Chitosan Edible Films and Coatings with Added Bioactive Compounds: Antibacterial and Antioxidant Properties and Their Application to Food Products: A Review
Abstract
:1. Introduction
2. Chitosan Edible Films with Added Antimicrobial Bioactive Compounds
| Bioactive Compound | Bioactive Molecules | Concentration | Antimicrobial Effect | Reference |
|---|---|---|---|---|
| Eucalyptus globulus EO | Monoterpenes and sesquiterpenes | 1.5% | Log reduction of 2.28–4.71 for Staphylococcus aureus; 1.85–4.55 for Bacillus cereus; 1.81–4.22 for Escherichia coli and 1.73–3.98 for Salmonella enteritis | [16] |
| Thymus Piperella or Thymus moroderi EOs | Monoterpenes and sesquiterpenes | 0.5, 1 and 2% | Inhibition halo of 13.50–19.50 mm for Serratia marcenscens; 12.00–19.50 mm for Aeromonas hydrophila; 13.50–25.00 mm for Alcaligenes faecalis; 12.00–19.00 mm for Listeria innocua and 20.00–30.00 mm for Achromobacter denitrificans | [17] |
| Thymus mastichina EO | Monoterpenes and sesquiterpenes | 1 and 2% | Inhibition halo of: 21.15–32.36 mm for S. marcescens; 17.92–21.51 mm for L. innocua; and 18.42–28.29 mm for A. faecalis | [18] |
| Hop extract | Polyphenolic compounds | 0.1, 0.5, 1.0 and 1.5% | Inhibition zones of 1.4–3.0 mm for Bacillus subtilis | [19] |
| Ellagic acid | Polyphenolic compounds | 0.5, 1.0, 2.5 and 5.0% | Total inhibition of S. aureus and Pseudomonas aeruginosa growth | [20] |
| Maqui Berry | Polyphenolic compounds | 0.5 and 1% | Inhibition halo of: 14.65–17.07 mm for S. marcescens; 16.82–18.59 mm for A. hydrophila; 21.43–22.94 mm for A. denitrificans; 20.05–21.03 mm for A. faecalis; 12.86–22.03 mm for Pseudomonas fluorescens; 17.10–18.67 mm for Citrobacter freundii and 14.32–21.89 mm for Shewanella putrefaciens | [9] |
| Tea extracts | Polyphenolic compounds | 0.1% | Inhibition halo of 3.7 and 4.8 mm for S. aureus and 4.8–9.3 mm for E. coli | [21] |
| Nisin | Bacteriocins | 2.5 μg/mL | Inhibition halo of 11.5–12.8 mm for Listeria monocytogenes; 10.8–13.3 mm for B. cereus; 12.5 mm for E. coli; 10.0–12.1 mm for S. aureus; 9.5–10.8 mm for Salmonella enteritidis and 10.6–11.5 mm for Clostridium perfringens | [22] |
| Nisin | Bacteriocins | Nisin 6000 I.U/mL | L. monocytogenes: average inhibition diameter of 15 mm | [23] |
2.1. Essential Oils (EOs) from Aromatic Plants with Antimicrobial Properties
2.2. Plants and Vegetal Extracts with Antimicrobial Properties
2.3. Bacteriocins with Antimicrobial Properties
3. Chitosan Edible Films with Added Antioxidant Bioactive Compounds
3.1. Essential Oils with Antioxidant Properties
3.2. Plants and Vegetal Extracts with Antioxidant Properties
4. Application of Chitosan Edible Films and Coatings in Foods
4.1. Meat Products
4.2. Fish and Seafood Products
4.3. Dairy Products
4.4. Fruits and Vegetables
5. Major Challenges and Future Perspectives of Bioactive Chitosan-Based Coatings and Films
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Reddy, C.S.K.; Ghai, R.; Rashmi; Kalia, V.C. Polyhydroxyalkanoates: An overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.L.C.D.; Rico, B.P.M.; Cruz, R.M.S.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Preparation and characterization of a chitosan film with grape seed extract-carvacrol microcapsules and its effect on the shelf-life of refrigerated Salmon (Salmo salar). LWT Food Sci. Technol. 2018, 89, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Chitosan nanocomposite coatings for food, paints, and water treatment applications. Appl. Sci. 2019, 9, 2409. [Google Scholar] [CrossRef] [Green Version]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.T.; De La Caba, K. Characterization and antimicrobial analysis of chitosan-based films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P.; Prinyawiwatkul, W.; Xu, Z. Applications of chitosan for improvement of quality and shelf life of foods: A review. J. Food Sci. 2007, 72, R87–R100. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernandez-Lopez, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis). LWT Food Sci. Technol. 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Hossain, F.; Follett, P.; Salmieri, S.; Vu, K.D.; Fraschini, C.; Lacroix, M. Antifungal activities of combined treatments of irradiation and essential oils (EOs) encapsulated chitosan nanocomposite films in in vitro and in situ conditions. Int. J. Food Microbiol. 2019, 295, 33–40. [Google Scholar] [CrossRef]
- Hossain, I.S.; Sportelli, M.C.; Picca, R.A.; Gentile, L.; Palazzo, G.; Ditaranto, N.; Cioffi, N. Green Synthesis and Characterization of Antimicrobial Synergistic AgCl/BAC Nanocolloids. ACS Appl. Bio Mater. 2022, 5, 3230–3240. [Google Scholar]
- Ali, S.G.; Jalal, M.; Ahmad, H.; Sharma, D.; Ahmad, A.; Umar, K.; Khan, H.M. Green synthesis of silver nanoparticles from Camellia sinensis and Its antimicrobial and effect against clinical Isolates. Materials 2022, 15, 6978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wei, F.; Xu, W.; Han, X. Enhanced antibacterial performance of gelatin/chitosan film containing capsaicin loaded MOFs for food packaging. Appl. Surf. Sci. 2020, 510, 145418. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Azadbakht, E.; Maghsoudlou, Y.; Khomiri, M.; Kashiri, M. Development and structural characterization of chitosan films containing Eucalyptus globulus essential oil: Potential as an antimicrobial carrier for packaging of sliced sausage. Food Packag. Shelf Life 2018, 17, 65–72. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Viuda-Martos, M. Evaluation of the antibacterial and antioxidant activities of chitosan edible films incorporated with organic essential oils obtained from four Thymus species. J. Food Sci. Technol. 2016, 53, 3374–3379. [Google Scholar] [CrossRef] [Green Version]
