Enhanced Mechanical and Thermal Properties of Modified Oil Palm Fiber-Reinforced Polypropylene Composite via Multi-Objective Optimization of In Situ Silica Sol-Gel Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
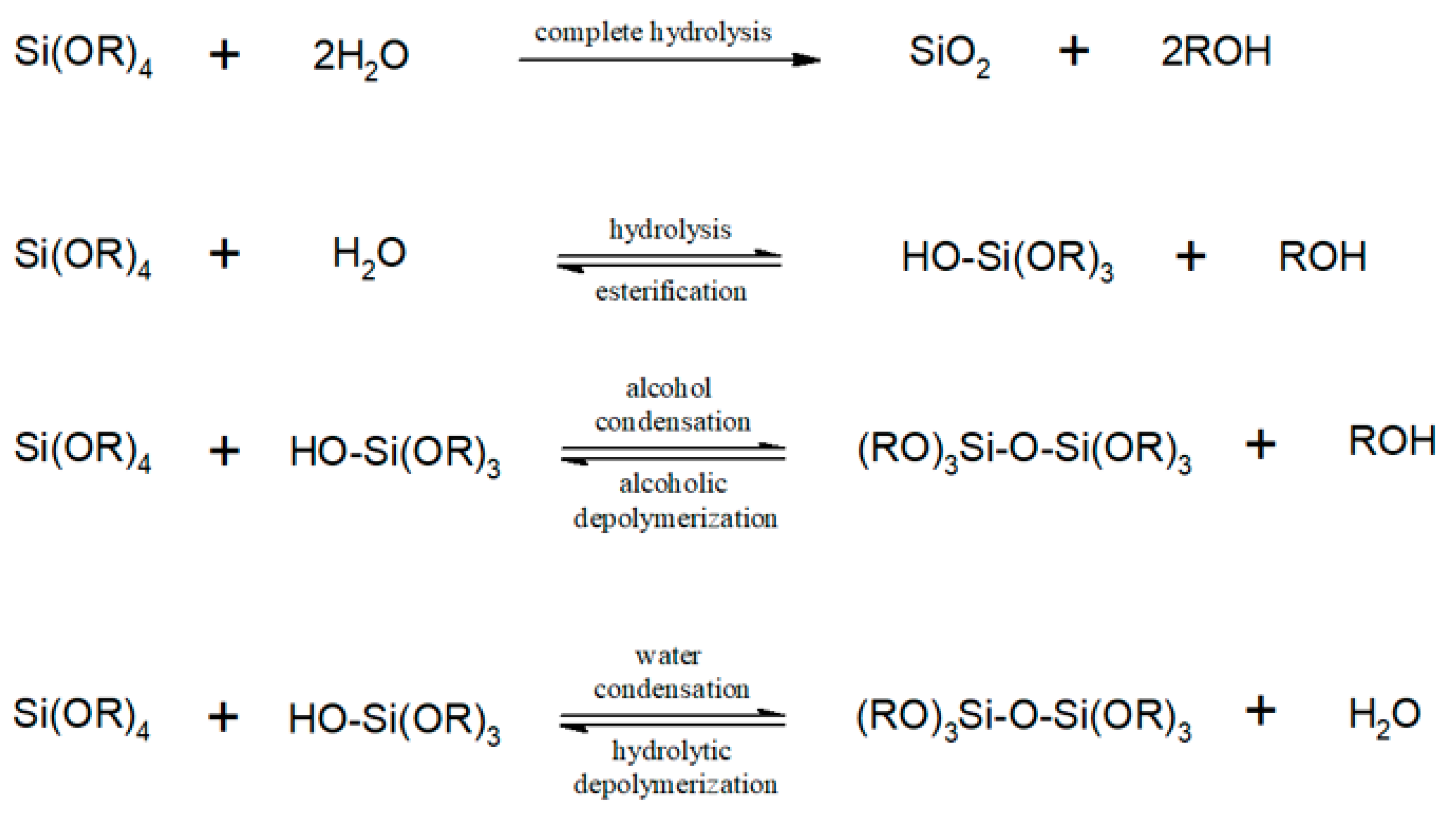
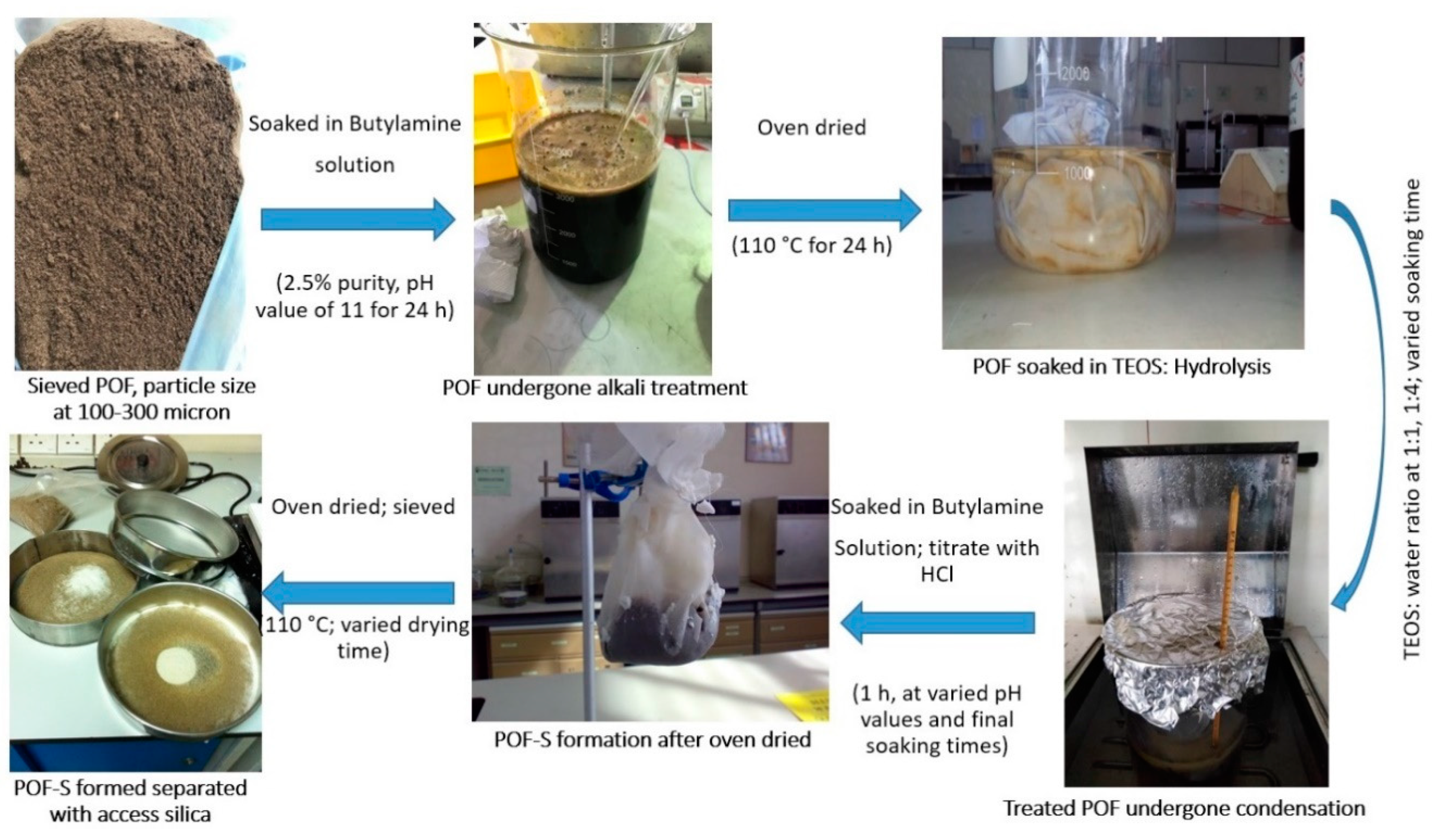
2.2. Design of Experiment and Optimization of In Situ Silica Sol-Gel Synthesis
2.3. Preparation of Natural Fiber Reinforced Composites
2.4. Mechanical Characterization
2.5. Termogravimetric Analysis (TGA)
