Production and Characterization of Biocomposite Films of Bacterial Cellulose from Kombucha and Coated with Chitosan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production Process of BC Films
Pretreatment and Performance of BC Films
2.2. Development of Chitosan-Coated BC Films
2.3. Rheological Characterization of CFSs
2.4. Characterization of BC and BC-CH Films
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. Thickness Measurements
2.4.3. Color Analysis
2.4.4. Opacity Analysis
2.4.5. Ultraviolet-Visible Spectroscopy (UV-Vis)
2.4.6. Water Solubility Tests
2.5. Biological Activity Determinations
2.5.1. Antimicrobial Activity Tests
2.5.2. Antioxidant Activity Tests
Obtaining Alcoholic Extracts
Total Phenolic Content
DPPH Radical Scavenging Activity
ABTS Radical Scavenging Activity
2.6. Statistical Analysis
3. Results and Discussion
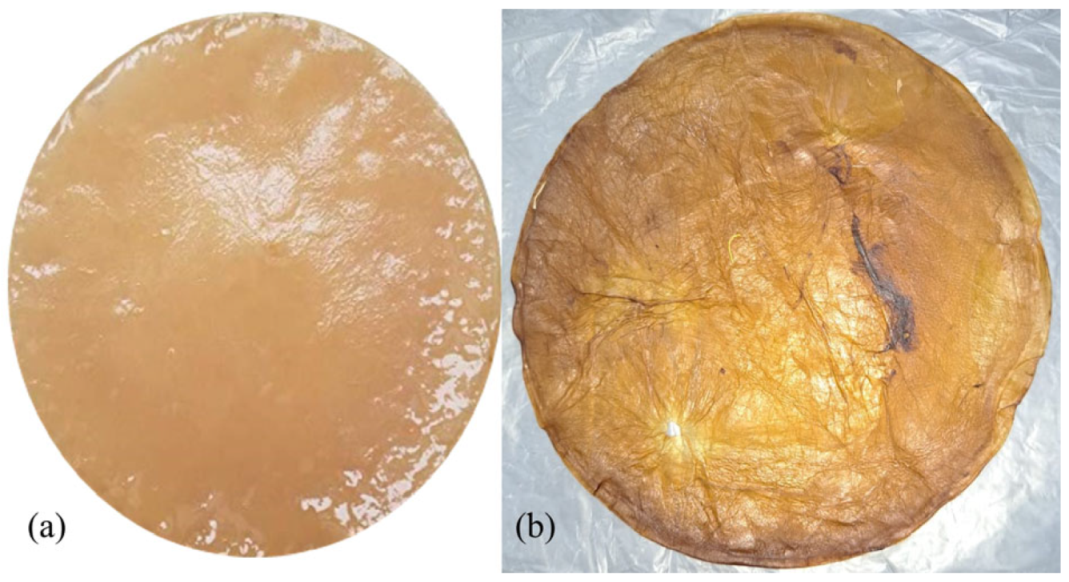
3.1. BC Films Yield
3.2. Rheological Analysis of CFS
3.3. Obtaining BC-CH Films
3.4. Physicochemical Characterization of BC and BC-CH Films
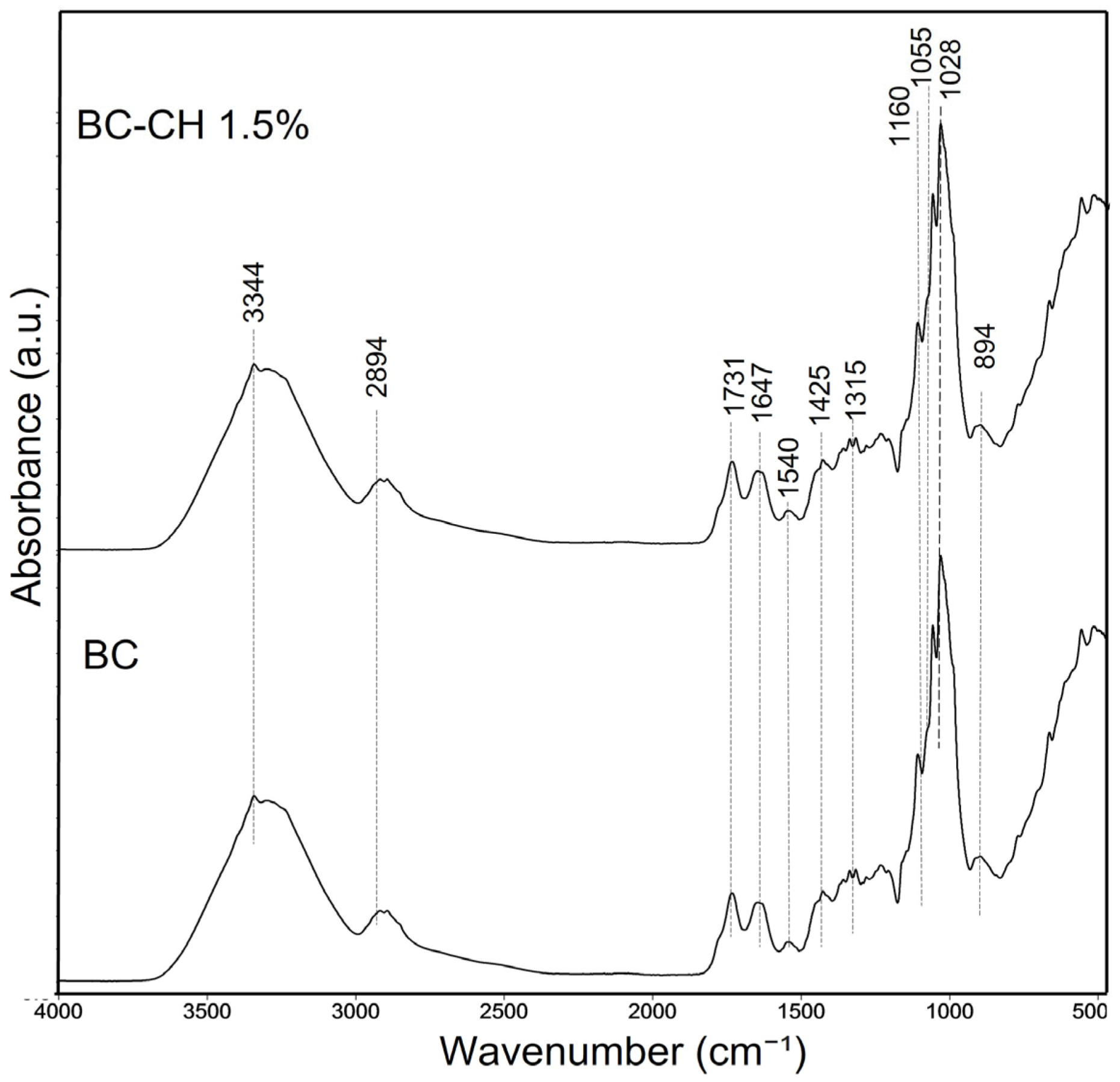
3.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.2. Thickness
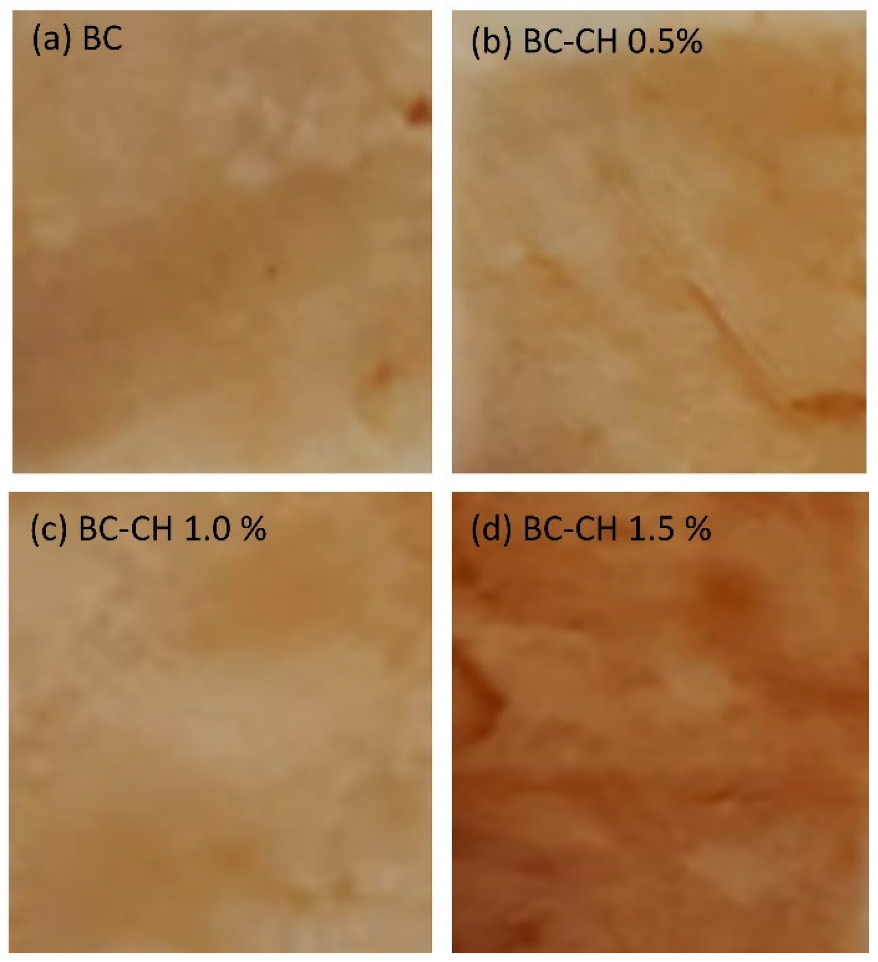
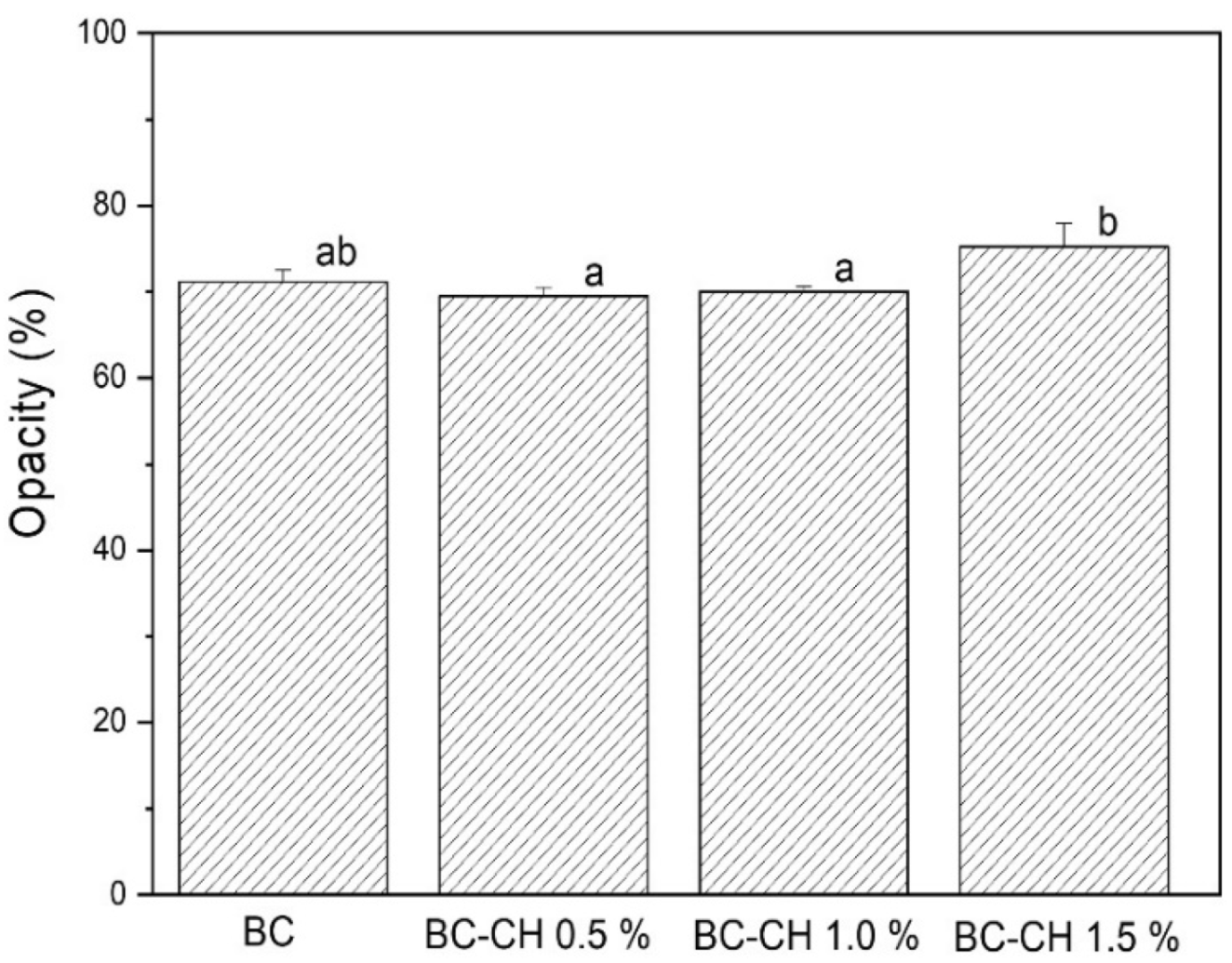
3.4.3. Color Properties and Opacity
3.4.4. UV Visible Spectroscopy
3.4.5. Solubility
3.5. Biological Activities
3.5.1. Antimicrobial Activity
3.5.2. Antioxidant Activity and Total Phenols Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bortolini, D.G.; Haminiuk, C.W.I.; Pedro, A.C.; Fernandes, I.d.A.A.F.; Maciel, G.M. Processing, chemical signature and food industry applications of Camellia sinensis teas: An overview. Food Chem. X 2021, 12, 100160. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; He, Y.; Xu, Y.; Li, P.; Zhang, J.; Zhao, J. Triterpenoid saponins in tea (Camellia sinensis) plants: Biosynthetic gene expression, content variations, chemical identification and cytotoxicity. Int. J. Food Sci. Nutr. 2021, 72, 308–323. [Google Scholar] [CrossRef]