- Bajić, M.; Jalšovec, H.; Travan, A.; Novak, U.; Likozar, B. Chitosan-based films with incorporated supercritical CO2 hop extract: Structural, physicochemical, and antibacterial properties. Carbohydr. Polym. 2019, 219, 261–268. [Google Scholar] [CrossRef]
- Vilela, C.; Pinto, R.J.B.; Coelho, J.; Domingues, M.R.M.; Daina, S.; Sadocco, P.; Santos, S.A.O.; Freire, C.S.R. Bioactive chitosan/ellagic acid films with UV-light protection for active food packaging. Food Hydrocoll. 2017, 73, 120–128. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. Antioxidant and antibacterial chitosan film with tea polyphenols-mediated green synthesis silver nanoparticle via a novel one-pot method. Int. J. Biol. Macromol. 2020, 155, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Cé, N.; Noreña, C.P.Z.; Brandelli, A. Antimicrobial activity of chitosan films containing nisin, peptide P34 and natamycin. CYTA J. Food 2012, 10, 21–26. [Google Scholar] [CrossRef]
- Zimet, P.; Mombrú, Á.W.; Mombrú, D.; Castro, A.; Villanueva, J.P.; Pardo, H.; Rufo, C. Physico-chemical and antilisterial properties of nisin-incorporated chitosan/carboxymethyl chitosan films. Carbohydr. Polym. 2019, 219, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Anis, A.; Pal, K.; Al-Zahrani, S.M. Essential oil-containing polysaccharide-based edible films and coatings for food security applications. Polymers 2021, 13, 575. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Palou, E.; Jiménez Munguía, M.T.; Nevárez-Moorillón, G.V.; Navarro Cruz, A.R.; López-Malo, A. Antifungal activity by vapor contact of essential oils added to amaranth, chitosan, or starch edible films. Int. J. Food Microbiol. 2012, 153, 66–72. [Google Scholar] [CrossRef]
- Raphaël, K.J.; Meimandipour, A. Antimicrobial activity of chitosan film forming solution enriched with essential oils; an in vitro assay. Iran. J. Biotechnol. 2017, 15, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Souza, V.G.L.; Rodrigues, C.; Ferreira, L.; Pires, J.R.A.; Duarte, M.P.; Coelhoso, I.; Fernando, A.L. In vitro bioactivity of novel chitosan bionanocomposites incorporated with different essential oils. Ind. Crops Prod. 2019, 140, 111563. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Wu, T.; Yuan, C.; Hu, Y. Antioxidant and antibacterial properties of coating with chitosan–citrus essential oil and effect on the quality of pacific mackerel during chilled storage. Food Sci. Nutr. 2019, 7, 1131–1143. [Google Scholar] [CrossRef] [Green Version]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of novel active packaging films based on whey protein isolate incorporated with chitosan nanofiber and nano-formulated cinnamon oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef]
- Odjo, K.; Al-Maqtari, Q.A.; Yu, H.; Xie, Y.; Guo, Y.; Li, M.; Du, Y.; Liu, K.; Chen, Y.; Yao, W. Preparation and characterization of chitosan-based antimicrobial films containing encapsulated lemon essential oil by ionic gelation and cranberry juice. Food Chem. 2022, 397, 133781. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Rawtani, D.; Rao, P.K. Antibacterial activity of chitosan film containing Syzygium aromaticum (clove) oil encapsulated halloysite nanotubes against foodborne pathogenic bacterial strains. Mater. Today Commun. 2022, 32, 104132. [Google Scholar] [CrossRef]
- Khan, U.A.; Rahman, H.; Niaz, Z.; Qasim, M.; Khan, J.; Bushra Rehman, T. Antibacterial activity of some medicinal plants against selected human pathogenic bacteria. Eur. J. Microbiol. Immunol. 2013, 3, 272–274. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lian, H.; Shi, J.; Meng, W.; Peng, Y. Plant extracts such as pine nut shell, peanut shell and jujube leaf improved the antioxidant ability and gas permeability of chitosan films. Int. J. Biol. Macromol. 2020, 148, 1242–1250. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Kabir, F.; Katayama, S.; Tanji, N.; Nakamura, S. Antimicrobial effects of chlorogenic acid and related compounds. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 359–365. [Google Scholar] [CrossRef]
- Kaya, M.; Khadem, S.; Cakmak, Y.S.; Mujtaba, M.; Ilk, S.; Akyuz, L.; Salaberria, A.M.; Labidi, J.; Abdulqadir, A.H.; Deligöz, E. Antioxidative and antimicrobial edible chitosan films blended with stem, leaf and seed extracts of Pistacia terebinthus for active food packaging. RSC Adv. 2018, 8, 3941–3950. [Google Scholar] [CrossRef] [Green Version]
- Ortiz de Elguea-Culebras, G.; Bourbon, A.I.; Costa, M.J.; Muñoz-Tebar, N.; Carmona, M.; Molina, A.; Sánchez-Vioque, R.; Berruga, M.I.; Vicente, A.A. Optimization of a chitosan solution as potential carrier for the incorporation of Santolina chamaecyparissus L. solid by-product in an edible vegetal coating on ‘Manchego’ cheese. Food Hydrocoll. 2019, 89, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Liu, Y.; Yuan, L.; Yong, H.; Liu, J. Preparation and characterization of antioxidant, antimicrobial and pH-sensitive films based on chitosan, silver nanoparticles and purple corn extract. Food Hydrocoll. 2019, 96, 102–111. [Google Scholar] [CrossRef]
