109, 307–314. [Google Scholar] [CrossRef]
- Minakov, A.A.; Mordvintsev, D.A.; Schick, C. Melting and reorganization of poly(ethylene terephthalate) on fast heating (1000 K/s). Polymer 2004, 45, 3755–3763. [Google Scholar] [CrossRef]
- Schawe, J.E.K. An analysis of the meta stable structure of poly(ethylene terephthalate) by conventional DSC. Thermochim. Acta 2007, 461, 145–152. [Google Scholar] [CrossRef]
- Scherer, G.W. Theories of relaxation. J. Non-Cryst. Solids 1990, 123, 75–89. [Google Scholar] [CrossRef]
- Hodge, I.M. Physical aging in polymer glasses. Science 1995, 267, 1945–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez Ribelles, J.L.; Monleón Pradas, M.; Vidaurre Garayo, A.; Romero Colomer, F.; Más Estellés, J.; Meseguer Dueñas, J.M. Structural relaxation of glass-forming polymers based on an equation for configurational entropy: 3. On the states attained at infinite time in the structural relaxation process. Results on poly(ether imide). Polymer 1997, 38, 963–969. [Google Scholar]
- Saiter, J.M.; Grenet, J.; Dargent, E.; Saiter, A.; Delbreilh, L. Glass transition temperature and value of the relaxation time at Tg in vitreous polymers. Macromol. Symp. 2007, 258, 152–161. [Google Scholar] [CrossRef]
- Yeh, G.S.Y.; Geil, P.H. Crystallization of polyethylene terephthalate from the glassy amorphous state. J. Macromol. Sci. Part. B Phys. 1967, 1, 235–249. [Google Scholar] [CrossRef]
- Nguyen, H.Y.; Ishida, H.J. Molecular analysis of the crystallization behavior of Poly(acryl-ether-ether-ketone). Polym. Sci. Part. B Polym. Phys. 1986, 24, 1079–1091. [Google Scholar] [CrossRef]
- Vittoria, V.; Petrillo, E.; Russo, R. Influence of aging on the crystallization phenomenon of isotactic polystyrene. J. Macromol. Sci. Part. B Phys. 1996, 35, 147–155. [Google Scholar] [CrossRef]
- Bove, L.; D’Aniello, C.; Gorrasi, G.; Guadagno, L.; Vittoria, V. Influence of ageing on the cold crystallization of glassy poly(ethyleneterephthalate). Polym. Bull. 1997, 38, 579–585. [Google Scholar] [CrossRef]
- Lu, X.; Hay, N.J. The effect of physical aging on the rates of cold crystallization of poly(ethylene terephthalate). Polymer 2000, 41, 7427–7436. [Google Scholar] [CrossRef]
- Tsuji, H.; Sawada, M. Accelerated crystallization of Poly(L-lactide) by physical aging. J. Appl. Polym. Sci. 2020, 116, 1190–1196. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R.; Righetti, M.C. Low-temperature crystallization of poly(butylene succinate). Eur. Polym. J. 2017, 94, 384–391. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schmelzer, J.W.P.; Wunderlich, B.; Schick, C. Kinetics of nucleation and crystallization in poly(ε-caprolactone) (PCL). Polymer 2011, 52, 1983–1997. