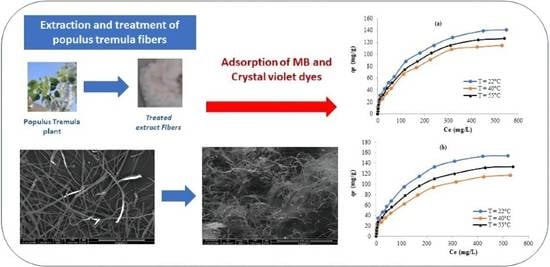
Extraction of Cellulose Polymeric Material from Populus tremula Fibers: Characterization and Application to the Adsorption of Methylene Blue and Crystal Violet
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Reagents
2.2. Extraction of Cellulose
2.3. Characterization Instruments
2.4. Adsorption Experiments
3. Results and Discussion
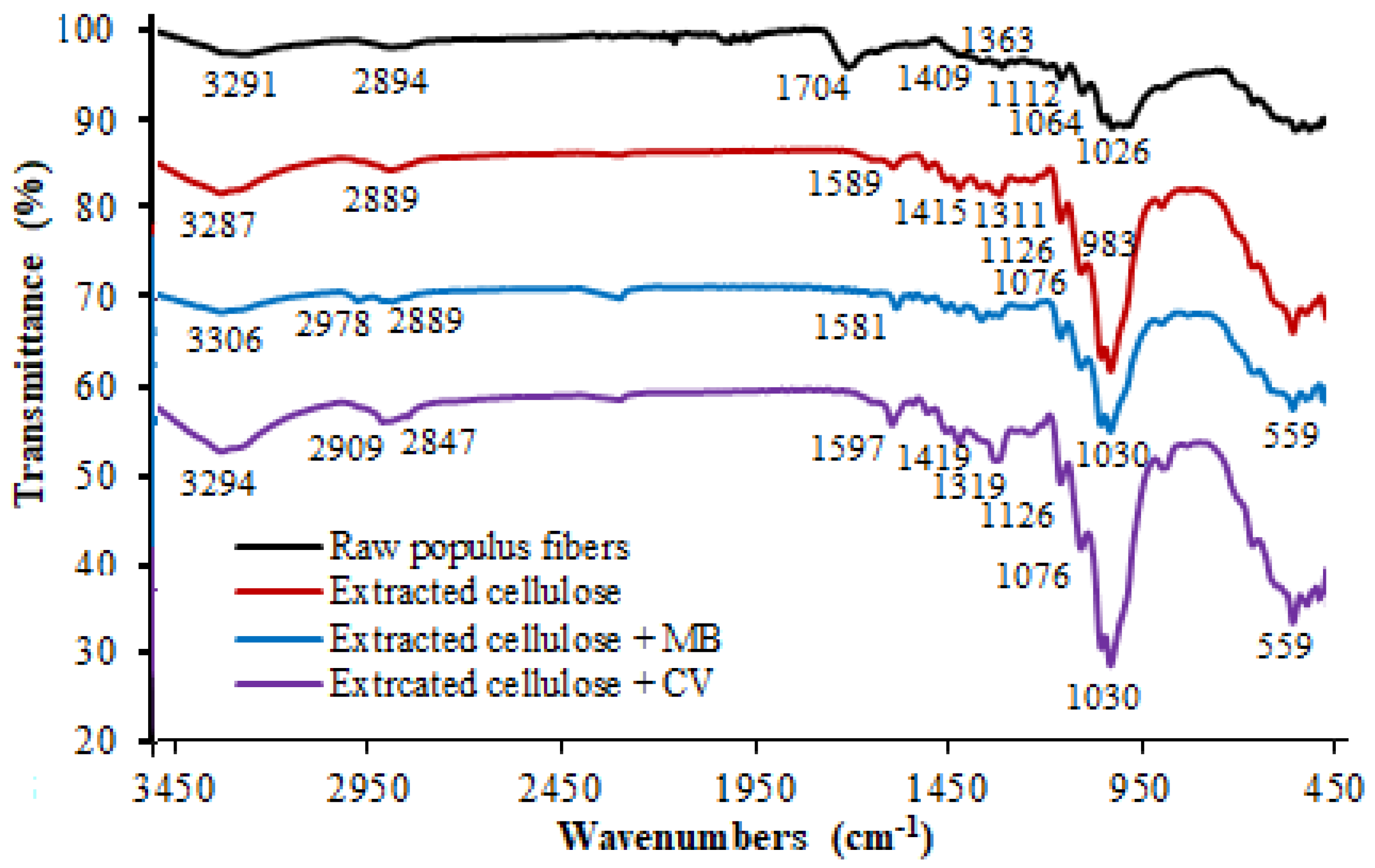
3.1. FT-IR Spectroscopy Characterization
3.2. SEM Images
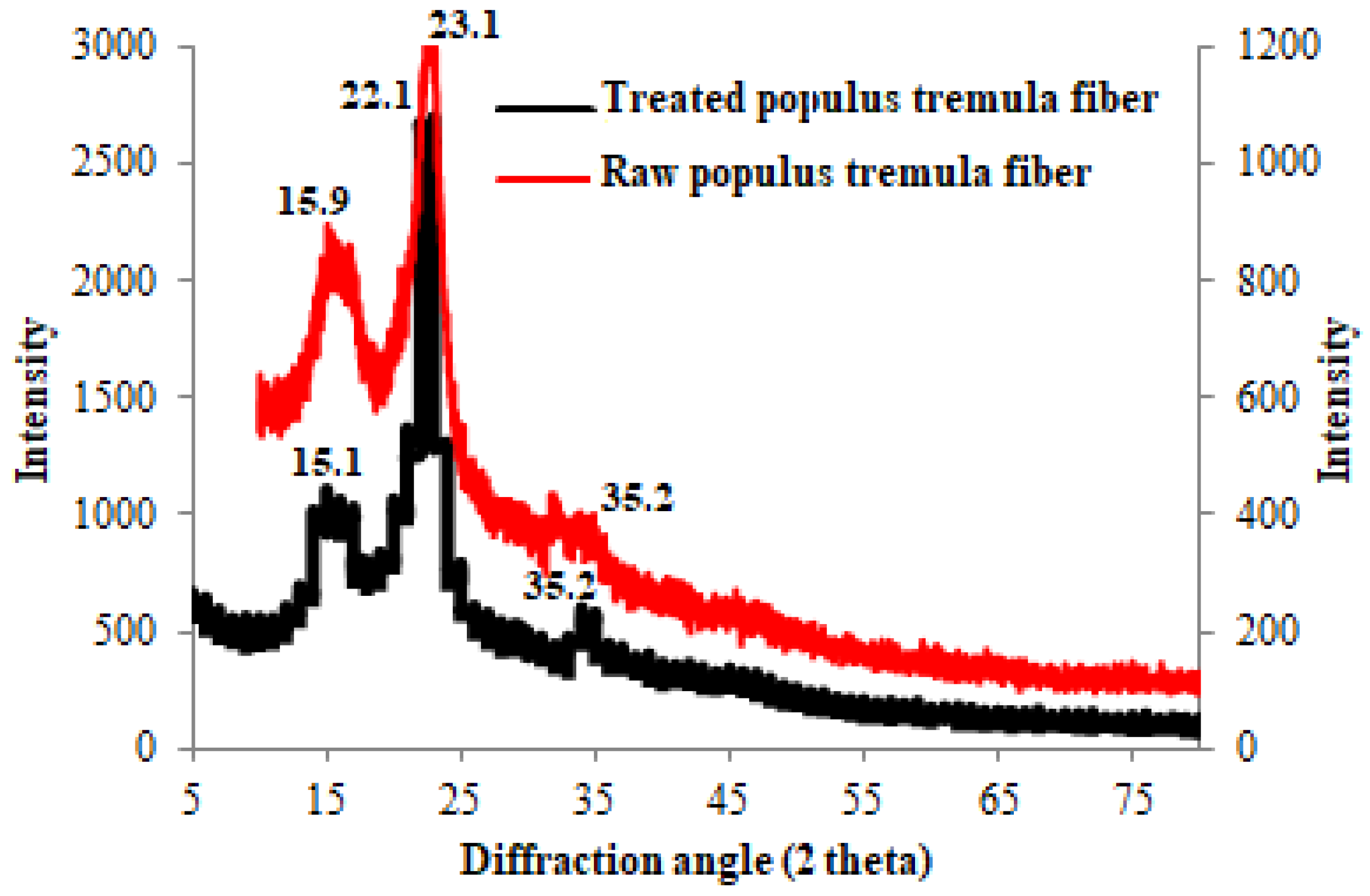
3.3. XRD Patterns
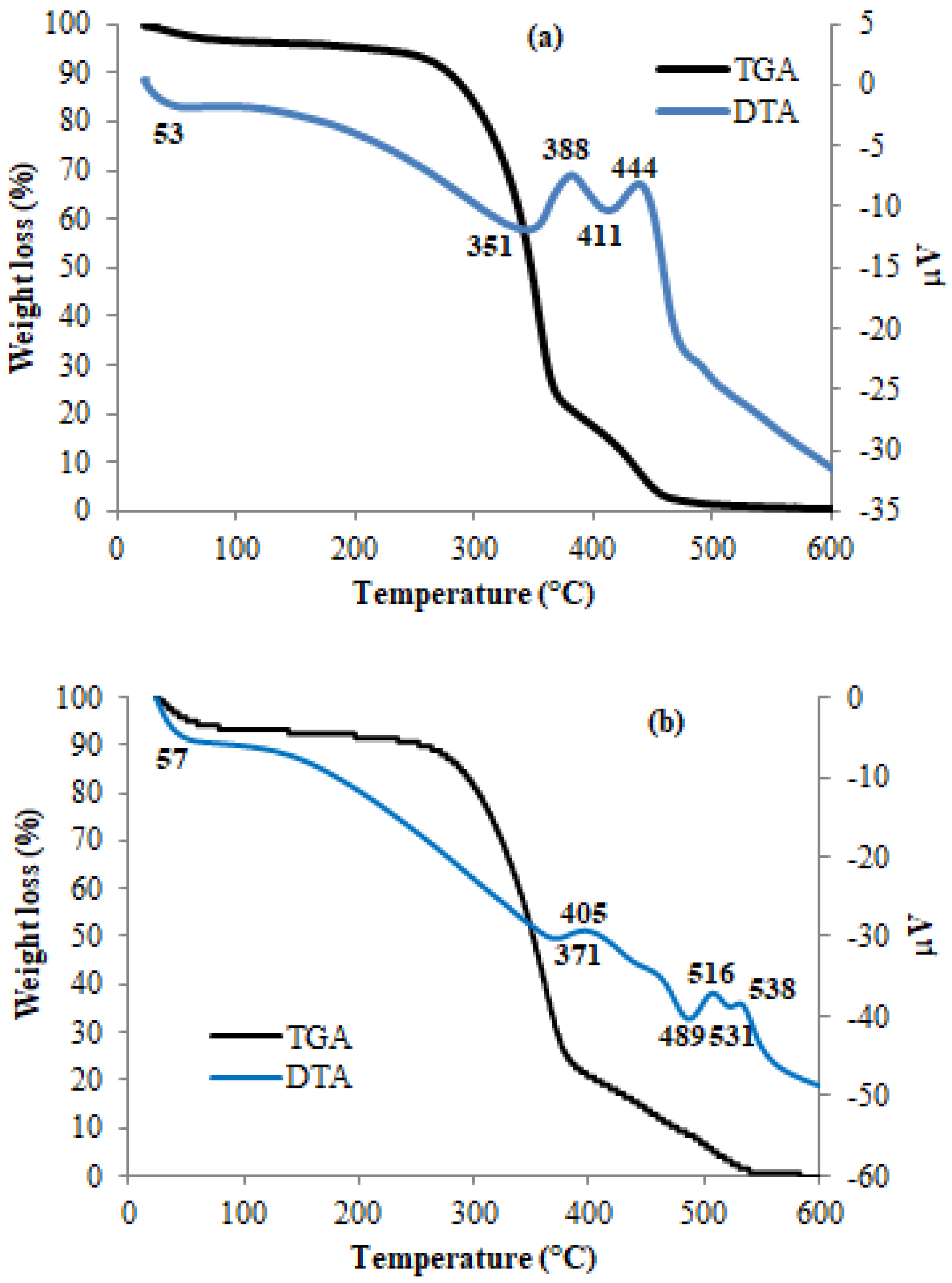
3.4. Thermal Analyses: TGA/DTA
3.5. Application to the Adsorption of Methylene Blue and Crystal Violet
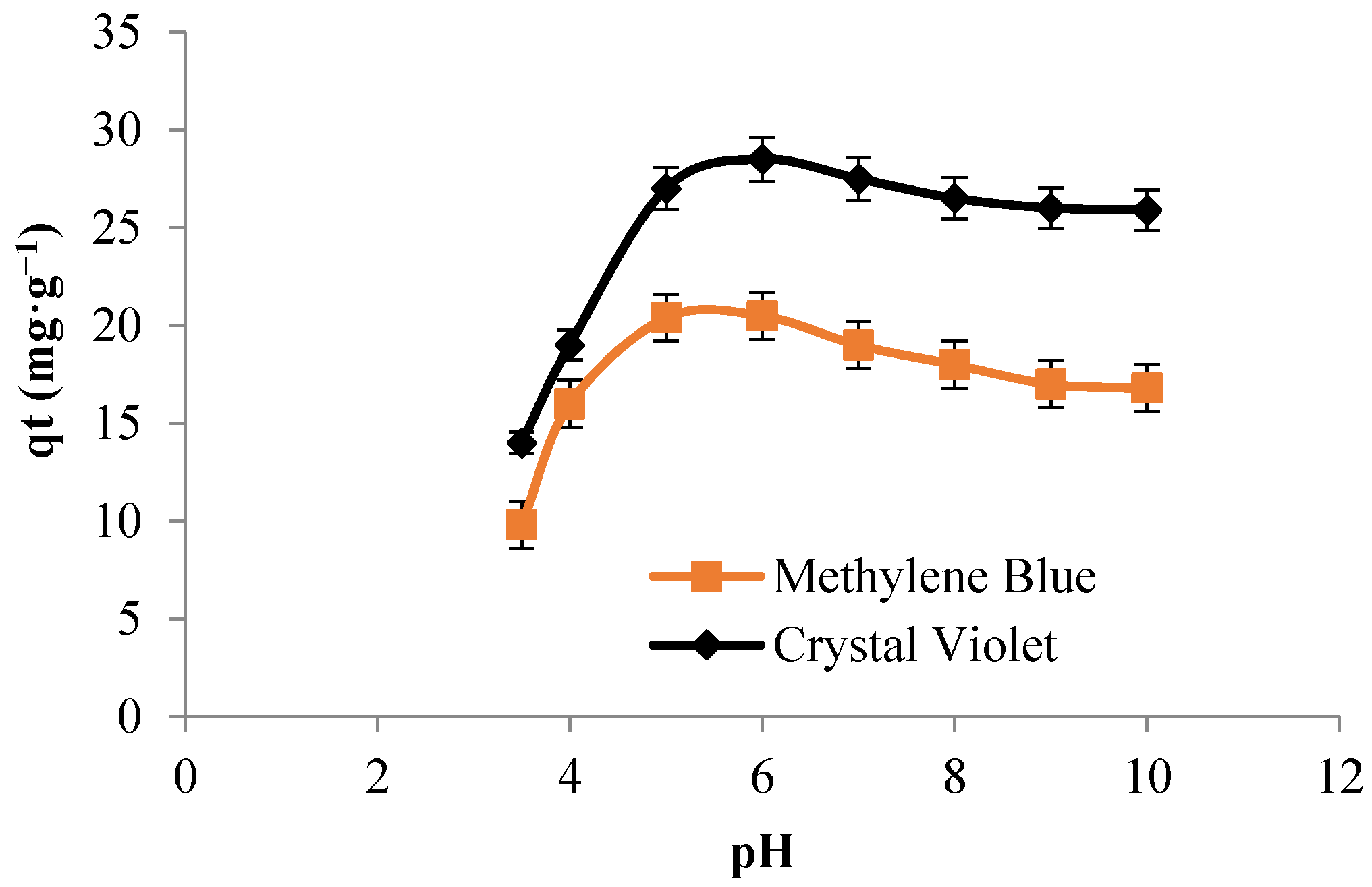
3.5.1. Effect of Some Experimental Parameters on Adsorption
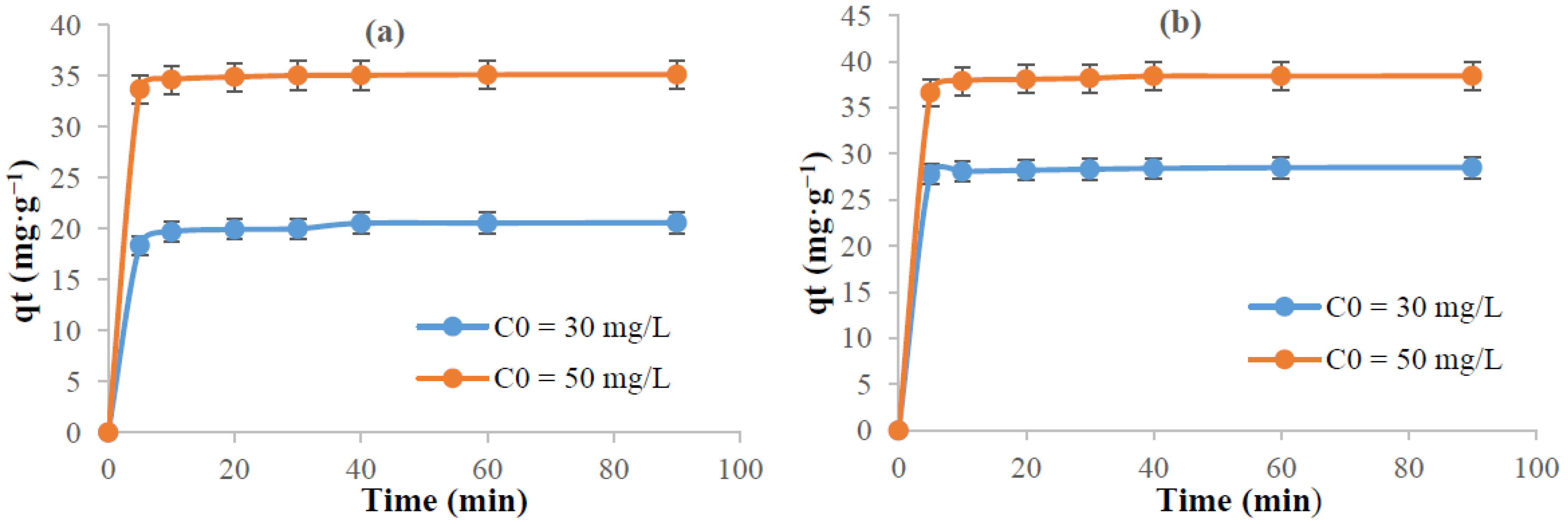
3.5.2. Kinetic Study
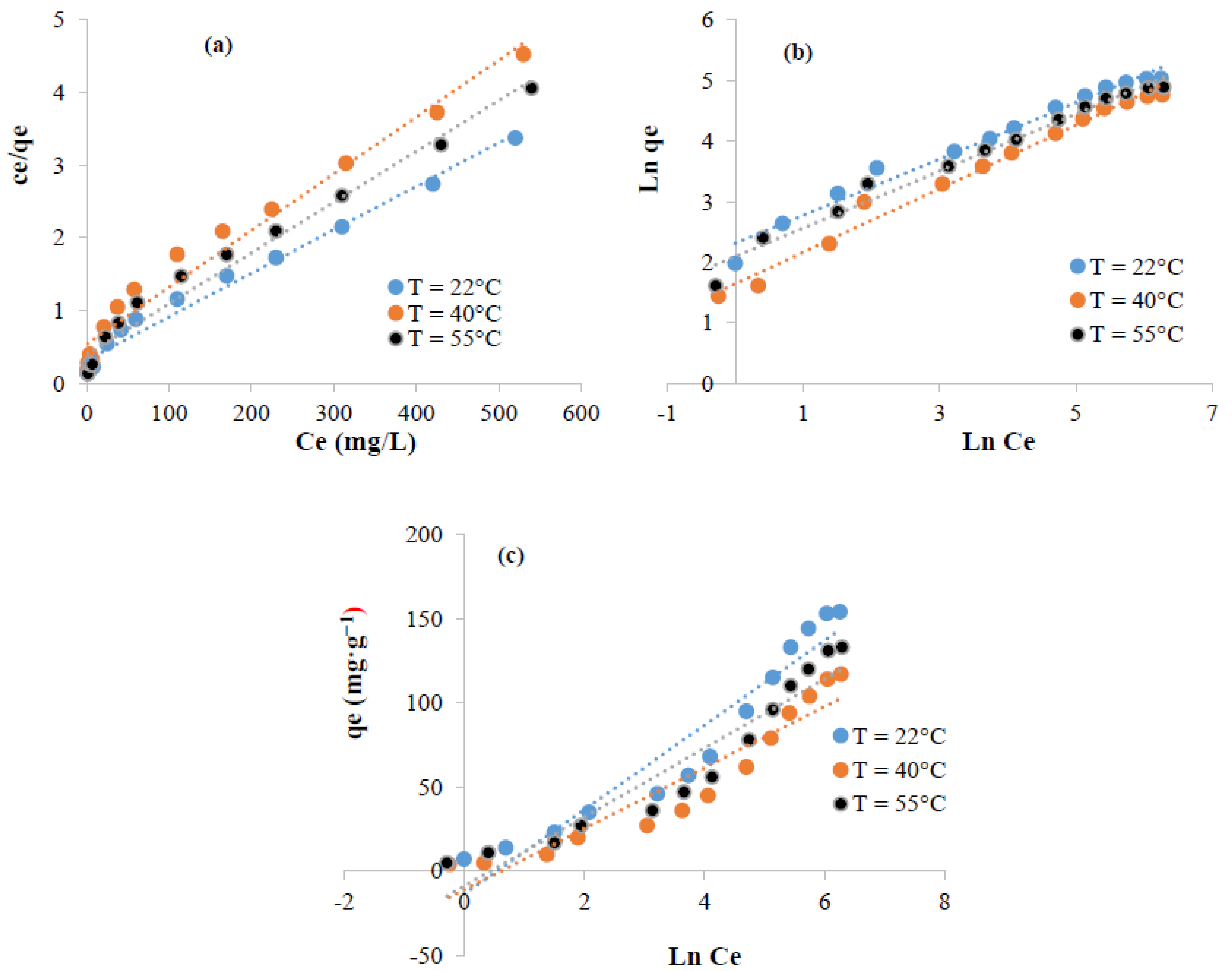
3.5.3. Isotherms Study and Thermodynamic Parameters Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cherubini, F.; Guest, G.; Strømman, A.H. Application of probability distributions to the modeling of biogenic CO2 fluxes in life cycle assessment. GCB Bioenergy 2012, 4, 784–798. [Google Scholar] [CrossRef] [Green Version]
- Ammar, A.; El-Ghoul, Y.; Jabli, M. Characterization and valuable use of Calotropis gigantea seedpods as a biosorbent of methylene blue. Int. J. Phytoremediat. 2021, 23, 1085–1094. [Google Scholar] [CrossRef]
- Dibyajyoti, H.; Kumar, P.M. Micro and nanocrystalline cellulose derivatives of lignocellulosic biomass: A review on synthesis, applications and advancements. Carbohydr. Polym. 2020, 250, 116937. [Google Scholar]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial Application of Cellulose Nano-composites–A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Balakrishnan, P.; Chandradhara, D.; Poovathankandy, D.; Thomas, S. General scenarios of cellulose and its use in the biomedical field. Mater. Today Chem. 2019, 13, 59–78. [Google Scholar] [CrossRef]
