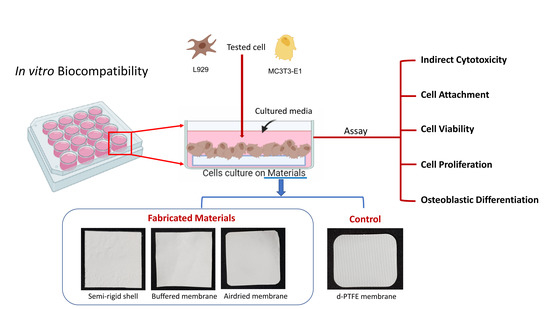
In Vitro Biocompatibility of a Novel Semi-Rigid Shell Barrier System: As a New Application for Guided Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Biphasic Calcium Phosphate (BCP) (HA30: β-TCP:70) with particle size < 75 µm was obtained in the cooperation with the Assistive Technology and Medical Devices Research Center (A-MED), Thailand.
- Poly ε-caprolactone (PCL), 80,000 by GPC was purchased from Sigma-Aldrich, Gillingham, UK.
- A high-density polytetrafluoroethylene (d-PTFE) membrane (Cytoplast® TXT-200, Osteogenics Biomedical, Lubbock, TX, USA), which is a non-resorbable membrane, was selected as a control.
2.2. Fabrication of the Semi-Rigid Shell Barrier System
2.3. Material Morphology Evaluation
2.3.1. Gross Observation
2.3.2. Surface Morphology
2.4. In Vitro Biocompatibility
2.4.1. Osteoblast and Fibroblast Cell Culture
2.4.2. Indirect Cytotoxicity Test
2.4.3. Direct Biocompatibility Test
2.4.4. Preparing the Cell-Sheath Structure
2.4.5. Cell Attachment and Morphologies
2.4.6. Cell Viability
2.4.7. Cell Proliferation
2.4.8. Osteoblast Cell Differentiation
- Total intracellular protein content
- Alkaline phosphatase activity (ALP assay)
- Osteocalcin assay
- Mineralization assay with Alizarin red staining
2.5. Statistical Analysis
3. Results
3.1. Material Morphology
3.2. Indirect Cell Cytotoxicity
3.3. Cell Attachment and Morphology
3.4. Cell Viability
3.5. Cells Proliferation
3.6. Osteoblast Cell Differentiation
4. Discussion
4.1. Material Morphology
4.2. In Vitro Biocompatibility and Cell Cytotoxicity
4.3. Cell Viability, Proliferation, and Osteoblast Cell Differentiation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iglhaut, G.; Schwarz, F.; Grundel, M.; Mihatovic, I.; Becker, J.; Schliephake, H. Shell technique using a rigid resorbable barrier system for localized alveolar ridge augmentation. Clin. Oral Implants Res. 2014, 25, E149–E154. [Google Scholar] [CrossRef] [PubMed]
- Pikos, M.A. Block autografts for localized ridge augmentation: Part II. The posterior mandible. Implant Dent. 2000, 9, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of bone defects by guided tissue regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, C.; Sennerby, L.; Lekholm, U.; Linde, A.; Nyman, S. Generation of New Bone Around Titanium Implants Using a Membrane Technique: An Experimental Study in Rabbits. Int. J. Oral Maxillofac. Implants 1989, 4, 33. [Google Scholar]
- Becker, W.; Dahlin, C.; Becker, B.E.; Lekholm, U.; van Steenberghe, D.; Higuchi, K.; Kultje, C. The use of e-PTFE barrier membranes for bone promotion around titanium implants placed into extraction sockets: A prospective multicenter study. Int. J. Oral Maxillofac. Implants 1994, 9, 31–40. [Google Scholar] [PubMed]
- Simion, M.; Baldoni, M.; Rossi, P.; Zaffe, D. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int. J. Periodontics Restor. Dent. 1994, 14, 166–180. [Google Scholar]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Hua, N.; Ti, V.L.; Xu, Y. Biodegradable effect of PLGA membrane in alveolar bone regeneration on beagle dog. Cell Biochem. Biophys. 2014, 70, 1051–1055. [Google Scholar] [CrossRef]
- Jung, R.E.; Kokovic, V.; Jurisic, M.; Yaman, D.; Subramani, K.; Weber, F.E. Guided bone regeneration with a synthetic biodegradable membrane: A comparative study in dogs. Clin. Oral Implants Res. 2011, 22, 802–807. [Google Scholar] [CrossRef] [Green Version]