- Pedro, A.C.; Maciel, G.M.; Rampazzo Ribeiro, V.; Haminiuk, C.W.I. Fundamental and applied aspects of catechins from different sources: A review. Int. J. Food Sci. 2019, 55, 429–442. [Google Scholar] [CrossRef]
- McNamara, J.T.; Morgan, J.L.W.; Zimmer, J. A molecular description of cellulose biosynthesis. Annu. Rev. Biochem. 2015, 84, 895–921. [Google Scholar] [CrossRef]
- Åkerholm, M.; Hinterstoisser, B.; Salmén, L. Characterization of the crystalline structure of cellulose using static and dynamic FT-IR spectroscopy. Carbohydr. Res. 2004, 339, 569–578. [Google Scholar] [CrossRef]
- Moniri, M.; Boroumand Moghaddam, A.; Azizi, S.; Abdul Rahim, R.; Bin Ariff, A.; Saad, W.Z.; Mohamad, R. Production and status of bacterial cellulose in biomedical engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Kadier, A.; Ilyas, R.A.; Huzaifah, M.R.M.; Harihastuti, N.; Sapuan, S.M.; Harussani, M.M.; Azlin, M.N.M.; Yuliasni, R.; Ibrahim, R.; Atikah, M.S.N.; et al. Use of industrial wastes as sustainable nutrient sources for bacterial cellulose (BC) production: Mechanism, advances, and future perspectives. Polymers 2021, 13, 3365. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef]
- Ojagh, S.M.A.; Vahabzadeh, F.; Karimi, A. Synthesis and characterization of bacterial cellulose-based composites for drug delivery. Carbohydr. Polym. 2021, 273, 118587. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose a masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Gautama, K.; Vishvakarma, R.; Sharma, P.; Singh, A.V.; Gaurad, V.K.; Varjani, S.; Srivastava, J.K. Production of biopolymers from food waste: Constrains and perspectives. Bioresour. Technol. 2022, 361, 127650. [Google Scholar] [CrossRef] [PubMed]