- Dutta, S.; Kim, J.; Ide, Y.; Kim, J.H.; Hossain, M.S.; Bando, Y.; Yamauchi, Y.; Wu, K.C. 3D network of cellulose-based energy storage devices and related emerging applications. Mater. Horiz. 2017, 4, 522–545. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Wang, Y.; Wu, M.; Zhang, L.; Wang, L.; Ni, H. Surface functionalization of cellulose with hyperbranched polyamide for efficient adsorption of organic dyes and heavy metals. J. Clean. Prod. 2019, 232, 774–783. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Biofibers from agricultural byproducts for industrial applications. Trends Biotechnol. 2005, 23, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Saravanakumar, S.S.; Kumaravel, A.; Nagarajan, T.; Sudhakar, P.; Baskaran, R. Characterization of a novel natural cellulosic fiber from Prosopis juliflora bark. Carbohydr. Polym. 2013, 92, 1928–1933. [Google Scholar] [CrossRef]
- Binoj, J.S.; Edwin, R.R.; Sreenivasan, V.S.; Thusnavis, G.R. Morphological, Physical, Mechanical, Chemical and Thermal Characterization of Sustainable Indian Areca Fruit Husk Fibers (Areca Catechu L.) as Potential Alternate for Hazardous Synthetic Fibers. J. Bionic Eng. 2016, 13, 156–165. [Google Scholar] [CrossRef]
- Balaji, A.N.; Nagarajan, K.J. Characterization of alkali treated and untreated new cellulosic fiber from Saharan aloe vera cactus leaves. Carbohydr. Polym. 2017, 174, 200–208. [Google Scholar]
- Khan, A.; Vijay, R.; Singaravelu, D.L.; Sanjay, M.R.; Siengchin, S.; Jawaid, M.; Alamry, K.A.; Asiri, A.M. Extraction and Characterization of Natural Fibers from Citrullus lanatus Climber. J. Nat. Fibers 2020, 1–9. [Google Scholar] [CrossRef]
- Saravanan, N.; Ganeshan, P.; Prabu, B.; Yamunadevi, V.; NagarajaGanesh, B.; Raja, K. Physical, Chemical, Thermal and Surface Characterization of Cellulose Fibers Derived from Vachellia Nilotica Ssp. Indica Tree Barks. J. Nat. Fibers 2021, 1–13. [Google Scholar] [CrossRef]