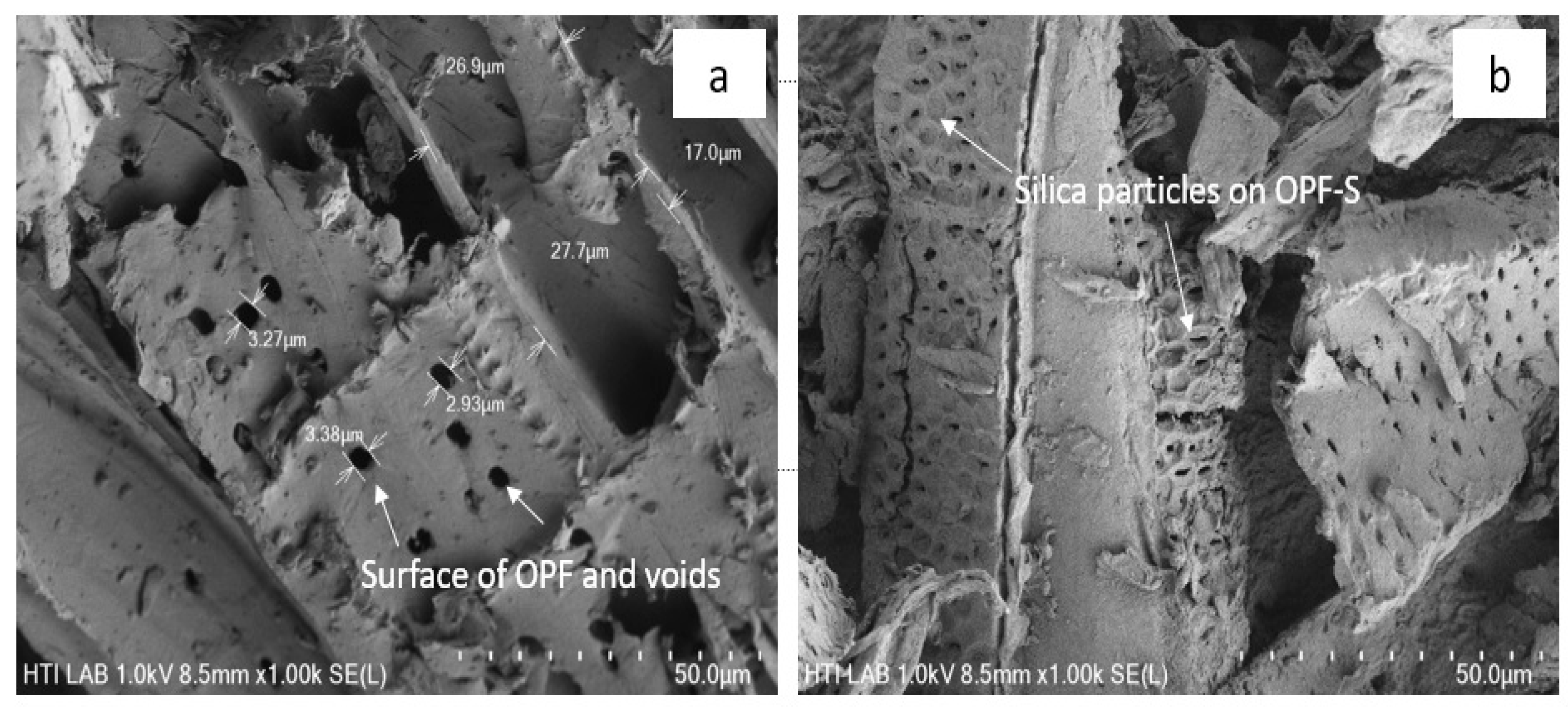
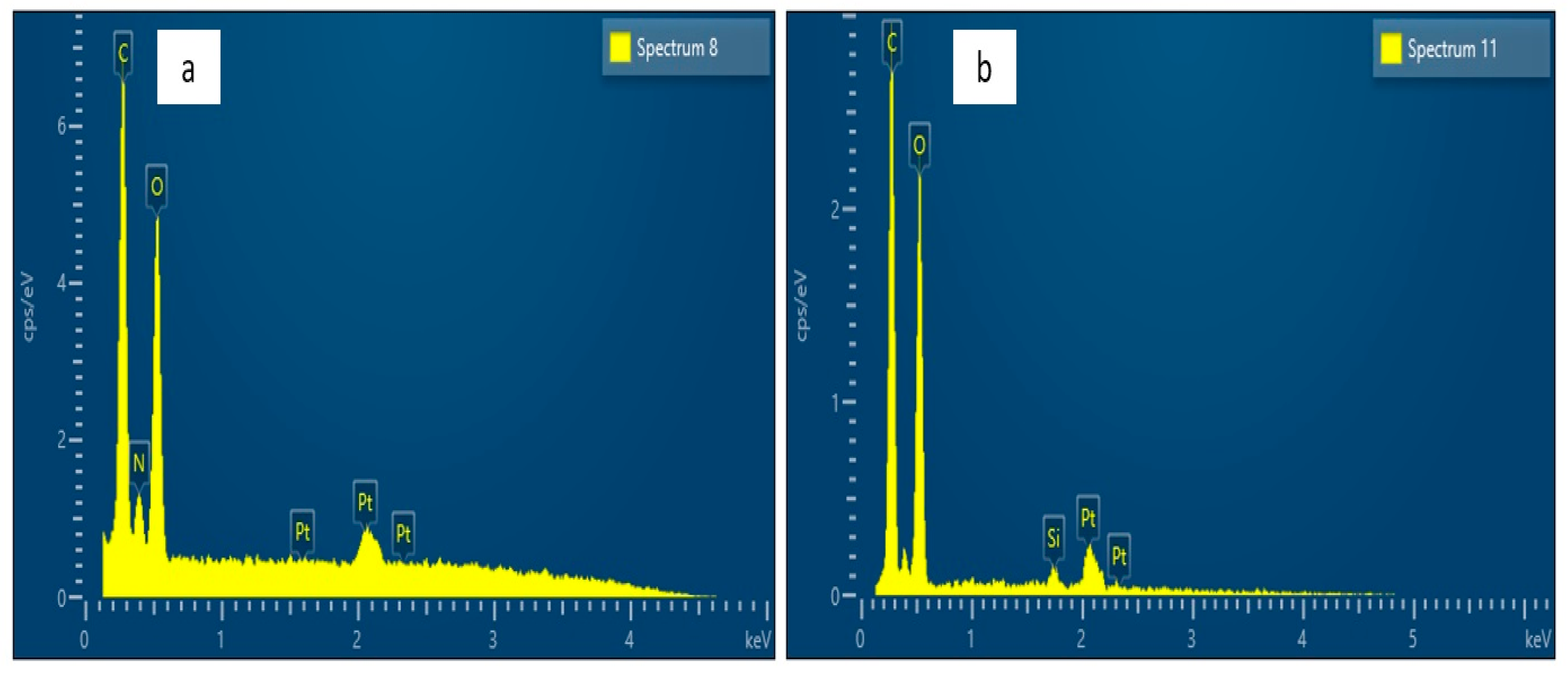
2.6. Surface Morphology Analyses
3. Results and Discussion
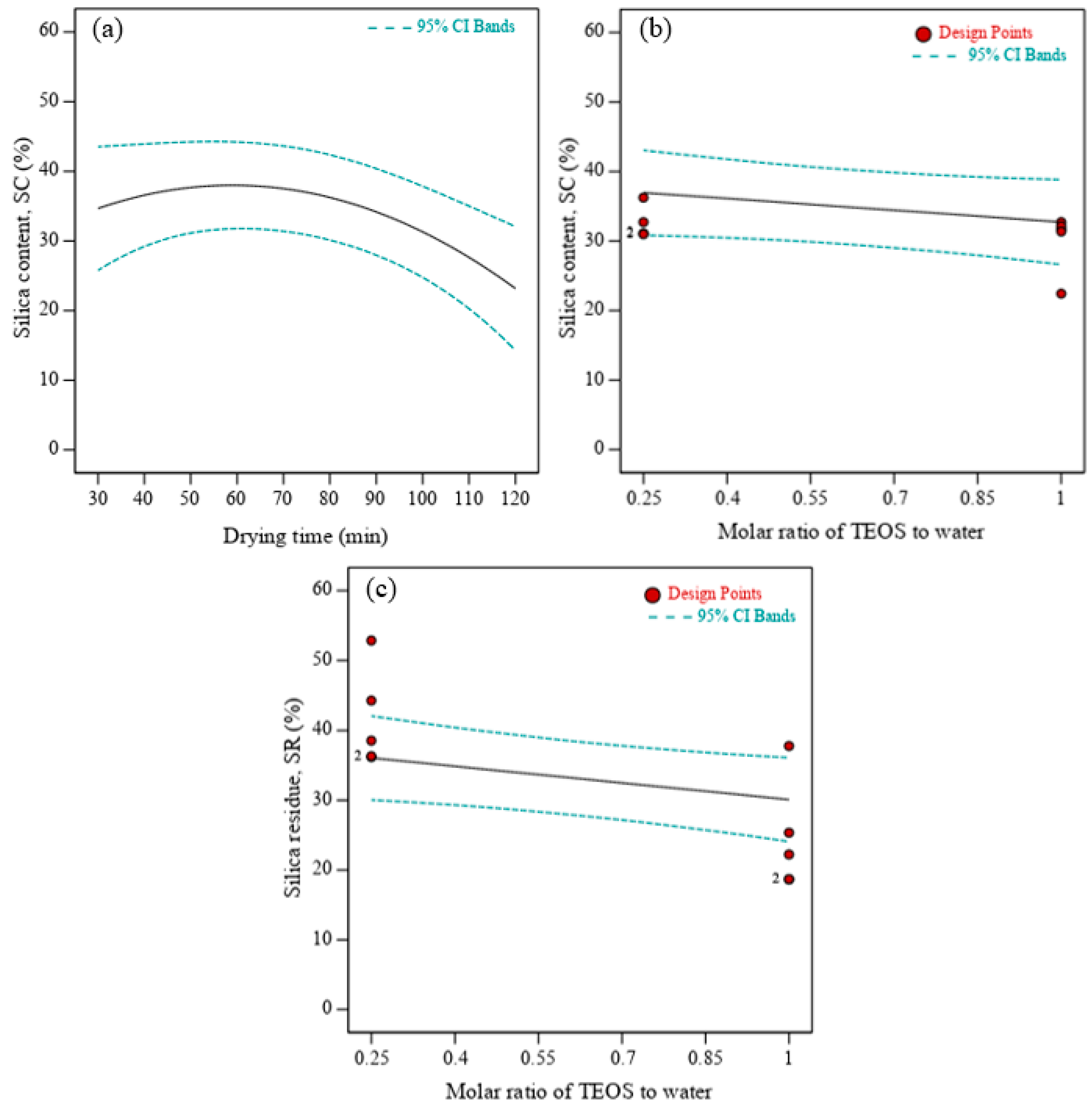
3.1. Multi-Objective Optimization of In Situ Silica Sol-Gel Synthesis
0.184 BD − 0.0829 CD − 3.645 A2 − 0.325 B2 + 0.003429C2 + 0.902 D2
0.786 BD − 0.03744 CD − 4.082 A2 + 0.0149 B2 + 0.000594 C2 + 1.459 D2
3.2. Mechanical Properties
3.3. Thermal Decomposition Analysis
4. Conclusions
- In situ silica sol-gel process on OPF was an effective method to fill the voids of OPF, which enhanced the mechanical and thermal properties of oil palm fiber-reinforced polypropylene composite.
- The optimum silica content of 34.1% and silica residue of 35.9% were achieved under optimum conditions of 21.3 h soaking time, 50 min drying time, pH value of 9.26, and 1:4 molar ratio of TEOS to water. The drying time after in situ silica sol-gel process on OPF was the most significant independent variable on silica content, while silica residue from TGA was highly influenced by molar ratio of TEOS to water.
- MAgPP was introduced for better interfacial adhesion between OPF-S and PP. The tensile strength of OPF-S-PP-MAgPP was significantly improved at 30.9 MPa, but other mechanical properties showed insignificant differences as compared to OPF-S-PP.
- The OPF-S-PP-MAgPP showed the highest thermal stability with the highest inflection point of 477 °C. However, the degradation difference between OPF-S-PP and OPF-S-PP-MAgPP was marginal.