- Koosha, M.; Hamedi, S. Intelligent Chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog. Org. Coatings 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Yong, H.; Qin, Y.; Liu, J.; Jin, C. Development of antioxidant and antimicrobial packaging films based on chitosan and mangosteen (Garcinia mangostana L.) rind powder. Int. J. Biol. Macromol. 2020, 145, 1129–1139. [Google Scholar] [CrossRef]
- Nadira, P.P.; Mujeeb, V.M.A.; Rahman, P.M.; Muraleedharan, K. Effects of cashew leaf extract on physicochemical, antioxidant, and antimicrobial properties of N, O–Carboxymethyl chitosan films. Carbohydr. Polym. Technol. Appl. 2022, 3, 100191. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Le, T.Q.; Nguyen, T.T.; Nguyen, L.T.M.; Nguyen, D.T.C.; Tran, T.V. Characterizations and antibacterial activities of passion fruit peel pectin/chitosan composite films incorporated Piper betle L. leaf extract for preservation of purple eggplants. Heliyon 2022, 8, e10096. [Google Scholar] [CrossRef]
- Nes, I.F.; Brede, D.A.; Diep, D.B. Class II Non-Lantibiotic Bacteriocins, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780123850959. [Google Scholar]
- Settanni, L.; Corsetti, A. Application of bacteriocins in vegetable food biopreservation. Int. J. Food Microbiol. 2008, 121, 123–138. [Google Scholar] [CrossRef]
- Mills, S.; Serrano, L.M.; Griffin, C.; Connor, P.M.O.; Schaad, G.; Bruining, C.; Hill, C.; Ross, R.P.; Meijer, W.C. Inhibitory activity of Lactobacillus plantarum LMG-26358 against Listeria innocua when used as an adjunct starter in the manufacture of cheese. Microb. Cell Factories 2011, 10, S7. [Google Scholar] [CrossRef] [Green Version]
- Pereira de Abreu, D.A.; Cruz, J.M.; Paseiro Losada, P. Active and intelligent packaging for the food industry. Food Rev. Int. 2012, 28, 146–187. [Google Scholar] [CrossRef]
- Preciado, G.M.; Michel, M.M.; Villarreal-Morales, S.L.; Flores-Gallegos, A.C.; Aguirre-Joya, J.; Morlett-Chávez, J.; Aguilar, C.N.; Rodríguez-Herrera, R. Bacteriocins and its use for multidrug-resistant bacteria control. In Antibiotic Resistence: Mechanisms and New Antimicrobial Approaches; Kon, K., Rai, M., Eds.; Academic Press: London, UK, 2016; pp. 329–349. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a food preservative: Part 1: Physicochemical properties, antimicrobial activity, and main uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef]
- Pranoto, Y.; Rakshit, S.K.; Salokhe, V.M. Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT Food Sci. Technol. 2005, 38, 859–865. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, T.; Wu, C.; Fu, S.; Yuan, C.; Chen, S. Formation and optimization of chitosan-nisin microcapsules and its characterization for antibacterial activity. Food Control 2017, 72, 43–52. [Google Scholar] [CrossRef]
- Divsalar, E.; Tajik, H.; Moradi, M.; Forough, M.; Lotfi, M.; Kuswandi, B. Characterization of cellulosic paper coated with chitosan-zinc oxide nanocomposite containing nisin and its application in packaging of UF cheese. Int. J. Biol. Macromol. 2018, 109, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Liu, D.; Zhang, C.; Yi, H.; Liu, D. Preparation, characterization, and application of edible antibacterial three-layer films based on gelatin–chitosan–corn starch–incorporated nisin. Food Packag. Shelf Life 2022, 34, 100980. [Google Scholar] [CrossRef]
- Saad, B.; Sing, Y.Y.; Nawi, M.A.; Hashim, N.H.; Mohamed Ali, A.S.; Saleh, M.I.; Sulaiman, S.F.; Talib, K.M.; Ahmad, K. Determination of synthetic phenolic antioxidants in food items using reversed-phase HPLC. Food Chem. 2007, 105, 389–394. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Cadet, J.; Wagner, J.R. Oxidatively generated base damage to cellular DNA by hydroxyl radical and one-electron oxidants: Similarities and differences. Arch. Biochem. Biophys. 2014, 557, 47–54. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Omoni, A.O.; Aluko, R.E. The anti-carcinogenic and anti-atherogenic effects of lycopene: A review. Trends Food Sci. Technol. 2005, 16, 344–350. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Effects of chitosan coating on shelf-life of ready-to-cook meat products during chilled storage. LWT Food Sci. Technol. 2013, 53, 321–326. [Google Scholar] [CrossRef]
- Hafsa, J.; Smach, M.A.; Ben Khedher, M.R.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Poloni, T.; Lourenço, R.V.; Sobral, P.J.A. Antioxidant potential of eugenol and ginger essential oils with gelatin/chitosan films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J.A. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Zheng, K.; Li, W.; Fu, B.; Fu, M.; Ren, Q.; Yang, F.; Qin, C. Physical, antibacterial and antioxidant properties of chitosan films containing hardleaf oatchestnut starch and Litsea cubeba oil. Int. J. Biol. Macromol. 2018, 118, 707–715. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E. Essential oils from herbs and spices as natural antioxidants: Diversity of promising food applications in the past decade. Food Rev. Int. 2021, 38, 403–433. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Graça Miguel, M. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Himani, K.; Mahawer, S.K.; Arya, S.; Kumar, R.; Prakash, O. Essential Oil: Source of antioxidants and role in food preservation. In Essential Oils Applications and Trends in Food Science and Technology; Santana de Oliveira, M., Ed.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2022; pp. 173–189. [Google Scholar]