- Khan, A.; Vijay, R.; Singaravelu, D.L.; Sanjay, M.R.; Siengchin, S.; Verpoort, F.; Alamry, K.A.; Asiri, A.M. Characterization of natural fibers from Cortaderia selloana grass (pampas) as reinforcement material for the production of the composites. J. Nat. Fibers 2020, 1–9. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr. Polym. 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- NagarajaGanesh, B.; Ganeshan, P.; Ramshankar, P.; Raja, K. Assessment of natural cellulosic fibers derived from Senna auriculata for making light weight industrial biocomposites. Ind. Crop. Prod. 2019, 139, 111546. [Google Scholar] [CrossRef]
- EL-Ghoul, Y.; Ammar, C.; Alminderej, F.M.; Shafiquzzaman, M. Design and Evaluation of a New Natural Multi-Layered Biopolymeric Adsorbent System-Based Chitosan/Cellulosic Nonwoven Material for the Biosorption of Industrial Textile Effluents. Polymers 2021, 13, 322. [Google Scholar] [CrossRef]
- Luo, J.; Yu, D.; Hristovski, K.D.; Westerhoff, P.; Crittenden, J.C. Review of Advances in Engineering Nanomaterial Adsorbents for Metal Removal and Recovery from Water: Synthesis and Microstructure Impacts. ACS EST Engg. 2021, 1, 623–661. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.-H.; Ibrahim, I.; Arun, D.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid-scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Sebeia, N.; Jabli, M.; Ghith, A.; Elghoul, Y.; Alminderej, F.M. Production of cellulose from Aegagropila Linnaei macro-algae: Chemical modification, characterization and application for the bio-sorption of cationic and anionic dyes from water. Int. J. Biol. Macromol. 2019, 135, 152–162. [Google Scholar] [CrossRef]
- Park, S.H.; Alammar, A.; Fulop, F.; Pulido, B.A.; Nunes, S.P.; Szekely, G. Hydrophobic thin film composite nanofiltration membranes derived solely from sustainable sources. Green Chem. 2021, 23, 1175–1184. [Google Scholar] [CrossRef]
- Jabli, M.; Ka, N.; Khiari, R.; Saleh, T.A. Physicochemical characteristics and dyeing properties of lignin-cellulosic fibers derived from Nerium oleander. J. Mol. Liq. 2018, 249, 1138–1144. [Google Scholar] [CrossRef]