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C.; Schmelzer, J.W.P. Sequence of enthalpy relaxation, homogeneous crystal nucleation and crystal growth in glassy polyamide 6. Europ. Polym. J. 2014, 53, 100–108. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Zhuravlev, E.; Papageorgiou, G.Z.; Bikiaris, D.; Chrissafis, K.; Schick, C. Kinetics of nucleation and crystallization in poly(butylene succinate) nanocomposites. Polymer 2014, 55, 6725–6734. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C. Interplay between the Relaxation of the Glass of Random L/D-Lactide Copolymers and Homogeneous Crystal Nucleation: Evidence for Segregation of Chain Defects. J. Phys. Chem. B 2016, 120, 4522–4528. [Google Scholar] [CrossRef]
- Quattrosoldi, S.; Androsch, R.; Janke, A.; Soccio, M.; Lotti, N. Enthalpy Relaxation, Crystal Nucleation and Crystal Growth of Biobased Poly(butylene Isophthalate). Polymers 2020, 12, 235. [Google Scholar] [CrossRef] [Green Version]
- Androsch, R.; Zhuravlev, E.; Schmelzer, J.W.P.; Schick, C. Relaxation and crystal nucleation in polymer glasses. Eur. Polym. J. 2018, 102, 195–208. [Google Scholar] [CrossRef]
- Loo, Y.-L.; Register, R.A.; Ryan, A.J.; Dee, G.T. Polymer Crystallization Confined in One, Two, or Three Dimensions. Macromolecules 2001, 34, 8968–8977. [Google Scholar] [CrossRef]
- Michell, R.M.; Blaszczyk-Lezak, I.; Mijangos, C.; Müller, A.J. Confinement effects on polymer crystallization: From droplets to alumina nanopores. Polymer 2013, 54, 4059–4077. [Google Scholar] [CrossRef]
- Zha, L.; Hu, W. Molecular simulations of confined crystallization in the microdomains of diblock copolymers. Progr. Polym. Sci. 2016, 54-55, 232–258. [Google Scholar] [CrossRef]
- Hu, W.; Mathot, V.B.F.; Alamo, R.G.; Gao, H.; Chen, X. Crystallization of statistical copolymers. Adv. Polym. Sci. 2017, 276, 1–44. [Google Scholar]
- Flory, P.J. Theory of crystallization in copolymers. Trans. Faraday Soc. 1955, 51, 848–857. [Google Scholar] [CrossRef]
- Hayashi, S.; Sakakida, H.; Oyama, M.; Nakagawa, T. Low-Temperature Properties of Hydrogenated Nitrile Rubber (HNBR). Rubber Chem. Technol. 1991, 64, 534–544. [Google Scholar] [CrossRef]
- van Herwaarden, S.; Iervolino, E.; van Herwaarden, F.; Wijffels, T.; Leenaers, A.; Mathot, V. Design, performance and analysis of thermal lag of the UFS1 twin-calorimeter chip for fast scanning calorimetry using the Mettler-Toledo Flash DSC 1. Thermochim. Acta 2011, 522, 46–52. [Google Scholar] [CrossRef]
- Schawe, J.E.K.; Pogatscher, S. Material Characterization by Fast Scanning Calorimetry: Practice and Applications. In Fast Scanning Calorimetry; Schick, C., Mathot, V.B.F., Eds.; Springer: Cham, Switzerland, 2016; pp. 3–80. [Google Scholar] [CrossRef]