- Go, E.J.; Song, K.B. Effect of java citronella essential oil addition on the physicochemical properties of Gelidium corneum-chitosan composite films. Food Sci. Biotechnol. 2020, 29, 909–915. [Google Scholar] [CrossRef]
- Tügen, A.; Ocak, B.; Özdestan-Ocak, Ö. Development of gelatin/chitosan film incorporated with lemon essential oil with antioxidant properties. J. Food Meas. Charact. 2020, 14, 3010–3019. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Azizi, M.H. Nanoencapsulation approach to improve antimicrobial and antioxidant activity of thyme essential oil in beef burgers during refrigerated storage. Food Bioprocess Technol. 2016, 9, 1187–1201. [Google Scholar] [CrossRef]
- Su, H.; Huang, C.; Liu, Y.; Kong, S.; Wang, J.; Huang, H.; Zhang, B. Preparation and characterization of cinnamomum essential oil-chitosan nanocomposites: Physical, structural, and antioxidant activities. Processes 2020, 8, 834. [Google Scholar] [CrossRef]
- Cazón, P.; Antoniewska, A.; Rutkowska, J.; Vázquez, M. Evaluation of easy-removing antioxidant films of chitosan with Melaleuca alternifolia essential oil. Int. J. Biol. Macromol. 2021, 186, 365–376. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Y. Physicochemical and antioxidant properties based on fish sarcoplasmic protein/chitosan composite films containing ginger essential oil nanoemulsion. Food Bioprocess Technol. 2021, 14, 151–163. [Google Scholar] [CrossRef]
- Roshandel-Hesari, N.; Mokaber-Esfahani, M.; Taleghani, A.; Akbari, R. Investigation of physicochemical properties, antimicrobial and antioxidant activity of edible films based on chitosan/casein containing Origanum vulgare L. essential oil and its effect on quality maintenance of cherry tomato. Food Chem. 2022, 396, 133650. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Pabón-Baquero, L.C.; Otálvaro-Álvarez, Á.M.; Fernández, M.R.R.; Chaparro-González, M.P. Plant Extracts as antioxidant additives for food industry. In Antioxidants in Foods and Its Applications; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Pateiro, M.; Gómez-Salazar, J.A.; Jaime-Patlán, M.; Sosa-Morales, M.E.; Lorenzo, J.M. Plant extracts obtained with green solvents as natural antioxidants in fresh meat products. Antioxidants 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Characterization of chitosan film incorporated with curcumin extract. Polymers 2021, 13, 963. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Petkoska, A.T.; Khojah, E.; Sami, R.; Al-Mushhin., A.A. Chitosan edible films enhanced with pomegranate peel extract: Study on physical, biological, thermal, and barrier properties. Materials 2021, 14, 3305. [Google Scholar] [CrossRef]
- Moalla, S.; Ammar, I.; Fauconnier, M.L.; Danthine, S.; Blecker, C.; Besbes, S.; Attia, H. Development and characterization of chitosan films carrying Artemisia campestris antioxidants for potential use as active food packaging materials. Int. J. Biol. Macromol. 2021, 183, 254–266. [Google Scholar] [CrossRef]
- Kahya, N.; Kestir, S.M.; Öztürk, S.; Yolaç, A.; Torlak, E.; Kalaycıoğlu, Z.; Akın-Evingür, G.; Erim, F.B. Antioxidant and antimicrobial chitosan films enriched with aqueous sage and rosemary extracts as food coating materials: Characterization of the films and detection of rosmarinic acid release. Int. J. Biol. Macromol. 2022, 217, 470–480. [Google Scholar] [CrossRef]
- Ma, J.; Ye, G.; Jia, S.; Ma, H.; Jia, D.; He, J.; Lv, J.; Chen, X.; Liu, F.; Gou, K.; et al. Preparation of chitosan/peony (Paeonia suffruticosa Andr.) leaf extract composite film and its application in sustainable active food packaging. Int. J. Biol. Macromol. 2022, 222, 2200–2211. [Google Scholar] [CrossRef]
- Alamed, J.; Chaiyasit, W.; McClements, D.J.; Decker, E.A. Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 2009, 57, 2969–2976. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar] [CrossRef] [Green Version]
- Quesada, J.; Sendra, E.; Navarro, C.; Sayas-Barberá, E. Antimicrobial active packaging including chitosan films with Thymus vulgaris L. essential oil for ready-to-eat meat. Foods 2016, 5, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xia, Y.; Zhang, P.; Ye, L.; Wu, L.; He, S. Physical characterization and pork packaging application of chitosan films incorporated with combined essential oils of cinnamon and ginger. Food Bioprocess Technol. 2017, 10, 503–511. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H.; Gardini, F. Chitosan-cinnamon essential oil nano-formulation: Application as a novel additive for controlled release and shelf life extension of beef patties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Karimnezhad, F.; Razavilar, V.; Anvar, A.A.; Eskandari, S. Study the antimicrobial effects of chitosan-based edible film containing the Trachyspermum ammi essential oil on shelf-life of chicken meat. Microbiol. Res. 2017, 8, 7226. [Google Scholar] [CrossRef] [Green Version]