- Tka, N.; Jabli, M.; Saleh, T.A.; Ghazwan, A.S. Amines modified fibers obtained from natural Populus tremula and their rapid biosorpt on of Acid Blue 25. J. Mol. Liq. 2018, 250, 423–432. [Google Scholar] [CrossRef]
- Sebeia, N.; Jabli, M.; Ghith, A.; Elghoul, Y.; Alminderej, F.M. Populus tremula, Nerium oleander and Pergularia tomentosa seed fibers as sources of cellulose and lignin for the bio-sorption of methylene blue. Int. J. Biol. Macromol. 2019, 121, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Worrell, R. European aspen (Populus tremula L.): A review with particular reference to Scotland I. Distribution, ecology and genetic variation. Forestry 1995, 68, 93–105. [Google Scholar] [CrossRef]
- Sezgin, K.G.; Saduman, T. Effect of chip mixing ratio of pinus pinaster and Populus tremula on kraft pulp and paper properties. Ind. Eng. Chem. Res. 2013, 52, 2304–2308. [Google Scholar]
- Niemczyk, M.; Przybysz, P.; Przybysz, K.; Karwański, M.; Kaliszewski, A.; Wojda, T.; Liesebach, M. Productivity, Growth Patterns, and Cellulosic Pulp Properties of Hybrid Aspen Clones. Forests 2019, 10, 450. [Google Scholar] [CrossRef] [Green Version]
- Rooni, V.; Sjulander, N.; Cristobal-Sarramian, A.; Raud, M.; Rocha-Meneses, L.; Kika, T. The efficiency of nitrogen explosion pretreatment on common aspen–Populus tremula: N2– VS steam explosion. Energy 2021, 220, 119741. [Google Scholar] [CrossRef]
- Gao, A.; Chen, H.; Tang, J.; Xie, K.; Hou, A. Efficient extraction of cellulose nanocrystals from waste Calotropis gigantean fiber by SO42−/TiO2 nano-solid superacid catalyst combined with ball milling Exfoliation. Ind. Crop. Prod. 2020, 152, 112524. [Google Scholar] [CrossRef]
- Saleh, T.A.; Ali, I. Synthesis of polyamide grafted carbon microspheres for removal of rhodamine B dye and heavy metals. J. Environ. Chem. Eng. 2018, 6, 5361–5368. [Google Scholar] [CrossRef]
- Saleh, T.A.; Al-Saadi, A.A. Surface characterization and sorption efficacy of tireobtained carbon: Experimental and semiempirical study of rhodamine B adsorption. Surf. Interface Anal. 2015, 47, 785–792. [Google Scholar] [CrossRef]