- Elsabeea, M.Z.; Abdo, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Negro, C.; Blanco, A. Chitosan grafted/cross-linked with biodegradable polymers: A review. Int. J. Biol. Macromol. 2021, 178, 325–343. [Google Scholar] [CrossRef]
- Treviño-Garza, M.; Guerrero-Medina, A.; González-Sánchez, R.; García-Gómez, C.; Guzmán-Velasco, A.; Báez-González, J.; Márquez-Reyes, J. Production of microbial cellulose films from green tea (Camellia Sinensis) kombucha with various carbon sources. Coatings 2020, 10, 1132. [Google Scholar] [CrossRef]
- Betlej, I.; Salerno-Kochan, R.; Krajewski, K.; Zawadzki, J.; Boruszewski, P. The influence of culture medium components on the physical and mechanical properties of cellulose synthesized by kombucha microorganisms. Bioresources 2020, 15, 3125–3135. [Google Scholar] [CrossRef]
- Amorim, L.F.A.; Fangueiro, R.; Gouveia, I.C. Characterization of bioactive colored materials produced from bacterial cellulose and bacterial pigments. Materials 2022, 15, 2069. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Jean, B. Nanocellulose/polymer multilayered thin films: Tunable architectures towards tailored physical properties. Nord. Pulp Pap. Res. J. 2014, 29, 19–30. [Google Scholar] [CrossRef]
- Tanjung, F.A.; Arifin, Y.; Abdullah, A.H.; Tahir, I. Bilayer-structured regenerated cellulose/chitosan films prepared with ionic liquid. Indones. J. Chem. 2017, 17, 351–359. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jokar, M.; Nafchia, A.M. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Tanpichai, S.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Chitosan coating for the preparation of multilayer coated paper for food-contact packaging: Wettability, mechanical properties, and overall migration. Int. J. Biol. Macromol. 2022, 213, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Orelma, H.; Hokkanen, A.; Leppänen, I.; Kammiovirta, K.; Kammiovirta, K.; Kapulainen, M.; Harlin, A. Optical cellulose fiber made from regenerated cellulose and cellulose acetate for water sensor applications. Cellulose 2020, 27, 1543–1553. [Google Scholar] [CrossRef]