- Serrano-León, J.S.; Bergamaschi, K.B.; Yoshida, C.M.P.; Saldaña, E.; Selani, M.M.; Rios-Mera, J.D.; Alencar, S.M.; Contreras-Castillo, C.J. Chitosan active films containing agro-industrial residue extracts for shelf life extension of chicken restructured product. Food Res. Int. 2018, 108, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jokar, M.; Mohammadi Nafchi, A. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef]
- Mahdavi, V.; Hosseini, S.E.; Sharifan, A. Effect of edible chitosan film enriched with anise (Pimpinella anisum L.) essential oil on shelf life and quality of the chicken burger. Food Sci. Nutr. 2018, 6, 269–279. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Kang, H. Chitosan coatings incorporated with free or nano-encapsulated Paulownia Tomentosa essential oil to improve shelf-life of ready-to-cook pork chops. LWT Food Sci. Technol. 2019, 116, 108580. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Effect of chitosan-gelatin coating containing nano-encapsulated tarragon essential oil on the preservation of pork slices. Meat Sci. 2020, 166, 108137. [Google Scholar] [CrossRef] [PubMed]
- Mojaddar Langroodi, A.; Nematollahi, A.; Sayadi, M. Chitosan coating incorporated with grape seed extract and Origanum vulgare essential oil: An active packaging for turkey meat preservation. J. Food Meas. Charact. 2021, 15, 2790–2804. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Ayaseh, A.; Alirezalu, K.; Nemati, Z.; Pateiro, M.; Lorenzo, J.M. Effect of chitosan coating incorporated with Artemisia fragrans essential oil on fresh chicken meat during refrigerated storage. Polymers 2021, 13, 716. [Google Scholar] [CrossRef]
- Gaba, A.B.M.; Hassan, M.A.; Abd El-Tawab, A.A.; Abdelmonem, M.A.; Morsy, M.K. Protective impact of chitosan film loaded oregano and thyme essential oil on the microbial profile and quality attributes of beef meat. Antibiotics 2022, 11, 583. [Google Scholar] [CrossRef]
- Pérez-Córdoba, L.J.; Pinheiro, A.C.; de Núñez de Villavicencio-Ferrer, M.; Trindade, M.A.; Sobral, P.J.A. Applying gelatine:chitosan film loaded with nanoemulsified garlic essential oil/α-tocopherol as active packaging of sliced omega-3-rich mortadella. Int. J. Food Sci. Technol. 2022, 57, 6378–6388. [Google Scholar] [CrossRef]
- Hussain, M.A.; Sumon, T.A.; Mazumder, S.K.; Ali, M.M.; Jang, W.J.; Abualreesh, M.H.; Sharifuzzaman, S.M.; Brown, C.L.; Lee, H.T.; Lee, E.W.; et al. Essential oils and chitosan as alternatives to chemical preservatives for fish and fisheries products: A review. Food Control 2021, 129, 108244. [Google Scholar] [CrossRef]
- Losada, V.; Barros-Velázquez, J.; Aubourg, S.P. Rancidity development in frozen pelagic fish: Influence of slurry ice as preliminary chilling treatment. LWT 2007, 40, 991–999. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Natural preservatives for extending the shelf-life of seafood: A Revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Regenstein, J.M.; Xia, W. Bio-based edible coatings for the preservation of fishery products: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2481–2493. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of pacific white shrimp during iced storage. Food Control 2016, 59, 818–823. [Google Scholar] [CrossRef]
- Kakaei, S.; Shahbazi, Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil on survival of Listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT Food Sci. Technol. 2016, 72, 432–438. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of fungal chitosan incorporated with pomegranate peel extract as edible coating for microbiological, chemical and sensorial quality enhancement of nile tilapia fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Sun, J.; Liu, D.; Fu, M.; Yang, X.; Guo, Y. The preservative effects of chitosan film incorporated with thinned young apple polyphenols on the quality of grass carp (Ctenopharyngodon idellus) fillets during cold storage: Correlation between the preservative effects and the active properties of the film. Food Packag. Shelf Life 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Ramírez-Guerra, H.E.; Castillo-Yañez, F.J.; Montaño-Cota, E.A.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Canizales-Rodríguez, D.F.; Torres-Arreola, W.; Montoya-Camacho, N.; Ocaño-Higuera, V.M. Protective effect of an edible tomato plant extract/chitosan coating on the quality and shelf life of sierra fish fillets. J. Chem. 2018, 2018, 2436045. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Leng, L.; Cao, A.; Cheng, X.; Li, J. The effect of chitosan-essential oils complex coating on physicochemical, microbiological, and quality change of grass carp (Ctenopharyhgodon idella) fillets. J. Food Saf. 2018, 38, e12399. [Google Scholar] [CrossRef]
- Ebadi, Z.; Khodanazary, A.; Hosseini, S.M.; Zanguee, N. The shelf life extension of refrigerated Nemipterus japonicus fillets by chitosan coating incorporated with propolis extract. Int. J. Biol. Macromol. 2019, 139, 94–102. [Google Scholar] [CrossRef]