- Cecone, C.; Hoti, G.; Krabicová, I.; Appleton, S.L.; Caldera, F.; Bracco, P.; Zanetti, M.; Trotta, F. Sustainable synthesis of cyclodextrin-based polymers exploiting natural deep eutectic solvents. Green Chem. 2020, 22, 5806–5814. [Google Scholar] [CrossRef]
- Topuz, F.; Holtzl, T.; Szekely, G. Scavenging organic micropollutants from water with nanofibrous hypercrosslinked cyclodextrin membranes derived from green resources. Chem. Eng. J. 2021, 419, 129443. [Google Scholar] [CrossRef]
- Abhilash, V.; Jun, Y.C.; Sung-Ho, S.; Akshaykumar, K.P.; Tae, G.Y.; Jaehyeong, B.; Stanisław, W.; Miroslav, Č.; Seema, A.; Andreas, G.; et al. Recycling non-food-grade tree gum wastes into nanoporous carbon for sustainable energy harvesting. Green Chem. 2020, 22, 1198–1208. [Google Scholar]
- Yulin, Z.; Guozhao, J.; Changjing, L.; Xuexue, W.; Aimin, L. Templating synthesis of hierarchical porous carbon from heavy residue of tire pyrolysis oil for methylene blue removal. Chem. Eng. J. 2020, 390, 124398. [Google Scholar]
- Tanobe, V.O.A.; Sydenstricker, T.H.D.; Munaro, M.; Amico, S.C. A comprehensive characterization of chemically treated Brazilian sponge-gourds (Luffa cylindrica). Polym. Test. 2005, 24, 474–482. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.Y. Optimum plasma surface treatment of luffa fibers. J. Macromol. Sci. Part B Phys. 2012, 51, 662–670. [Google Scholar] [CrossRef]
- Morản, J.I.; Alvarez, V.A.; Cyras, V.P.; Vảzquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149. [Google Scholar] [CrossRef]
- Maaloul, N.; Ben Arfi, R.; Rendueles, M.; Ghorbal, A.M.; Diaz, M. Dialysis-free extraction and characterization of cellulose crystals from almond (Prunus dulcis) shells. J. Mater. Environ. Sci. 2017, 8, 4171–4181. [Google Scholar]