- Tunthasen, R.; Pripatnanont, P.; Meesane, J. Fabrication and characterization of a semi-rigid shell barrier system made of polycaprolactone and biphasic calcium phosphate: A novel barrier system for bone regeneration. J. Mech. Behav. Biomed. Mater. 2021, 124, 104841. [Google Scholar] [CrossRef]
- Maryam Farzad, M.M. Guided bone regeneration: A literature review. Oral Health Oral Epidemiol. 2012, 1, 3–18. [Google Scholar]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Engelberg, I.; Kohn, J. Physicomechanical properties of degradable polymers used in medical applications: A comparative study. Biomaterials 1991, 12, 292–304. [Google Scholar] [CrossRef]
- Thuaksuban, N.; Nuntanaranont, T.; Suttapreyasri, S.; Pattanachot, W.; Sutin, K.; Cheung, L.K. Biomechanical properties of novel biodegradable poly epsilon-caprolactone-chitosan scaffolds. J. Investig. Clin. Dent. 2013, 4, 26–33. [Google Scholar] [CrossRef]
- Suneelkumar, C.; Datta, K.; Srinivasan, M.R.; Kumar, S.T. Biphasic calcium phosphate in periapical surgery. J. Conserv. Dent. 2008, 11, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, M.; Pripatnanont, P.; Monmaturapoj, N.; Suttapreyasri, S. Fabrication and characterization of novel nano hydroxyapatite/beta-tricalcium phosphate scaffolds in three different composition ratios. J. Biomed. Mater. Res. A 2012, 100, 2260–2268. [Google Scholar] [CrossRef]
- Houmard, M.; Fu, Q.; Genet, M.; Saiz, E.; Tomsia, A.P. On the structural, mechanical, and biodegradation properties of HA/beta-TCP robocast scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Pripatnanont, P.; Praserttham, P.; Suttapreyasri, S.; Leepong, N.; Monmaturapoj, N. Bone Regeneration Potential of Biphasic Nanocalcium Phosphate with High Hydroxyapatite/Tricalcium Phosphate Ratios in Rabbit Calvarial Defects. Int. J. Oral Maxillofac. Implants 2016, 31, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Thuaksuban, N.; Luntheng, T.; Monmaturapoj, N. Physical characteristics and biocompatibility of the polycaprolactone-biphasic calcium phosphate scaffolds fabricated using the modified melt stretching and multilayer deposition. J. Biomater. Appl. 2016, 30, 1460–1472. [Google Scholar] [CrossRef]
- Subedi, D.P. Contact Angle Measurement for The Surface Characterization of Solids. Himal. Phys. 2011, 2, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Assad, M.; Jackson, N. Biocompatibility Evaluation of Orthopedic Biomaterials and Medical Devices: A Review of Safety and Efficacy Models. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 281–309. [Google Scholar]
- Omidi, M.; Fatehinya, A.; Farahani, M.; Akbari, Z.; Shahmoradi, S.; Yazdian, F.; Tahriri, M.; Moharamzadeh, K.; Tayebi, L.; Vashaee, D. 7-Characterization of biomaterials. In Biomaterials for Oral and Dental Tissue Engineering; Tayebi, L., Moharamzadeh, K., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 97–115. [Google Scholar]
- Grimm, J.B.; Heckman, L.M.; Lavis, L.D. Chapter One—The Chemistry of Small-Molecule Fluorogenic Probes. In Progress in Molecular Biology and Translational Science; Morris, M.C., Ed.; Academic Press: New York, NY, USA, 2013; Volume 113, pp. 1–34. [Google Scholar]