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Innocentini, M.L.H. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Haghighi, H.; Gullo, M.; La China, S.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Characterization of bio-nanocomposite films based on gelatin/polyvinyl alcohol blend reinforced with bacterial cellulose nanowhiskers for food packaging applications. Food Hydrocoll. 2021, 113, 106454. [Google Scholar] [CrossRef]
- Treviño-Garza, M.Z.; Yañez-Echeverría, S.A.; García, S.; Mora-Zúñiga, A.E.; Arévalo-Niño, K. Physico-mechanical, barrier and antimicrobial properties of linseed mucilage films incorporated with H. virginiana extract. Rev. Mex. Ing. Quim. 2020, 19, 983–996. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Yen, H.Y.; Chen, G.C. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of antimicrobial and antioxidant gelatin/curcumin composite films for active food packaging application. Colloids Surf. B Biointerfaces 2020, 188, 110761. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.W.; Bagheri, R. Gelatin-based functional films integrated with grapefruit seed extract and TiO2 for active food packaging applications. Food Hydrocoll. 2021, 112, 106314. [Google Scholar] [CrossRef]
- Hwang, J.; Hae, S. Rheological properties of chitosan solutions. Korea Aust. Rheol. J. 2000, 12, 175–179. [Google Scholar]
- Abu-Jdayil, B.; Ghannam, M.; Alsayyed Ahmed, K.; Djama, M. The effect of biopolymer chitosan on the rheology and stability of Na-bentonite drilling mud. Polymers 2021, 13, 3361. [Google Scholar] [CrossRef]