- Lindemann, N.; Schawe, J.E.K.; Lacayo-Pineda, J. Kinetics of the glass transition of styrene-butadiene-rubber: Dielectric spectroscopy and fast differential scanning calorimetry. J. Appl. Polym. Sci. 2020, e49769. [Google Scholar] [CrossRef]
- Toda, A.; Konishi, M. An evaluation of thermal lags of fast-scan microchip DSC with polymer film samples. Thermochim. Acta 2014, 589, 262–269. [Google Scholar] [CrossRef]
- Schawe, J.E.K. Measurement of the thermal glass transition of polystyrene in a cooling rate range of more than six decades. Thermochim. Acta 2015, 603, 128–134. [Google Scholar] [CrossRef]
- Wrana, C.; Schawe, J.E.K. Isothermal crystallization of cis-1.4-polybutadiene at low temperatures. Thermochim. Acta 2020, 690, 178669. [Google Scholar] [CrossRef]
- Cebe, P.; Partlow, B.P.; Kaplan, D.L.; Wurm, A.; Zhuravlev, E.; Schick, C. Using flash DSC for determining the liquid state heat capacity of silk fibroin. Thermochim. Acta 2015, 615, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Quick, C.; Schawe, J.E.K.; Uggowitzer, P.J.; Pogatscher, S. Measurement of specific heat capacity via fast scanning calorimetry –– accuracy and loss corrections. Thermochim. Acta 2019, 677, 12–20. [Google Scholar] [CrossRef]
- Schawe, J.E.K.; Pogatscher, S.; Löffler, J.F. Thermodynamics of polymorphism in a bulk metallic glass: Heat capacity measurements by fast differential scanning calorimetry. Thermochim. Acta 2020, 685, 178518. [Google Scholar] [CrossRef]
- Kim, J.; Mok, M.M.; Sandoval, R.W.; Woo, D.J.; Torkelson, J.M. Uniquely broad glass temperatures of gradient copolymers relative to random and block copolymers containing repulsive comonomers. Macromolecules 2006, 39, 6152–6160. [Google Scholar] [CrossRef]
- Wong, C.L.H.; Kim, J.; Torkelson, J.M. Breadth of glass transition temperature in styrene/acrylic acid block, random, and gradient copolymers: Unusual sequence distribution effects. J. Polym. Sci. Part. B Polym. Phys. 2007, 45, 2842–2849. [Google Scholar] [CrossRef]
- Psarras, G.C.; Sofos, G.A.; Vradis, A.; Anastassopoulos, D.L.; Georga, S.N.; Krontiras, C.A.; Karger-Kocsis, J. HNBR and its MWCNT reinforced nanocomposites: Crystalline morphology and electrical response. Eur. Polym. J. 2014, 54, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Wrana, C.; Reinartz, K.; Winkelbach, H.R. Therban® - The high performance elastomer for the new millennium. Macromol. Mater. Eng. 2001, 286, 657–662. [Google Scholar] [CrossRef]
- Severe, G.; White, J.L. Physical properties and blend miscibility of hydrogenated acrylonitrile-butadiene rubber. J. Appl. Polym. Sci. 2000, 78, 1521–1529. [Google Scholar] [CrossRef]