- The outcomes of this study will be useful for future works on formulations of modified oil palm fiber-reinforced polypropylene composite in many potential applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balla, V.; Kate, K.H.; Satyavolu, J.; Singh, P.; Tadimeti, J.G.D. Additive manufacturing of natural fiber reinforced polymer composites: Processing and prospects. Compos. Part B Eng. 2019, 174, 106956. [Google Scholar] [CrossRef]
- Asim, M.; Abdan, K.; Jawaid, M.; Nasir, M.; Dashtizadeh, Z.; Ishak, M.R.; Hoque, M.E. A Review on Pineapple Leaves Fibre and Its Composites. Int. J. Polym. Sci. 2015, 2015, 950567. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, K.G.; Arizaga, G.G.C.; Wypych, F. Biodegradable composites based on lignocellulosic fibers—An overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar] [CrossRef]
- Singh, T.J.; Samanta, S. Characterization of Kevlar Fiber and Its Composites: A Review. Mater. Today Proc. 2015, 2, 1381–1387. [Google Scholar] [CrossRef]
- Sood, M.; Dwivedi, G. Effect of fiber treatment on flexural properties of natural fiber reinforced composites: A review. Egypt. J. Pet. 2018, 27, 775–783. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, S.; Li, Y.; Wang, Z.; Long, Y.; Yu, T.; Shen, Y. High performances of plant fiber reinforced composites—A new insight from hierarchical microstructures. Compos. Sci. Technol. 2020, 194, 108151. [Google Scholar] [CrossRef]
- Shah, D.U. Developing plant fibre composites for structural applications by optimising composite parameters: A critical review. J. Mater. Sci. 2013, 48, 6083–6107. [Google Scholar] [CrossRef]
- Ahmad, F.; Choi, H.S.; Park, M.K. A Review: Natural Fiber Composites Selection in View of Mechanical, Light Weight, and Economic Properties. Macromol. Mater. Eng. 2015, 300, 10–24. [Google Scholar] [CrossRef]
- Sumathi, S.; Chai, S.; Mohamed, A.R. Utilization of oil palm as a source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2008, 12, 2404–2421. [Google Scholar] [CrossRef]
- Talero, G.; Rincón, S.; Gómez, A. Biomass torrefaction in a standard retort: A study on oil palm solid residues. Fuel 2019, 244, 366–378. [Google Scholar] [CrossRef]
- Abdullah, N.; Sulaiman, N.A.A.F. The Oil Palm Wastes in Malaysia. In Biomass Now-Sustainable Growth and Use; Intech Open: Rijeka, Croatia, 2013; Volume 1, pp. 75–93. [Google Scholar] [CrossRef] [Green Version]
- USDA, World Agricultural Production, Circular Series—WAP 5-20. 2020. Available online: https://usda.library.cornell.edu/concern/publications/5q47rn72z?locale=en (accessed on 14 September 2021).
- Huda, M.S.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Chopped glass and recycled newspaper as reinforcement fibers in injection molded poly(lactic acid) (PLA) composites: A comparative study. Compos. Sci. Technol. 2006, 66, 1813–1824. [Google Scholar] [CrossRef]
- Fang, T.W.; Malaysia, M.U.S.; Asyikin, N.S.S.N.; Shawkataly, A.K.H.P.; Kassim, M.H.M.; Syakir, M. Water Absorption and Thickness Swelling of Oil Palm Empty Fruit Bunch (OPEFB) and Seaweed Composite for Soil Erosion Mitigation. J. Phys. Sci. 2017, 28, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ramlee, N.A.; Jawaid, M.; Zainudin, E.S.; Yamani, S.A.K. Tensile, physical and morphological properties of oil palm empty fruit bunch/sugarcane bagasse fibre reinforced phenolic hybrid composites. J. Mater. Res. Technol. 2019, 8, 3466–3474. [Google Scholar] [CrossRef]
- Gassan, J.; Bledzki, A.K. Thermal degradation of flax and jute fibers. J. Appl. Polym. Sci. 2001, 82, 1417–1422. [Google Scholar] [CrossRef]
- Dicker, M.; Duckworth, P.F.; Baker, A.B.; Francois, G.; Hazzard, M.K.; Weaver, P. Green composites: A review of material attributes and complementary applications. Compos. Part A Appl. Sci. Manuf. 2014, 56, 280–289. [Google Scholar] [CrossRef]