- Jacek, P.; Kubiak, K.; Ryngajłło, M.; Rytczak, P.; Paluch, P.; Bielecki, S. Modification of bacterial nanocellulose properties through mutation of motility related genes in Komagataeibacter Hansenii ATCC 53582. New Biotechnol. 2019, 52, 60–68. [Google Scholar] [CrossRef]
- Lin, S.P.; Huang, Y.H.; Hsu, K.D.; Lai, Y.J.; Chen, Y.K.; Cheng, K.C. Isolation and identification of cellulose-producing strain Komagataeibacter intermedius from fermented fruit juice. Carbohydr. Polym. 2016, 20, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, K.; Jarosz, M.; Grudzień, J.; Pawlik, J.; Zastawnik, F.; Pandyra, P.; Kołodziejczyk, A. Hydrogel bacterial cellulose: A path to improved materials for new eco-friendly textiles. Cellulose 2020, 27, 5353–5365. [Google Scholar] [CrossRef]
- Wang, S.S.; Han, Y.H.; Ye, Y.X.; Shi, X.X.; Xiang, P.; Chen, D.L.; Li, M. Physicochemical characterization of high-quality bacterial cellulose produced by Komagataeibacter sp. strain w1 and identification of the associated genes in bacterial cellulose production. RSC Adv. 2017, 7, 45145–45155. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. IR study on cellulose with the varied moisture contents: Insight into the supramolecular structure. Materials 2020, 13, 4573. [Google Scholar] [CrossRef]
- Neera, K.; Ramana, V.; Batra, V.H. Occurrence of cellulose-producing Gluconacetobacter spp. in fruit samples and kombucha tea, and production of the biopolymer. Appl. Biochem. Biotechnol. 2015, 176, 1162–1173. [Google Scholar] [CrossRef]
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing cellulose (in) solubility: Reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 2012, 19, 581–587. [Google Scholar] [CrossRef]
- Khoshgozaran-Abras, S.; Azizi, M.H.; Hamidy, Z.; Bagheripoor-Fallah, N. Mechanical, physicochemical and color properties of chitosan based-films as a function of Aloe vera gel incorporation. Carbohydr. Polym. 2012, 87, 2058–2062. [Google Scholar] [CrossRef]