- Zhang, X.; Xiao, N.; Wang, H.; Liu, C.; Pan, X. Preparation and Characterization of Regenerated Cellulose Film from a Solution in Lithium Bromide Molten Salt Hydrate. Polymers 2018, 10, 614. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.O.; Zhang, J.; Zhang, J.; Rajulu, A.V. Preparation and properties of self-reinforced cellulose composite films from Agave microfibrils using an ionic liquid. Carbohydr. Polym. 2014, 114, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Millogo, Y.; Aubert, J.-E.; Hamard, E.; Morel, J.C. How Properties of Kenaf Fibers from Burkina Faso Contribute to the Reinforcement of Earth Blocks. Materials 2015, 8, 2332–2345. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mishra, M.; Oksman, K. Chemical composition, crystallinity and thermal degradation of bleached and unbleached kenaf bast (Hibiscus cannabinus) pulp and nanofiber. BioResources 2009, 4, 626–639. [Google Scholar]
- Ramesh, G.K.; Subramanian, S.; Sathiyamurthy, S.; Prakash, M. Calotropis Gigantea fiber-epoxy composites: Influence of fiber orientation on mechanical properties and thermal behavior. J. Nat. Fibers 2020, 1–13. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.-S.; Lee, D.J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Banerjee, S.; Dastidar, M.G. Use of jute processing wastes for treatment of wastewater contaminated with dye and other organics. Bioresour. Technol. 2005, 96, 1919–1928. [Google Scholar] [CrossRef]
- Belala, Z.; Jeguirim, M.; Belhachemi, M.; Addoun, F.; Trouve, G. Biosorption of basic dye from aqueous solutions by date palm trees: Kinetic, equilibrium and thermodynamic studies. Desalination 2011, 271, 80–87. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef]
- Sajab, M.S.; Chia, C.H.; Zakaria, S.; Jani, S.M.; Ayob, M.K.; Chee, K.L.; Khiew, P.S.; Chiu, W.S. Citric acid modified kenaf core fibres for removal of methylene blue from aqueous solution. Bioresour. Technol. 2011, 102, 7237–7243. [Google Scholar] [CrossRef]
- Kumar, K.V.; Kumaran, A. Removal of methylene blue by mango seed kernel powder. Biochem. Eng. J. 2005, 27, 83–93. [Google Scholar] [CrossRef]