- Merlo, T.C.; Contreras-Castillo, C.J.; Saldaña, E.; Barancelli, G.V.; Dargelio, M.D.B.; Yoshida, C.M.P.; Ribeiro Junior, E.E.; Massarioli, A.; Venturini, A.C. Incorporation of pink pepper residue extract into chitosan film combined with a modified atmosphere packaging: Effects on the shelf life of salmon fillets. Food Res. Int. 2019, 125, 108633. [Google Scholar] [CrossRef]
- Vieira, B.B.; Mafra, J.F.; da Rocha Bispo, A.S.; Ferreira, M.A.; de Lima Silva, F.; Rodrigues, A.V.N.; Evangelista-Barreto, N.S. Combination of chitosan coating and clove essential oil reduces lipid oxidation and microbial growth in frozen stored tambaqui (Colossoma macropomum) fillets. LWT 2019, 116, 108546. [Google Scholar] [CrossRef]
- Rezaeifar, M.; Mehdizadeh, T.; Mojaddar Langroodi, A.; Rezaei, F. Effect of chitosan edible coating enriched with lemon verbena extract and essential oil on the shelf life of vacuum rainbow trout (Oncorhynchus mykiss). J. Food Saf. 2020, 40, e12781. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Afshari, A.; Aminzare, M.; Raeisi, M.; Zeinali, T. Effect of different types of active biodegradable films containing lactoperoxidase system or sage essential oil on the shelf life of fish burger during refrigerated storage. LWT Food Sci. Technol. 2020, 117, 108633. [Google Scholar] [CrossRef]
- Demircan, B.; Özdestan-Ocak, Ö. Effects of lemon essential oil and ethyl lauroyl arginate on the physico-chemical and mechanical properties of chitosan films for mackerel fillet coating application. J. Food Meas. Charact. 2021, 15, 1499–1508. [Google Scholar] [CrossRef]
- Gasti, T.; Dixit, S.; D’souza, O.J.; Hiremani, V.D.; Vootla, S.K.; Masti, S.P.; Chougale, R.B.; Malabadi, R.B. Smart biodegradable films based on chitosan/methylcellulose containing Phyllanthus reticulatus anthocyanin for monitoring the freshness of fish fillet. Int. J. Biol. Macromol. 2021, 187, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.; Khoshkhoo, Z.; Hosseini, S.E.; Basti, A.A.Z.; Azizi, M.H. Chitosan nano-coating incorporated with green cumin (Cuminum cyminum) extracts: An active packaging for rainbow trout (Oncorhynchus mykiss) preservation. J. Food Meas. Charact. 2022, 16, 1228–1240. [Google Scholar] [CrossRef]
- Surendhiran, D.; Roy, V.; Park, J.S.; Chun, B.S. Fabrication of chitosan-based food packaging film impregnated with turmeric essential oil (TEO)-loaded magnetic-silica nanocomposites for surimi preservation. Int. J. Biol. Macromol. 2022, 203, 650–660. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Salehabadi, A.; Mohammadi Nafchi, A.; Oladzadabbasabadi, N.; Mahdi Jafari, S. Cheese packaging by edible coatings and biodegradable nanocomposites; improvement in shelf life, physicochemical and sensory properties. Trends Food Sci. Technol. 2021, 116, 218–231. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2022, 20, 875–900. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Nazari, M.; Davudian, S.H.; Roufegarinejad, L. Application of reinforced ZnO nanoparticle-incorporated gelatin bionanocomposite film with chitosan nanofiber for pckaging of chicken fillet and cheese as food models. Food Bioprocess Technol. 2019, 12, 1205–1219. [Google Scholar] [CrossRef]
- Vinod, A.; Sanjay, M.R.; Siengchin, S.; Parameswaranpillai, J. Renewable and sustainable biobased materials:An Assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Cano Embuena, A.I.; Cháfer Nácher, M.; Chiralt Boix, A.; Molina Pons, M.P.; Borrás Llopis, M.; Beltran Martínez, M.C.; González Martínez, C. Quality of goat’s milk cheese as affected by coating with edible chitosan-essential oil films. Int. J. Dairy Technol. 2017, 70, 68–76. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J.A. Gelatin-chitosan edible film activated with Boldo extract for improving microbiological and antioxidant stability of sliced Prato cheese. Int. J. Food Sci. Technol. 2019, 54, 1617–1624. [Google Scholar] [CrossRef]
- Hani Tabaie Zavareh, S.A.; Ardestani, F. Antibacterial effects of chitosan coating containing Mentha aquatica L. essence against Escherichia coli, Staphylococcus aureus and Listeria monocytogenes in Iranian white cheese. Int. J. Dairy Technol. 2020, 73, 585–593. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; El-Sayed, H.S.; Ibrahim, O.A.; Youssef, A.M. Rational design of chitosan/guar gum/zinc oxide bionanocomposites based on roselle calyx extract for Ras cheese coating. Carbohydr. Polym. 2020, 239, 116234. [Google Scholar] [CrossRef]
- Ríos-De-Benito, L.F.; Escamilla-García, M.; García-Almendárez, B.; Amaro-Reyes, A.; Di Pierro, P.; Regalado-González, C. Design of an active edible coating based on sodium caseinate, chitosan and oregano essential oil reinforced with silica particles and its application on panela cheese. Coatings 2021, 11, 1212. [Google Scholar] [CrossRef]
- Hassan, A.H.A.; Korany, A.M.; Zeinhom, M.M.A.; Mohamed, D.S.; Abdel-Atty, N.S. Effect of chitosan-gelatin coating fortified with papaya leaves and thyme extract on quality and shelf life of chicken breast fillet and Kareish cheese during chilled storage. Int. J. Food Microbiol. 2022, 371, 109667. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and incorporation of functional ingredients in edible films and coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Olivas, G.I.I.; Barbosa-Cánovas, G. Edible films and coatings for fruits and vegetables. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 211–244. [Google Scholar]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Improving the shelf-life stability of apple and strawberry fruits applying chitosan-incorporated olive oil processing residues coating. Food Packag. Shelf Life 2016, 9, 10–19. [Google Scholar] [CrossRef]