- Primiani, C.; Pujiati, N.; Mumtahanah, M.; Ardhi, W. Kombucha fermentation test used for various types of herbal teas. J. Phys. Conf. Ser. 2018, 1025, 012073. [Google Scholar] [CrossRef]
- Zubaidah, E.; Dewantari, F.J.; Novitasari, F.R.; Srianta, I.; Blanc, P.J. Potential of snake fruit (Salacca zalacca (Gaerth.) Voss) for the development of a beverage through fermentation with the Kombucha consortium. Biocatal. Agric. Biotechnol. 2018, 13, 198–203. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Health, Wellness, and safety aspects of the consumption of kombucha. J. Chem. 2015, 2015, 591869. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Bhattacharya, S.; Patra, M.M.; Chakravorty, S.; Sarkar, S.; Chakraborty, W.; Koley, H.; Gachhui, R. Antibacterial activity of polyphenolic fraction of kombucha against enteric bacterial pathogens. Curr. Microbiol. 2016, 73, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lim, L.; Lim, B.; Wum, G.; Tang, Q.; Ibrahim, M.; Li, H.; Xie, G.; Sun, G. Action of chitosan against Xanthomonas pathogenic bacteria isolated from Euphorbia pulcherrima. Molecules 2012, 17, 7028–7041. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, A.G.; de Menezes, J.L.; Dutra, T.V.; Ferreira, T.V.; Castro, J.C.; da Silva, C.A.J.; de Abreu Filho, B.A. Evaluation of antimicrobial activity of green tea kombucha at two fermentation time points against Alicyclobacillus spp. LWT 2020, 130, 109641. [Google Scholar] [CrossRef]
- Quihui-Cota, L.; Morales-Figueroa, G.G.; Valbuena-Gregorio, E.; Campos-García, J.C.; Silva-Beltrán, N.P.; López-Mata, M.A. Membrane of chitosan with essential oils of Romero and Tree of Tea: Potential as biomaterial. Rev. Mex. Ing. Biomed. 2017, 38, 255–264. [Google Scholar]





| Films Samples | Staphylococcus aureus Inhibition Zone (mm) | Escherichia coli Inhibition Zone (mm) |
|---|---|---|