- Degasne, I.; Baslé, M.F.; Demais, V.; Huré, G.; Lesourd, M.; Grolleau, B.; Mercier, L.; Chappard, D. Effects of roughness, fibronectin and vitronectin on attachment, spreading, and proliferation of human osteoblast-like cells (Saos-2) on titanium surfaces. Calcif. Tissue Int. 1999, 64, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Zaidel-Bar, R.; Cohen, M.; Addadi, L.; Geiger, B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem. Soc. Trans. 2004, 32, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Ploux, L.; Ponche, A. Cell/Material Interfaces: Influence of Surface Chemistry and Surface Topography on Cell Adhesion. J. Adhes. Sci. Technol. 2010, 24, 831–852. [Google Scholar] [CrossRef]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. Assay Guidance Manual [Internet]; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2013. [Google Scholar]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Cancer Cell Cult. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Schwartz, Z.; Lohmann, C.H.; Oefinger, J.; Bonewald, L.F.; Dean, D.D.; Boyan, B.D. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv. Dent. Res. 1999, 13, 38–48. [Google Scholar] [CrossRef]
- Godwin, S.L.; Soltoff, S.P. Extracellular Calcium and Platelet-derived Growth Factor Promote Receptor-mediated Chemotaxis in Osteoblasts through Different Signaling Pathways. J. Biol. Chem. 1997, 272, 11307–11312. [Google Scholar] [CrossRef] [Green Version]
- Beck, G.R.; Moran, E.; Knecht, N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp. Cell Res. 2003, 288, 288–300. [Google Scholar] [CrossRef]
- Bingham, P.J.; Raisz, L.G. Bone growth in organ culture: Effects of phosphate and other nutrients on bone and cartilage. Calcif. Tissue Res. 1974, 14, 31–48. [Google Scholar] [CrossRef]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Zimina, A.; Senatov, F.; Choudhary, R.; Kolesnikov, E.; Anisimova, N.; Kiselevskiy, M.; Orlova, P.; Strukova, N.; Generalova, M.; Manskikh, V.; et al. Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction. Polymers 2020, 12, 2938. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Both, S.K.; Yang, X.; Walboomers, X.F.; Jansen, J.A. Development of an electrospun nano-apatite/PCL composite membrane for GTR/GBR application. Acta Biomater. 2009, 5, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Sculean, A.; Herten, M.; Scherbaum, W.; Becker, J. Biocompatibility of various collagen membranes in cultures of human PDL fibroblasts and human osteoblast-like cells. Clin. Oral Implants Res. 2004, 15, 443–449. [Google Scholar] [CrossRef]
- Bunyaratavej, P.; Wang, H.-L. Collagen Membranes: A Review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef] [Green Version]
- Carbonell, J.M.; Martín, I.S.; Santos, A.; Pujol, A.; Sanz-Moliner, J.D.; Nart, J. High-density polytetrafluoroethylene membranes in guided bone and tissue regeneration procedures: A literature review. Int. J. Oral Maxillofac. Surg. 2014, 43, 75–84. [Google Scholar] [CrossRef]









| Study Groups | Abbreviation |
|---|---|
| Semi-rigid shell | SR shell |
| Semi-resorbable barrier membrane fabricated using a buffering technique | BF membrane |
| Semi-resorbable barrier membrane fabricated using an air-dry technique | AD membrane |
| Commercial d-PTFE membrane (Cytoplast®, USA) | CP membrane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tunthasen, R.; Pripatnanont, P.; Meesane, J. In Vitro Biocompatibility of a Novel Semi-Rigid Shell Barrier System: As a New Application for Guided Bone Regeneration. Polymers 2022, 14, 2451. https://doi.org/10.3390/polym14122451
Tunthasen R, Pripatnanont P, Meesane J. In Vitro Biocompatibility of a Novel Semi-Rigid Shell Barrier System: As a New Application for Guided Bone Regeneration. Polymers. 2022; 14(12):2451. https://doi.org/10.3390/polym14122451
Chicago/Turabian StyleTunthasen, Rudjit, Prisana Pripatnanont, and Jirut Meesane. 2022. "In Vitro Biocompatibility of a Novel Semi-Rigid Shell Barrier System: As a New Application for Guided Bone Regeneration" Polymers 14, no. 12: 2451. https://doi.org/10.3390/polym14122451