- Araújo, J.M.S.; de Siqueira, A.C.P.; Blank, A.F.; Narain, N.; de Aquino Santana, L.C.L. A Cassava Starch–chitosan edible coating enriched with Lippia sidoides Cham. essential oil and pomegranate peel extract for preservation of italian tomatoes (Lycopersicon esculentum Mill.) stored at room temperature. Food Bioprocess Technol. 2018, 11, 1750–1760. [Google Scholar] [CrossRef]
- Mannozzi, C.; Tylewicz, U.; Chinnici, F.; Siroli, L.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Effects of chitosan based coatings enriched with procyanidin by-product on quality of fresh blueberries during storage. Food Chem. 2018, 251, 18–24. [Google Scholar] [CrossRef]
- Zam, W. Effect of Alginate and chitosan edible coating enriched with olive leaves extract on the shelf life of sweet cherries (Prunus avium L.). J. Food Qual. 2019, 2019, 8192964. [Google Scholar] [CrossRef] [Green Version]
- Karagöz, Ş.; Demirdöven, A. Effect of chitosan coatings with and without Stevia rebaudiana and modified atmosphere packaging on quality of cold stored fresh-cut apples. LWT Food Sci. Technol. 2019, 108, 332–337. [Google Scholar] [CrossRef]
- Chen, C.; Nie, Z.; Wan, C.; Chen, J. Preservation of Xinyu tangerines with an edible coating using Ficus hirta Vahl. fruits extract-incorporated chitosan. Biomolecules 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Saucedo, A.; Barrera-Necha, L.L.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Hernández-López, M. Extension of the postharvest quality of bell pepper by applying nanostructured coatings of chitosan with Byrsonima crassifolia extract (L.) Kunth. Postharvest Biol. Technol. 2019, 149, 74–82. [Google Scholar] [CrossRef]
- Eldib, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Helal, M. Chitosan, nisin, silicon dioxide nanoparticles coating films effects on blueberry (Vaccinium myrtillus) quality. Coatings 2020, 10, 962. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef]
- Yang, C.; Lu, J.H.; Xu, M.T.; Shi, X.C.; Song, Z.W.; Chen, T.M.; Herrera-Balandrano, D.D.; Zhang, Y.J.; Laborda, P.; Shahriar, M.; et al. Evaluation of chitosan coatings enriched with turmeric and green tea extracts on postharvest preservation of strawberries. LWT Food Sci. Technol. 2022, 163, 113551. [Google Scholar] [CrossRef]
- Sarengaowa; Wang, L.; Liu, Y.; Yang, C.; Feng, K.; Hu, W. Screening of essential oils and effect of a chitosan-based edible coating containing cinnamon oil on the quality and microbial safety of fresh-cut potatoes. Coatings 2022, 12, 1492. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan composites in packaging industry-Current trends and future challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef] [PubMed]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- EL Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga, O.; Rorrer, G.L. Phosphate addition strategies for enhancing the co-production of lipid and chitin nanofibers during fed-batch cultivation of the diatom Cyclotella sp. Algal Res. 2019, 38, 101403. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2012, 33, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.R.V.; Alves, V.D.; Coelhoso, I.M. Polysaccharide-based membranes in food packaging applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvis-Sánchez, A.C.; Castro, M.C.R.; Biernacki, K.; Gonçalves, M.P.; Souza, H.K.S. Natural deep eutectic solvents as green plasticizers for chitosan thermoplastic production with controlled/desired mechanical and barrier properties. Food Hydrocoll. 2018, 82, 478–489. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ariffin, F.; Wijekoon, M.M.J.O.; Al-Hassan, A.A.; Dheyab, M.A.; Ghasemlou, M. Recent advances in extraction, modification, and application of chitosan in packaging industry. Carbohydr. Polym. 2022, 277, 118876. [Google Scholar] [CrossRef]

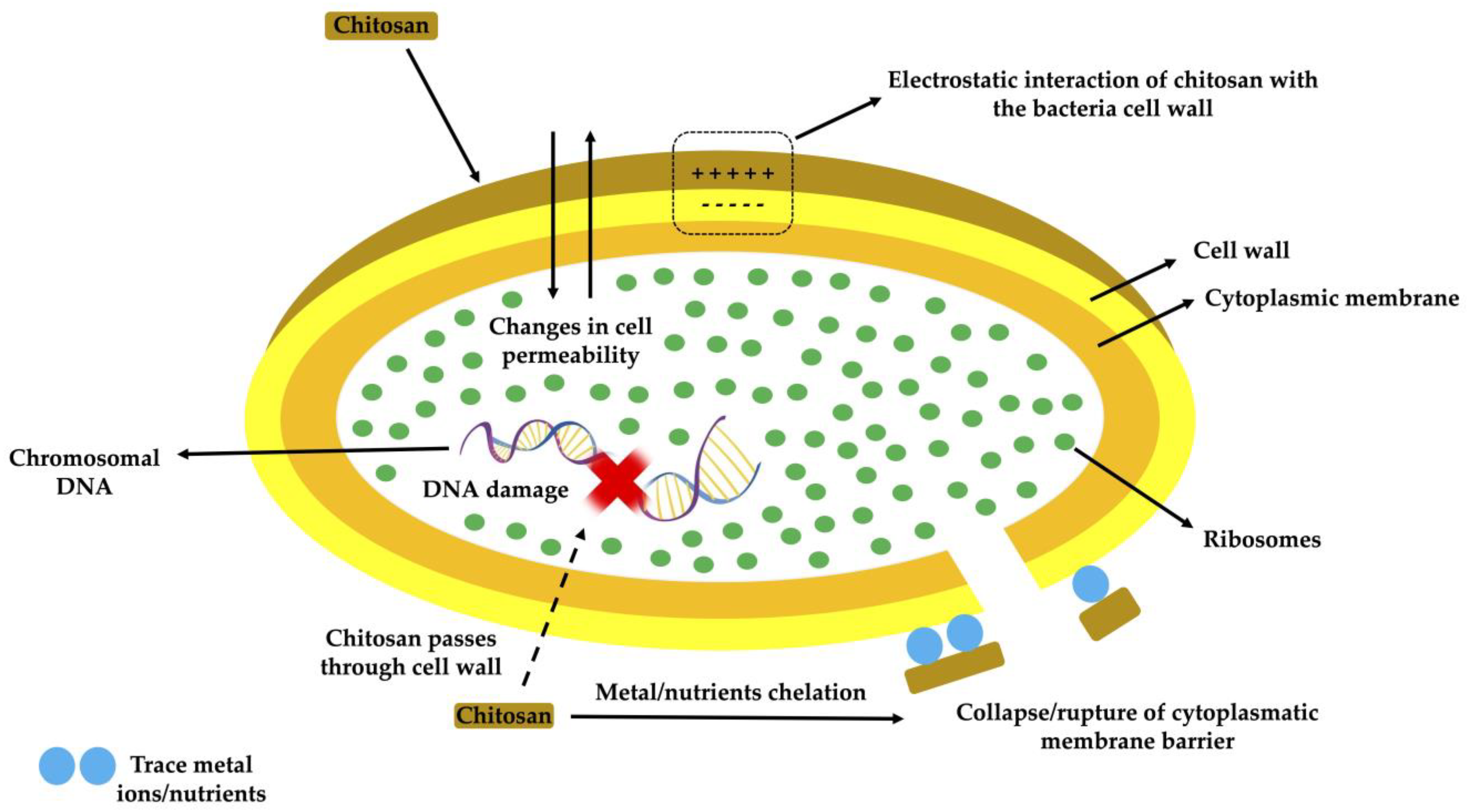
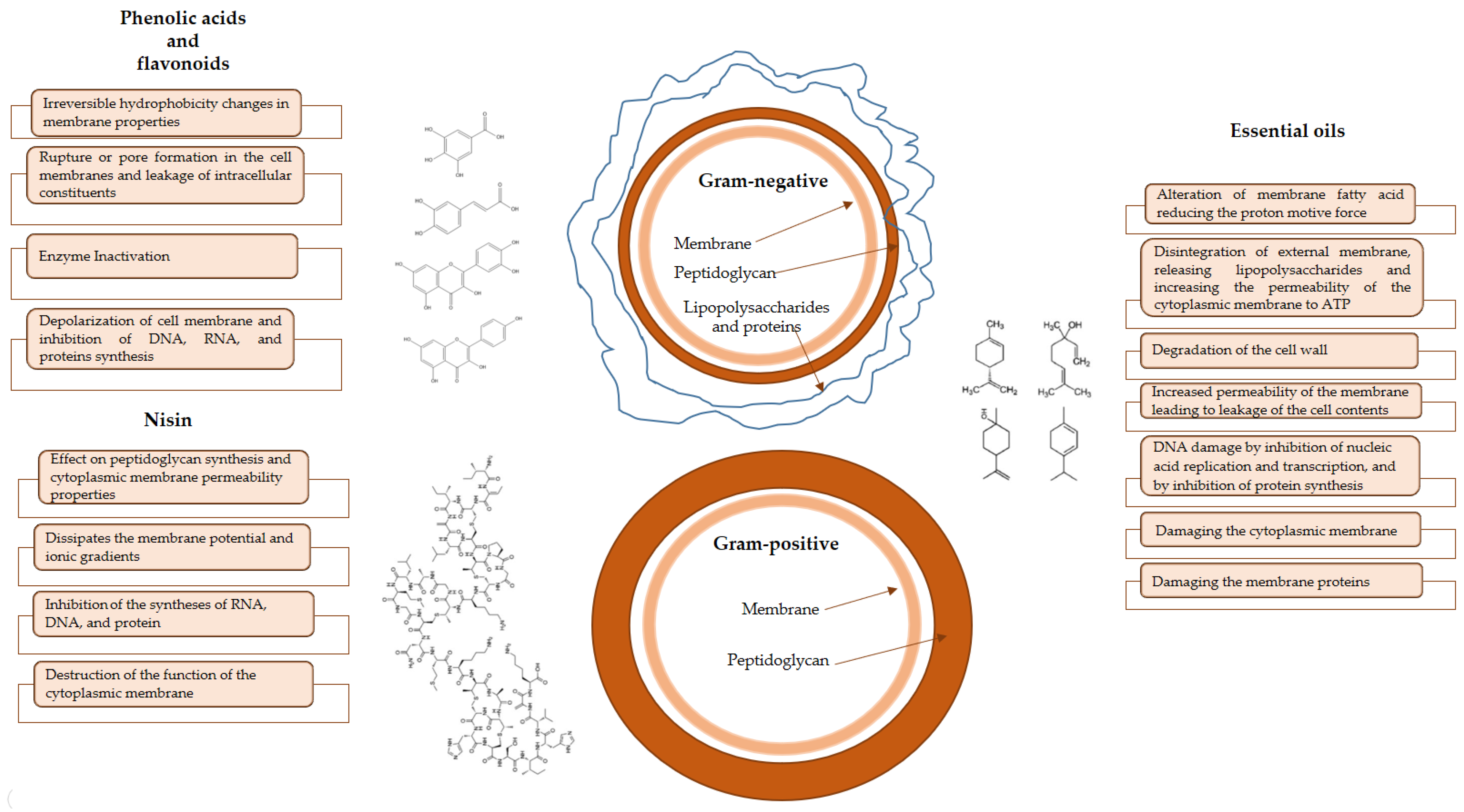

| Bioactive Compound | Bioactive Molecules | Concentration | Antioxidant Effect | Reference |
|---|---|---|---|---|
| Eucalyptus globulus EO | Monoterpenes and sesquiterpenes | 1, 2, 3, and 4% v/v | DPPH scavenging: 23.03–43.62% Nitric oxide radical scavenging: 35.23–70.47% H2O2 radical scavenging: 27.4-63.15% | [62] |
| Thymus EOs | Monoterpenes and sesquiterpenes | 1 and 2% | DPPH increased from 0.01 to 0.31–0.55 mg Trolox Equivalent (TE)/g film Ferric reducing antioxidant power (FRAP) increased from 0.08 to 1.98–4.70 mg TE/g film | [18] |
| Ziziphora clinopodioides EO | Monoterpenes and sesquiterpenes | 1% v/w | DPPH scavenging activity: ca. 29% | [63] |
| Ginger EO | Monoterpenes and sesquiterpenes | 0.5 g/g biopolymer | Trolox-equivalent-antioxidant capacity (TEAC): 3.3 mM | [64] |
| Cinnamon, guarana, rosemary, and boldo-do-chile ethanolic extracts | Polyphenolic compounds | 1% | TEAC cinnamon: 0.31–0.32 mg/L TEAC guarana: 1.5–1.6 mg/L TEAC rosemary: 0.27–9 mg/L TEAC boldo-do-chile: 1.5–1.8 mg/L | [65] |