| BC | 5.27 ± 0.16 a | 6.66 ± 0.72 a |
| BC-CH 0.5% | 6.35 ± 0.16 b | 7.78 ± 0.23 b |
| BC-CH 1.0% | 6.55 ± 0.06 c | 7.89 ± 0.17 b |
| BC-CH 1.5% | 6.31 ± 0.34 b | 8.25 ± 0.70 c |
| Films Samples | DPPH Radical Scavenging Activity (%) | ABTS Radical Scavenging Activity (%) | Total Phenolic Content (mg GAE/g) |
|---|---|---|---|
| BC | 13.56 ± 9.02 a | 3.90 ± 1.10 a | 0.64 ± 2.78 a |
| BC-CH 0.5% | 38.02 ± 7.5 c | 21.90 ± 1.43 c | 2.16 ± 0.15 b |
| BC-CH 1.0% | 57.71 ± 2.04 d | 24.57 ± 1.43 c | 2.45 ± 0.68 b |
| BC-CH 1.5% | 20.52 ± 0.22 b | 10.4 ± 0.90 b | 2.14 ± 0.68 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Reyes, J.M.; Rodríguez-Quiroz, R.E.; Hernández-Rodríguez, J.P.; Rodríguez-Romero, B.A.; Flores-Breceda, H.; Napoles-Armenta, J.; Romero-Soto, I.C.; Galindo-Rodríguez, S.A.; Báez-González, J.G.; Treviño-Garza, M.Z. Production and Characterization of Biocomposite Films of Bacterial Cellulose from Kombucha and Coated with Chitosan. Polymers 2022, 14, 3632. https://doi.org/10.3390/polym14173632
Márquez-Reyes JM, Rodríguez-Quiroz RE, Hernández-Rodríguez JP, Rodríguez-Romero BA, Flores-Breceda H, Napoles-Armenta J, Romero-Soto IC, Galindo-Rodríguez SA, Báez-González JG, Treviño-Garza MZ. Production and Characterization of Biocomposite Films of Bacterial Cellulose from Kombucha and Coated with Chitosan. Polymers. 2022; 14(17):3632. https://doi.org/10.3390/polym14173632
Chicago/Turabian StyleMárquez-Reyes, Julia M., Rubí E. Rodríguez-Quiroz, Juan P. Hernández-Rodríguez, Beatriz A. Rodríguez-Romero, Héctor Flores-Breceda, Juan Napoles-Armenta, Itzel C. Romero-Soto, Sergio A. Galindo-Rodríguez, Juan G. Báez-González, and Mayra Z. Treviño-Garza. 2022. "Production and Characterization of Biocomposite Films of Bacterial Cellulose from Kombucha and Coated with Chitosan" Polymers 14, no. 17: 3632. https://doi.org/10.3390/polym14173632