- Facca, A.G.; Kortschot, M.T.; Yan, N. Predicting the tensile strength of natural fibre reinforced thermoplastics. Compos. Sci. Technol. 2007, 67, 2454–2466. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Senthilraja, R.; Sarala, R.; Antony, A.G. Effect of acetylation technique on mechanical behavior and durability of palm fibre vinyl-ester composites. Mater. Today Proc. 2020, 21, 634–637. [Google Scholar] [CrossRef]
- Essabir, H.; Boujmal, R.; Bensalah, M.O.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.E.K. Mechanical and thermal properties of hybrid composites: Oil-palm fiber/clay reinforced high density polyethylene. Mech. Mater. 2016, 98, 36–43. [Google Scholar] [CrossRef]
- Ramli, R.; Yunus, R.; Beg, M. Effects of fiber loading, fiber type, its mesh sizes, and coupling agent on the properties of oil palm biomass/polypropylene composites. J. Compos. Mater. 2011, 45, 2165–2171. [Google Scholar] [CrossRef]
- Jawaid, M.; Khalil, H.A.; Abu Bakar, A.; Khanam, P.N. Chemical resistance, void content and tensile properties of oil palm/jute fibre reinforced polymer hybrid composites. Mater. Des. 2011, 32, 1014–1019. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Progress Report on Natural Fiber Reinforced Composites. Macromol. Mater. Eng. 2014, 299, 9–26. [Google Scholar] [CrossRef]
- Shinoj, S.; Visvanathan, R.; Panigrahi, S.; Kochubabu, M. Oil palm fiber (OPF) and its composites: A review. Ind. Crops Prod. 2011, 33, 7–22. [Google Scholar] [CrossRef]
- Karsli, N.G.; Aytac, A. Effects of maleated polypropylene on the morphology, thermal and mechanical properties of short carbon fiber reinforced polypropylene composites. Mater. Des. 2011, 32, 4069–4073. [Google Scholar] [CrossRef]
- Yi, S.; Xu, S.; Li, Y.; Gan, W.; Yi, X.; Liu, W.; Wang, Q.; Wang, H.; Ou, R. Synergistic toughening effects of grafting modification and elastomer-olefin block copolymer addition on the fracture resistance of wood particle/polypropylene/elastomer composites. Mater. Des. 2019, 181, 107918. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Ma, H.; Yang, J. The preparation of a green shape-stabilized composite phase change material of polyethylene glycol/SiO2 with enhanced thermal performance based on oil shale ash via temperature-assisted sol–gel method. Sol. Energy Mater. Sol. Cells 2015, 132, 29–39. [Google Scholar] [CrossRef]
- He, F.; Wang, X.; Wu, D. New approach for sol–gel synthesis of microencapsulated n-octadecane phase change material with silica wall using sodium silicate precursor. Energy 2014, 67, 223–233. [Google Scholar] [CrossRef]
- Wang, C.; Cai, Z.; Wang, T.; Chen, K. Preparation and thermal properties of shape-stabilized 1, 8-octanediol /SiO2 composites via sol gel methods. Mater. Chem. Phys. 2020, 250, 123041. [Google Scholar] [CrossRef]
- Zulkifli, M.; Hossain, S.; Khalil, N.A.; Yahaya, A.N.A.; Yusof, F.A.M.; Hashim, A.S. Preparation and Characterization of Sol-gel Silica-modified Kenaf Bast Microfiber/Polypropylene Composites. BioResources 2017, 13, 1977–1992. Available online: https://ojs.cnr.ncsu.edu/index.php/BioRes/article/view/BioRes_13_1_1977_Zulkifli_Sol_gel_Silica_Kenaf_Bast_Composites/5868 (accessed on 14 September 2021). [CrossRef]
- Yusof, F.; Tajudin, Z.; Ong, S.; Hashim, A. Optimization of Early Stage Hydrolysis of Silica Sol-Gel/Kenaf using Response Surface Methodology. Mater. Today Proc. 2019, 19, 1663–1672. [Google Scholar] [CrossRef]
- Agustín-Sáenz, C.; Machado, M.; Tercjak, A. Antireflective mesoporous silica coatings by optimization of water content in acid-catalyzed sol-gel method for application in glass covers of concentrated photovoltaic modules. J. Colloid Interface Sci. 2019, 534, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Luyt, A. Effect of sol–gel derived nano-silica and organic peroxide on the thermal and mechanical properties of low-density polyethylene/wood flour composites. Polym. Degrad. Stab. 2008, 93, 1–8. [Google Scholar] [CrossRef]
- Wu, C.-S.; Liao, H.-T. Modification of biodegradable polylactide by silica and wood flour through a sol–gel process. J. Appl. Polym. Sci. 2008, 109, 2128–2138. [Google Scholar] [CrossRef]