- Bieliński, D.M.; Ślusarski, L.; Włochowicz, A.; Ślusarczyk, C. Structure and mechanical properties of nitrile rubbers modified with lodine. J. Appl. Polym. Sci. 1998, 67, 501–512. [Google Scholar] [CrossRef]
- Wunderlich, B.; Czornyj, G. A study of equilibrium melting of polyethylene. Macromolecules 1977, 10, 906–913. [Google Scholar] [CrossRef]
- Furushima, Y.; Nakada, M.; Takahashi, H.; Ishikiriyama, K. Study of melting and crystallization behavior of polyacrylonitrile using ultrafast differential scanning calorimetry. Polymer 2014, 55, 3075–3081. [Google Scholar] [CrossRef]
- Androsch, A.; Wunderlich, B. The link between rigid amorphous fraction and crystal perfection in cold-crystallized poly(ethylene terephthalate). Polymer 2005, 46, 12556–12566. [Google Scholar] [CrossRef]
- Schawe, J.E.K. Mobile amorphous, rigid amorphous and crystalline fractions in isotactic polypropylene during fast cooling. J. Thermal. Anal. Calorim. 2017, 127, 931–937. [Google Scholar] [CrossRef]
- Kohlrausch, R. Theorie des elektrischen Rückstandes in der Leidner Flasche. Ann. Der Phys. Und Chem. (Poggendorff) 1854, 91, 179–214. [Google Scholar] [CrossRef] [Green Version]
- Kohlrausch, F. Beiträge zur Kenntnis der elastischen Nachwirkung. Ann. Der Phys. Und Chem. (Poggendorff) 1866, 207, 1–20. [Google Scholar]
- Kolmogorov, A.N. On the statistical theory of the crystallization of metals (in Russian). Izv. Akad. Nauk Sssr Ser. Math. 1937, 1, 355–359. [Google Scholar]
- Johnson, W.A.; Mehl, R.F. Reaction kinetics in processes of nucleation and growth. Trans. Am. Inst. Eng. 1939, 135, 416–458. [Google Scholar]
- Avrami, M. Kinetics of phase change, I. General theory. J. Chem. Phys. 1939, 7, 1103–1109. [Google Scholar] [CrossRef]
- Debenedetti, P.G.; Stillinger, F.H. Supercooled liquids and the glass transition. Nature 2001, 410, 259–267. [Google Scholar] [CrossRef]
- Rault, J. Glass: Kohlrausch exponent, fragility, anharmonicity. Eur. Phys. J. E 2012, 35, 26. [Google Scholar] [CrossRef]
- Seo, J.; Gohn, A.M.; Dubin, O.; Takahashi, H.; Hasegawa, H.; Sato, R.; Rhoades, A.M.; Schaake, R.P.; Colby, R.H. Isothermal crystallization of poly(ether ether ketone) with different molecular weights over a wide temperature range. Polym. Cryst. 2019, 2, e10055. [Google Scholar] [CrossRef]
- Evans, U.R. The laws of expanding circles and spheres in relation to the lateral growth of surface films and the grain size of metals. Trans. Faraday Soc. 1945, 14, 365–374. [Google Scholar] [CrossRef]
- Turnbull, D. The kinetics of precipitation of barium sulfate from aqueous solution. Acta Metall. 1953, 1, 684–691. [Google Scholar] [CrossRef]
- Aaron, H.B.; Fainstein, D.; Kotler, G.R. Diffusion-limited phase transformation: A comparison and critical evaluation of the mathematical approximations. J. Appl. Phys. 1970, 41, 4404–4410. [Google Scholar] [CrossRef]