- Nasuha, N.; Hameed, B.H.; Din, A.T. Rejected tea as a potential low-cost adsorbent for the removal of methylene blue. J. Hazard. Mater. 2010, 175, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Gucek, A.; Sener, S.; Bilgen, S.; Mazmanci, A. Adsorption and kinetic studies of cationic and anionic dyes on pyrophyllite from aqueous solutions. J. Coll. Interf. Sci. 2005, 286, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass-Transfer Operations, 3rd ed.; McGraw-Hill: New York, NY, USA, 1981. [Google Scholar]
- Ammar, C.; Alminderej, F.M.; EL-Ghoul, Y.; Jabli, M.; Shafiquzzaman, M. Preparation and characterization of a New polymeric multi-layered material-based K-carrageenan and alginate for efficient bio-sorption of methylene blue dye. Polymers 2021, 13, 411. [Google Scholar] [CrossRef]
- Omer, O.S.; Hussein, M.A.; Hussein, B.H.M.; Mgaidi, A. Adsorption thermodynamics of cationic dyes (methylene blue and crystal violet) to a natural clay mineral from aqueous solution between 293.15 and 323.15 K. Arab. J. Chem. 2018, 11, 615–623. [Google Scholar] [CrossRef]



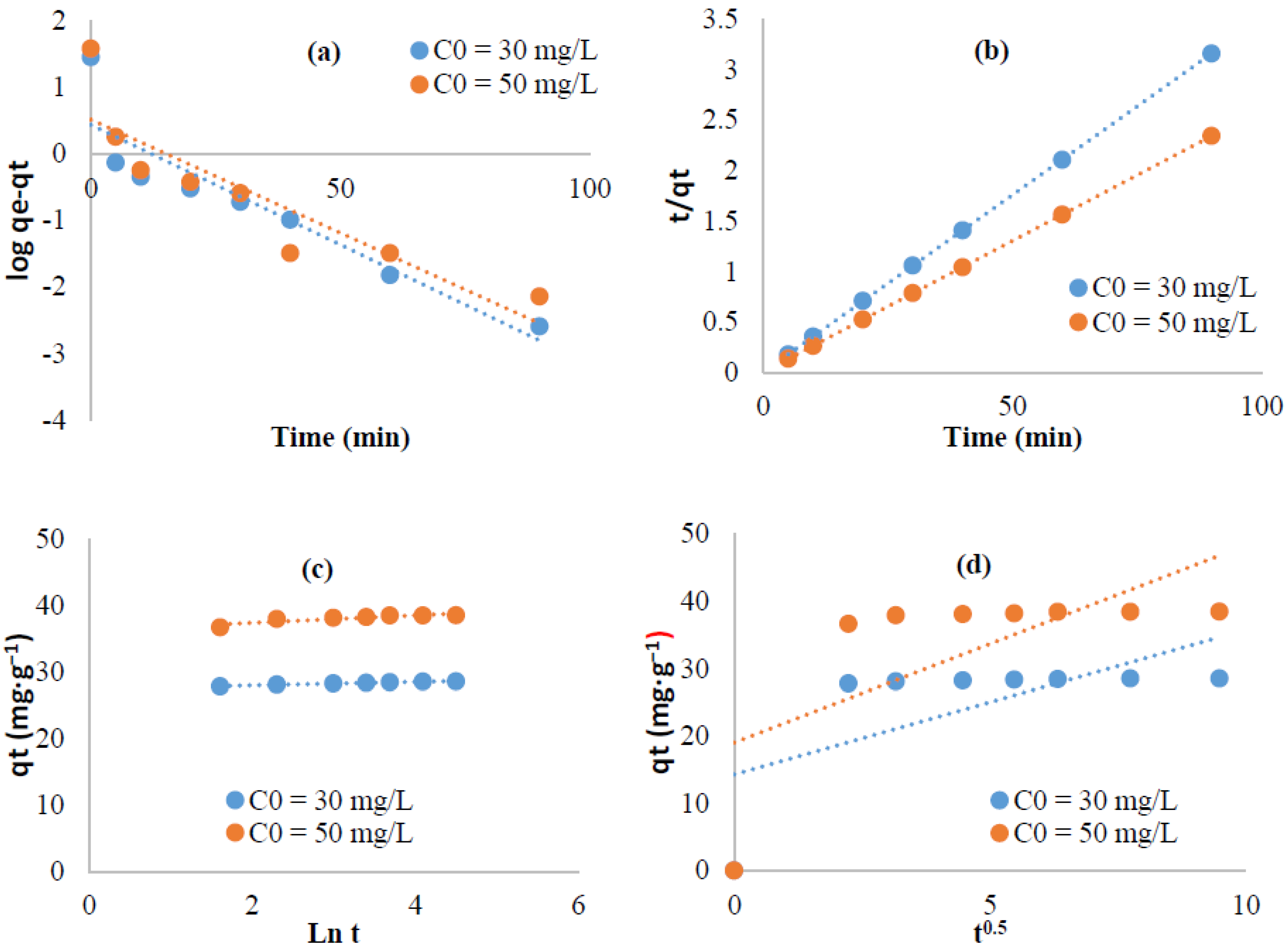
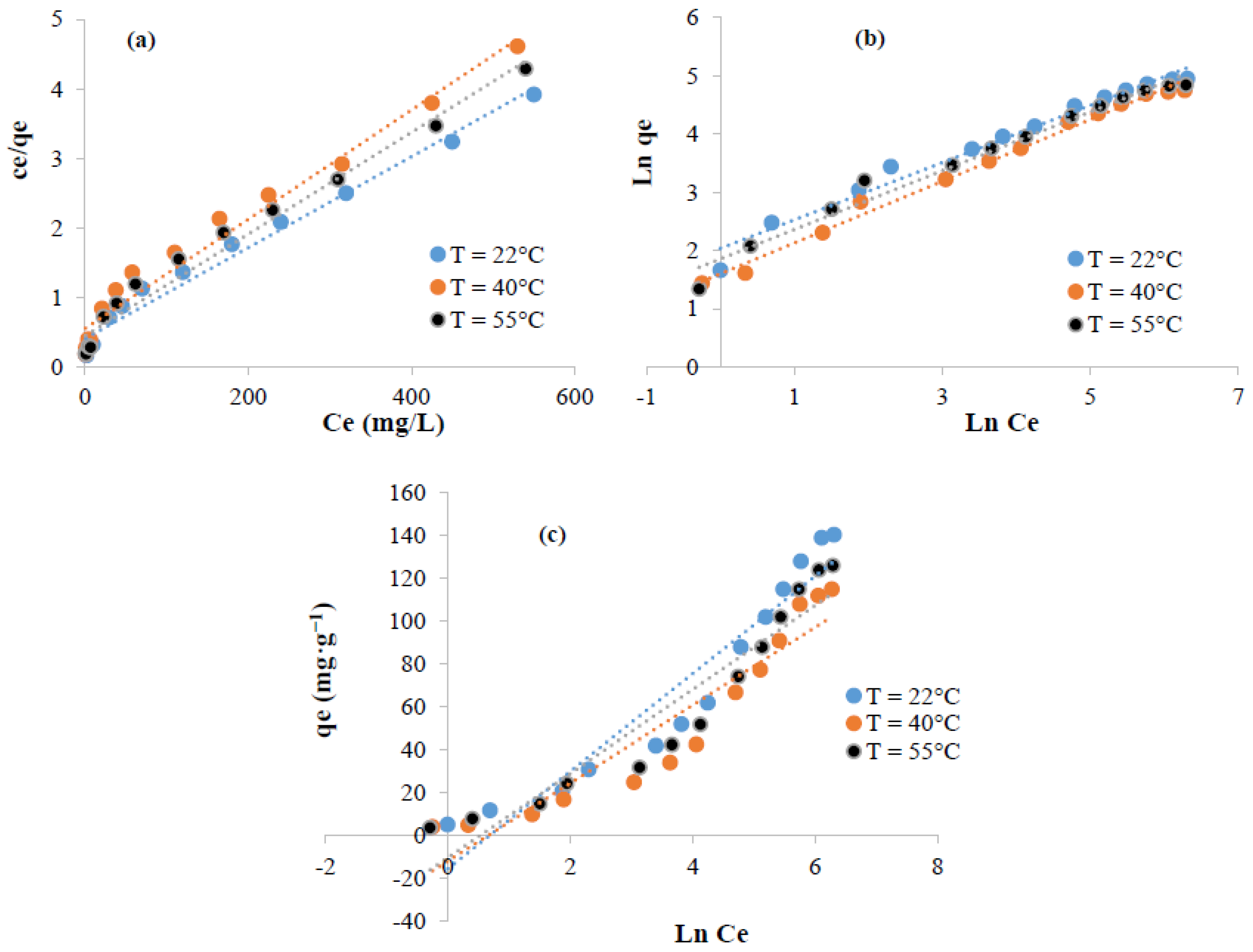

| Kinetic equation | Constants | Dye concentration | Isotherms | Parameters | Temperature | |||
| Pseudo first order | 30 mg L−1 | 50 mg L−1 | 22 | 40 | 55 | |||
| K1 (min−1) | 0.0328 | 0.0339 | qm (mg·g−1) | 153.84 | 126.58 | 136.98 | ||
| q (mg·g−1) | 4.22 | 2.76 | Langmuir | KL (L·g−1) | 0.016 | 0.014 | 0.016 | |
| R2 | 0.88 | 0.8 | R2 | 0.98 | 0.97 | 0.97 | ||
| Pseudo second order | K2 | 0.068 | 0.155 | Thermodynamic parameters | ΔH° (KJ mol−1) | −7.20 | ||
| q | 20.75 | 35.21 | ΔS° (J mol−1) | −33.049 | ||||
| R2 | 0.99 | 1 | ΔG° (KJ mol−1) | 2.55 | 3.14 | 3.64 | ||
| Freundlich | KF (L·g−1) | 108.26 | 39.67 | 71.53 | ||||
| Elovich | α (mg·g−1·min−1) | 2.68 × 1010 | 2.62 × 1031 | n | 2.036 | 1.88 | 1.98 | |
| β (mg·g−1·min−1) | 1.38 | 2.19 | R2 | 0.98 | 0.99 | 0.98 | ||
| R2 | 0.86 | 0.79 | Temkin | bT (J.mol−1) | 107.86 | 143.23 | 139.13 | |