- Çok, S.S.; Koç, F.; Balkan, F.; Gizli, N. Revealing the pore characteristics and physicochemical properties of silica ionogels based on different sol-gel drying strategies. J. Solid State Chem. 2019, 278, 120877. [Google Scholar] [CrossRef]
- Pouxviel, J.; Boilot, J.; Beloeil, J.; Lallemand, J. NMR study of the sol/gel polymerization. J. Non Cryst. Solids 1987, 89, 345–360. [Google Scholar] [CrossRef]
- Engelhardt, G. Silicon-29 NMR of Solid Silicates. In Encyclopedia of Magnetic Resonance; University of California: Los Angeles, CA, USA, 2007. [Google Scholar] [CrossRef]
- Peña, L.F.; Nanayakkara, C.E.; Mallikarjunan, A.; Chandra, H.; Xiao, M.; Lei, X.; Pearlstein, R.M.; Derecskei-Kovacs, A.; Chabal, Y.J. Atomic Layer Deposition of Silicon Dioxide Using Aminosilanes Di-sec-butylaminosilane and Bis(tert-butylamino)silane with Ozone. J. Phys. Chem. C 2016, 120, 10927–10935. [Google Scholar] [CrossRef]
- Bajuri, F.; Mazlan, N.; Ishak, M.R.; Imatomi, J. Flexural and Compressive Properties of Hybrid Kenaf/Silica Nanoparticles in Epoxy Composite. Procedia Chem. 2016, 19, 955–960. [Google Scholar] [CrossRef] [Green Version]
- Rane, L.R.; Savadekar, N.R.; Kadam, P.G.; Mhaske, S.T. Preparation and Characterization of K-Carrageenan/Nanosilica Biocomposite Film. J. Mater. 2014, 2014, 736271. [Google Scholar] [CrossRef] [Green Version]
- Dewi, I.R.; Indrajati, I.N.; Nurhajati, D.W. Effect of compatibilizers on the mechanical and morphological properties of polycarbonate/poly (acrylonitrile-butadiene-styrene) blends. IOP Conf. Ser. Mater. Sci. Eng. 2018, 432, 012039. [Google Scholar] [CrossRef]
- Sailaja, R.R.N.; Reddy, A.P.; Chanda, M. Effect of epoxy functionalized compatibilizer on the mechanical properties of low-density polyethylene/plasticized tapioca starch blends. Polym. Int. 2001, 50, 1352–1359. [Google Scholar] [CrossRef]
- Selvi, M.; Vengatesan, M.R.; Devaraju, S.; Kumar, M.; Alagar, M. In situ sol–gel synthesis of silica reinforced polybenzoxazine hybrid materials with low surface free energy. RSC Adv. 2014, 4, 8446. [Google Scholar] [CrossRef]




| Factor | Symbol | Levels | ||
|---|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | ||
| pH | A | 7 | 9 | 11 |
| Soaking time (h) | B | 18 | 22 | 26 |
| Drying time (min) | C | 30 | 75 | 120 |
| Molar ratio of TEOS/water | D | 1:1 | 1:2.5 | 1.:4 |
| Sample | Polypropylene, PP (wt%) | Oil Palm Fiber, OPF (wt%) | Silica Sol-Gel Modified-OPF (wt%) | MAgPP Coupling Agent (wt%) |
|---|---|---|---|---|
| OPF-PP | 70 | 30 | - | - |
| SiO2-OPF-PP | 70 | - | 30 | - |
| SiO2-OPF-PP-MAgPP | 70 | - | 30 | 10 |
| Run | A | B | C | D | Response | |||
|---|---|---|---|---|---|---|---|---|
| SC (%) | SR (%) | |||||||
| Actual | Predicted | Actual | Predicted | |||||
| 1 | 7 | 18 | 30 | 1:1 | 6.78 | 4.23 | 9.28 | 10.76 |
| 2 | 11 | 18 | 30 | 1:1 | 7.97 | 8.67 | 13.06 | 12.04 |
| 3 | 7 | 26 | 30 | 1:1 | 8.93 | 12.25 | 17.98 | 17.99 |
| 4 | 11 | 26 | 30 | 1:1 | 10.17 | 11.59 | 18.35 | 19.20 |
| 5 | 7 | 18 | 120 | 1:1 | 11.78 | 13.84 | 15.49 | 16.28 |
| 6 | 11 | 18 | 120 | 1:1 | 18.66 | 19.97 | 17.37 | 17.87 |
| 7 | 7 | 26 | 120 | 1:1 | 21.3 | 20.38 | 25.64 | 24.94 |
| 8 | 11 | 26 | 120 | 1:1 | 22.43 | 20.41 | 25.37 | 26.45 |
| 9 | 7 | 18 | 30 | 1:4 | 22.43 | 25.10 | 32.78 | 31.23 |
| 10 | 11 | 18 | 30 | 1:4 | 31.72 | 31.47 | 26.95 | 29.13 |
| 11 | 7 | 26 | 30 | 1:4 | 32.17 | 29.69 | 18.63 | 19.61 |
| 12 | 11 | 26 | 30 | 1:4 | 31.38 | 29.97 | 18.68 | 17.43 |
| 13 | 7 | 18 | 120 | 1:4 | 15.92 | 13.33 | 26.02 | 26.65 |
| 14 | 11 | 18 | 120 | 1:4 | 23.06 | 20.39 | 25.33 | 24.85 |
| 15 | 7 | 26 | 120 | 1:4 | 15.5 | 15.45 | 15.89 | 16.45 |
| 16 | 11 | 26 | 120 | 1:4 | 14.04 | 16.42 | 14.57 | 14.57 |
| 17 | 7 | 22 | 75 | 1:2.5 | 11.3 | 12.84 | 18.07 | 15.88 |
| 18 | 11 | 22 | 75 | 1:2.5 | 15.5 | 16.04 | 17.46 | 15.59 |
| 19 | 9 | 18 | 75 | 1:2.5 | 20.22 | 22.55 | 35.59 | 33.06 |
| 20 | 9 | 26 | 75 | 1:2.5 | 25.33 | 25.08 | 33.07 | 31.54 |
| 21 | 9 | 22 | 30 | 1:2.5 | 37.13 | 36.70 | 34.28 | 32.60 |