- Parker, R.L. Crystal growth mechanisms: Energetics, kinetics, and transport. Solid State Phys. 1970, 25, 152–299. [Google Scholar]
- Basamo, V.; Urdaneta, N.; Pérez, L.; Carrizales, P.; Abetz, V.; Müller, A.J. Effect of the polyethylene confinement and topology on its crystallization within simicrystalline ABC triblock copolymers. Eur. Polym. J. 2004, 40, 1033–1049. [Google Scholar] [CrossRef]
- Shiomi, T.; Tsukada, H.; Takeshita, H.; Takenaka, K.; Tezuk, Y. Crystallization of semicrystalline block copolymers containing a glassy amorphous component. Polymer 2001, 42, 4997–5004. [Google Scholar] [CrossRef]
- Xu, J.T.; Ryan, A.J.; Mai, S.M.; Yuan, J.J.; Cheng, S.Y. Diffusion control of homogeneous crystallization in nanoconfined domains of block copolymers. J. Macromol. Sci. Part. B 2004, 43, 685–694. [Google Scholar] [CrossRef]
- Cheng, S.Z.D. Kinetics of mesophase transitions in thermotropic copolyesters. 1. Calorimetric study. Macromolecules 1988, 21, 2475–2484. [Google Scholar] [CrossRef]
- Hedmark, P.G.; Werner, P.-E.; Westdahl, M.; Gedde, U.W. Cold-crystallization in liquid crystalline poly(p-hydroxybenzoic acid-co-etylene therephthalate). Polymer 1989, 30, 2068–2073. [Google Scholar] [CrossRef]
- Schlosser, E.; Schönhals, A. Dielectric relaxation during physical ageing. Polymer 1991, 32, 2135–2140. [Google Scholar] [CrossRef]
- Di Laurenzo, M.L.; Gazzano, M.; Righetti, M.C. The role of the rigid amorphous fraction on cold crystallization of poly(3-hydroxybutyrate). Macromolecules 2012, 45, 5684–5691. [Google Scholar] [CrossRef]
- Schawe, J.E.K. Remarks regarding the determination of the initial crystallinity by temperature modulated DSC. Thermochim. Acta 2017, 657, 151–155. [Google Scholar] [CrossRef]



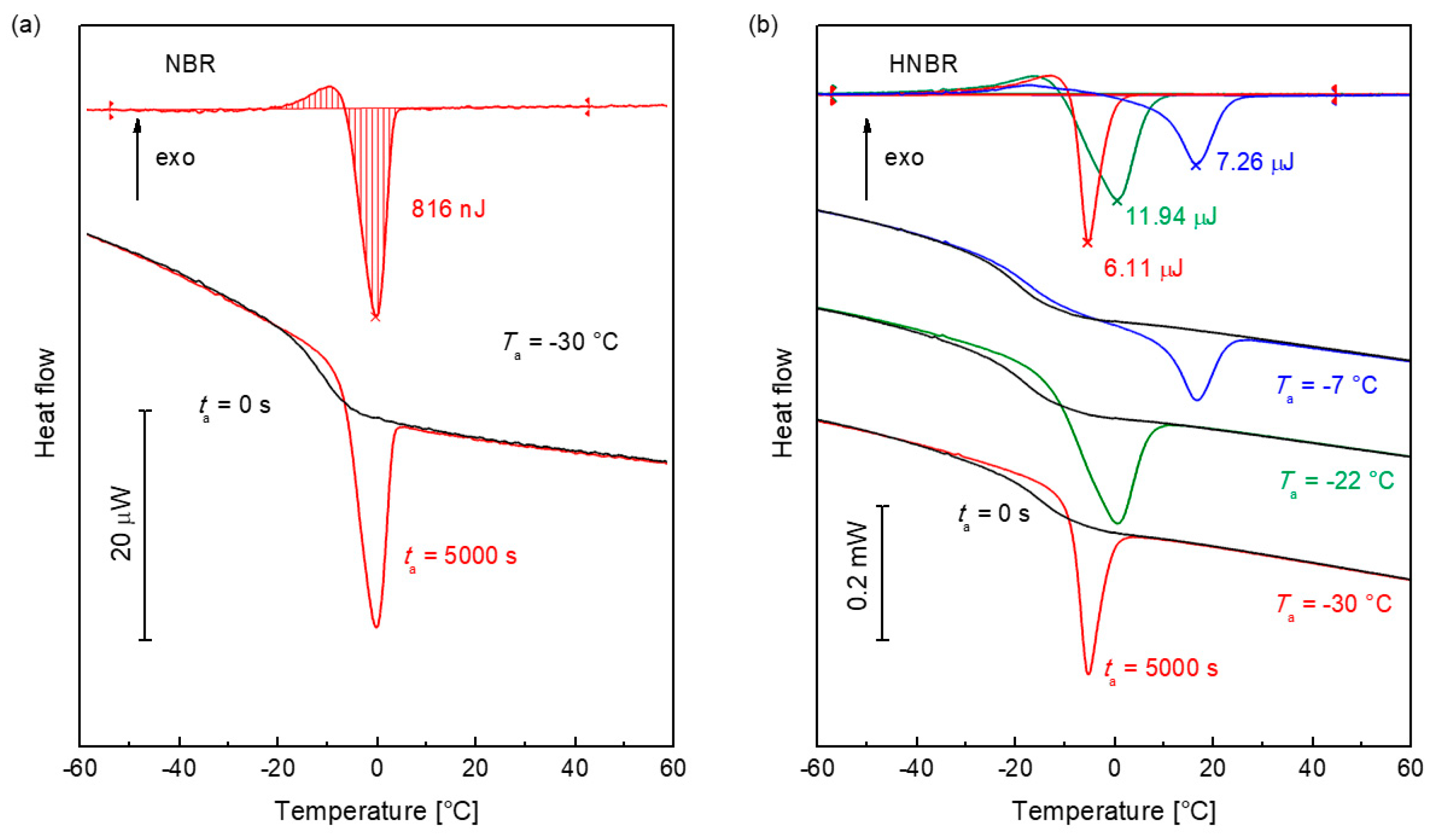



| Ta (°C) | ΔHmax (μJ) | ΔHa (5000 s) (μJ) |
|---|---|---|
| −30 | 6.66 | 6.11 |
| −22 | 0.95 | 11.94 |
| −7 | 0.00 | 7.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schawe, J.E.K.; Wrana, C. Competition between Structural Relaxation and Crystallization in the Glass Transition Range of Random Copolymers. Polymers 2020, 12, 1778. https://doi.org/10.3390/polym12081778
Schawe JEK, Wrana C. Competition between Structural Relaxation and Crystallization in the Glass Transition Range of Random Copolymers. Polymers. 2020; 12(8):1778. https://doi.org/10.3390/polym12081778
Chicago/Turabian StyleSchawe, Jürgen E. K., and Claus Wrana. 2020. "Competition between Structural Relaxation and Crystallization in the Glass Transition Range of Random Copolymers" Polymers 12, no. 8: 1778. https://doi.org/10.3390/polym12081778