| Intra-particular- Diffusion | K (mg·g1·min1/2) | 1.63 | 2.66 | A (L·g−1) | 1.96 | 1.91 | 1.68 | |
| R2 | 0.5 | 0.44 | R2 | 0.92 | 0.89 | 0.91 | ||
| Kinetic equation | Constants | Dye concentration | Isotherms | Parameters | Temperature | |||
| Pseudo first order | 30 mg L−1 | 50 mg L−1 | 22 | 40 | 55 | |||
| K1 (min−1) | 0.0361 | 0.0341 | qm (mg·g−1) | 166.66 | 128.20 | 142.85 | ||
| q (mg·g−1) | 2.75 | 3.29 | Langmuir | KL (L·g−1) | 0.019 | 0.014 | 0.017 | |
| R2 | 0.85 | 0.79 | R2 | 0.98 | 0.97 | 0.97 | ||
| Pseudo second order | K2 | 0.036 | 0.129 | Thermodynamic parameters | ΔH° (KJ mol−1) | −3.95 | ||
| q | 28.57 | 38.61 | ΔS° (J mol−1) | −23.23 | ||||
| R2 | 1 | 1 | ΔG° (KJ mol−1) | 2.90 | 3.32 | 3.66 | ||
| Freundlich | KF (L·g−1) | 203.89 | 43.79 | 123.96 | ||||
| Elovich | α (mg·g−1·min−1) | 4.75 × 1045 | 5.67 × 1027 | n | 2.15 | 1.90 | 2.12 | |
| β (mg·g−1·min−1) | 3.88 | 1.78 | R2 | 0.98 | 0.99 | 0.98 | ||
| R2 | 0.97 | 0.79 | Temkin | bT (J.mol−1) | 97.77 | 143.39 | 133.16 | |
| Intra-particular- Diffusion | K (mg·g1·min1/2) | 2.145 | 2.925 | A (L·g−1) | 1.713 | 1.833 | 1.534 | |
| R2 | 0.43 | 0.44 | R2 | 0.92 | 0.90 | 0.91 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, F.M.; El-Ghoul, Y.; Jabli, M. Extraction of Cellulose Polymeric Material from Populus tremula Fibers: Characterization and Application to the Adsorption of Methylene Blue and Crystal Violet. Polymers 2021, 13, 3334. https://doi.org/10.3390/polym13193334
Almutairi FM, El-Ghoul Y, Jabli M. Extraction of Cellulose Polymeric Material from Populus tremula Fibers: Characterization and Application to the Adsorption of Methylene Blue and Crystal Violet. Polymers. 2021; 13(19):3334. https://doi.org/10.3390/polym13193334
Chicago/Turabian StyleAlmutairi, Faisal Muteb, Yassine El-Ghoul, and Mahjoub Jabli. 2021. "Extraction of Cellulose Polymeric Material from Populus tremula Fibers: Characterization and Application to the Adsorption of Methylene Blue and Crystal Violet" Polymers 13, no. 19: 3334. https://doi.org/10.3390/polym13193334