| 22 | 9 | 22 | 120 | 1:2.5 | 32.72 | 35.23 | 36.31 | 33.93 |
| 23 | 9 | 22 | 75 | 1:1 | 28.91 | 26.58 | 36.18 | 33.19 |
| 24 | 9 | 22 | 75 | 1:4 | 31.11 | 35.52 | 38.57 | 37.49 |
| 25 | 9 | 22 | 75 | 1:2.5 | 31.21 | 29.00 | 32.85 | 32.10 |
| 26 | 9 | 22 | 75 | 1:2.5 | 28.75 | 29.00 | 30.66 | 32.10 |
| 27 | 9 | 22 | 75 | 1:2.5 | 25.50 | 29.00 | 28.59 | 32.10 |
| 28 | 9 | 22 | 75 | 1:2.5 | 29.67 | 29.00 | 27.85 | 32.10 |
| 29 | 9 | 22 | 75 | 1:2.5 | 32.54 | 29.00 | 31.28 | 32.10 |
| 30 | 9 | 22 | 75 | 1:2.5 | 32.59 | 29.00 | 29.18 | 32.10 |
| 31 | 9 | 22 | 75 | 1:2.5 | 30.69 | 29.00 | 31.86 | 32.10 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 2241.06 | 14 | 160.076 | 16.55 | 0.000 |
| A-pH value | 46.41 | 1 | 46.144 | 4.77 | 0.044 |
| B-Soaking time | 28.65 | 1 | 28.652 | 2.96 | 0.104 |
| C-Drying time | 9.78 | 1 | 9.783 | 1.01 | 0.329 |
| D-Molar ratio of TEOS to water | 359.12 | 1 | 359.120 | 37.14 | 0.000 |
| AB | 37.15 | 1 | 37.149 | 3.84 | 0.068 |
| AC | 0.48 | 1 | 0.476 | 0.05 | 0.827 |
| AD | 0.87 | 1 | 0.874 | 0.09 | 0.768 |
| BC | 6.13 | 1 | 6.126 | 0.63 | 0.438 |
| BD | 19.54 | 1 | 19.536 | 2.02 | 0.174 |
| CD | 500.64 | 1 | 500.64 | 51.77 | 0.000 |
| A2 | 551.69 | 1 | 551.691 | 57.05 | 0.000 |
| B2 | 70.32 | 1 | 70.316 | 7.27 | 0.016 |
| C2 | 125.16 | 1 | 125.162 | 12.94 | 0.002 |
| D2 | 10.69 | 0 | 10.691 | 1.11 | 0.309 |
| Residual | 154.73 | 17 | 9.670 | ||
| Lack of fit | 118.10 | 10 | 11.810 | 1.93 | 0.217 |
| Pure error | 36.63 | 8 | 6.105 | ||
| Total | 2395.79 | 31 | |||
| R2= 0.9354; R2Adj = 0.8789 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 1878.43 | 14 | 134.173 | 22.07 | 0.000 |
| A-pH value | 0.39 | 1 | 0.387 | 0.06 | 0.804 |
| B-Soaking time | 10.41 | 1 | 10.412 | 1.71 | 0.209 |
| C-Drying time | 8.00 | 1 | 8.000 | 1.32 | 0.268 |
| D-Molar ratio of TEOS to water | 83.20 | 1 | 83.205 | 13.69 | 0.002 |
| AB | 0.01 | 1 | 0.006 | 0.00 | 0.975 |
| AC | 0.09 | 1 | 0.095 | 0.02 | 0.902 |
| AD | 11.48 | 1 | 11.475 | 1.89 | 0.188 |
| BC | 2.02 | 1 | 2.024 | 0.33 | 0.572 |
| BD | 355.79 | 1 | 355.794 | 58.52 | 0.000 |
| CD | 102.16 | 1 | 102.162 | 16.80 | 0.001 |
| A2 | 691.82 | 1 | 691.821 | 113.79 | 0.000 |
| B2 | 0.15 | 1 | 0.147 | 0.02 | 0.879 |
| C2 | 3.75 | 1 | 3.754 | 0.62 | 0.443 |
| D2 | 27.97 | 27.965 | 4.60 | 0.048 | |
| Residual | 97.28 | 17 | 6.080 | ||
| Lack of fit | 79.67 | 10 | 7.967 | 2.72 | 0.117 |
| Pure error | 17.60 | 6 | 2.934 | ||
| Total | 1975.71 | 31 | |||
| R2= 0.9508; R2Adj = 0.9077 |
| Composite | Decomposition Temperature (°C) | ||||
|---|---|---|---|---|---|
| 2% | 5% | 10% | 20% | 30% | |
| OPF-PP | 369.8 | 414.4 | 434.4 | 449.7 | 456.1 |
| OPF-S-PP | 283.0 | 327.3 | 384.8 | 449.7 | 461.4 |
| OPF-S-PP-MAgPP | 288.4 | 331.0 | 389.3 | 449.3 | 460.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mat Rozi, N.; Hamid, H.A.; Hossain, M.S.; Khalil, N.A.; Ahmad Yahaya, A.N.; Syimir Fizal, A.N.; Haris, M.Y.; Ahmad, N.; Zulkifli, M. Enhanced Mechanical and Thermal Properties of Modified Oil Palm Fiber-Reinforced Polypropylene Composite via Multi-Objective Optimization of In Situ Silica Sol-Gel Synthesis. Polymers 2021, 13, 3338. https://doi.org/10.3390/polym13193338
Mat Rozi N, Hamid HA, Hossain MS, Khalil NA, Ahmad Yahaya AN, Syimir Fizal AN, Haris MY, Ahmad N, Zulkifli M. Enhanced Mechanical and Thermal Properties of Modified Oil Palm Fiber-Reinforced Polypropylene Composite via Multi-Objective Optimization of In Situ Silica Sol-Gel Synthesis. Polymers. 2021; 13(19):3338. https://doi.org/10.3390/polym13193338
Chicago/Turabian StyleMat Rozi, Nasrullah, Hamidah Abdul Hamid, Md. Sohrab Hossain, Nor Afifah Khalil, Ahmad Naim Ahmad Yahaya, Ahmad Noor Syimir Fizal, Mohd Yusoff Haris, Norkhairi Ahmad, and Muzafar Zulkifli. 2021. "Enhanced Mechanical and Thermal Properties of Modified Oil Palm Fiber-Reinforced Polypropylene Composite via Multi-Objective Optimization of In Situ Silica Sol-Gel Synthesis" Polymers 13, no. 19: 3338. https://doi.org/10.3390/polym13193338