| Propolis | Polyphenolic Compounds, vitamins, and minerals | 2.5, 5, 10, and 20% w/w | DPPH scavenging activity: ca. 29–65% | [66] |
| Ethanolic grape extract | Polyphenolic compounds | 1% | DPPH scavenging activity: ca. 25% | [63] |
| Hardleaf oatchestnut starch | Polyphenolic compounds | 0.5, 2, and 8 g/100 mL | DPPH radical scavenging: 88.45% | [67] |
| Methanol extracts of stem, leaf, and seed from Pistacia terebinthus | Polyphenolic compounds | 1 g | DPPH radical scavenging: 37.21–95.91% | [39] |
| Mango leaf extract | Polyphenolic compounds and carotenoids | 1, 3, and 5 wt% | DPPH radical scavenging: 87.16% FRAP: 3.47 μg ABTS: 8.29 μg GA at 5% of extract | [68] |
| Extract Added | Product | Concentration | Bioactive Molecule | Storage Temperature | Effect | Reference |
|---|---|---|---|---|---|---|
| Thyme EO | Cooked ham | 0.0%, 0.5%, 1.0%, and 2.0% | Monoterpenes and sesquiterpenes | 3 °C for 6 days | Yeast counts significantly decreased. | [96] |
| Cinnamon or ginger EOs | Lean pork slices | 0.05, 0.20, and 1.00% | Monoterpenes and sesquiterpenes | 4 °C for 9 days | Chitosan films inhibited the growth of total microbes and significantly reduced TBARs values at 9 days storage. | [97] |
| Cinnamon EO | Beef patties | Chitosan Cinnamon EO ratio 1:0.8 | Monoterpenes and sesquiterpenes | 4 °C for 8 days | Enterobacteriaceae counts were 3.23 and 3.86 log CFU/g lower than control. | [98] |
| Trachyspermum ammi EO | Chicken fillets | 1.0 and 2.0% w/w | Monoterpenes and sesquiterpenes | 4 °C for 12 days | Reduction in aerobic plate count (from 8.32 to 4.56–4.74 log CFU/g), total psychrophilic count, TPC (from 8.65 to 4.54–4.73 log CFU/g), and coliforms (from 5.62 to 2.14–2.24 CFU/g) at the end of storage | [99] |
| Peanut skin and pink pepper residue extracts | Restructured chicken product | 0.84% and 1.90% v/v | Polyphenolic compounds | 3 °C for 7 days | Films without extracts had a PV value 49% higher. Decreased TBARs values by 50 % (peanut skin) and 64% (pepper residue) compared to control and reduced microbial growth at the end of storage. | [100] |
| Satureja khuzestanica Jamzad EO | Lamb meat | 1.0% (v/v) | Monoterpenes and sesquiterpenes | 4 °C for 20 days | Shelf-life extension of 10 days by reduction of microbial growth and TBARS scores below 2.5 mg/kg until day 20 of storage. | [101] |
| Kombucha tea extract | Minced beef meat | 1.0, 2.0, and 3.0% (w/w) | Polyphenolic compounds | 4 °C for 15 days | Extended the shelf life by a reduction in Staphylococcus counts from 5.36 to 2.11 log CFU/g in 4 days storage and lower TBARs values than control. | [102] |
| Anise EO | Chicken burger | 0.5, 1.0, 1.5, and 2.0% (v/v) | Monoterpenes and sesquiterpenes | 4 °C for 12 days | Chitosan active films extended shelf life of chicken burgers by reducing TVC, S. aureus, TPC, and P. aeruginosa values and lipid oxidation with lower levels of TBARs values. | [103] |
| Rosemary EO | Fresh poultry meat | 0.5%, 1.0%, and 2.0% (v/v) | Monoterpenes and sesquiterpenes | 5 °C for 12 days | Improved shelf life by reducing TBARs values from 2.03 to 0.24–0.28 mg MDA/g meat, total mesophilic aerobic bacteria from 10.1 to 8.0–8.1 log CFU/ g meat, and total coliforms from 5.6 to 3.0–4.1 log most probable number (/g meat. | [104] |
| Paulownia tomentosa EOs | Ready to cook pork chops | Chitosan:EO of 1:1 (w/w) | Monoterpenes and sesquiterpenes | 4 °C for 16 days | Films kept TVC below the microbiological thresholds after 16 days of storage, decreased LAB counts by 2.15–2.61 log CFU/g, and inhibit lipid oxidation, maintaining TBARs values below 1.25 mg MDA/kg meat at the end of storage. | [105] |
| Extract Added | Product | Concentration | Bioactive Molecule | Storage Temperature | Effect | Reference |
|---|---|---|---|---|---|---|
| Pomegranate peel extract (PPE) | Pacific white shrimp | 1.5% | Polyphenolic compounds | Ice storage for 10 days | The increase in TPC TVB-N values over 10 days of storage was significantly reduced in shrimp treated with the chitosan+PPE coating | [116] |
| Ethanolic red grape seed extract and Ziziphora clinopodioides EO | Minced trout fillet | 1.0 and 2.0% | Polyphenolic compounds and monoterpenes and sesquiterpenes | 4 °C for 11 days | Enterobacteriaceae final population decreased by approximately 1–3 log CFU/g compared to untreated fish samples, and films reduced final LAB count (~2–3 log CFU/g) and L. monocytogenes growth | [117] |
| Pomegranate peel extract | Nile tilapia fillets | 0.5, 1.0, 1.5 and 2.0% | Polyphenolic compounds | 4 °C for 30 days | Reduction in the increase in Enterobacteriaceae, Salmonella, E. coli, yeast-molds, and S. aureus over the 30 days of storage. PV values were reduced from 8.35 to 1.17 mEq O2/lipid and TBARs from 0.32 to 0.21 mg MDA/kg at the end of storage. | [118] |
| Apple extract | Grass carp fillets | 0.25, 0.50, 0.7, and 1.0% (w/w) | Polyphenolic compounds | 4 °C for 15 days | Films reduced PV and TBARs values as well as showing an inhibition of the increase in TVB-N values. | [119] |
| Tomato plant ethanolic extract | Sierra fish fillets | 0.3% (v/v) | Polyphenolic compounds and carotenoids | Ice storage for 15 days | Treatments were capable of delaying the mesophyll counts from 6.98 to 4.81 log CFU/g. | [120] |
| Lemon or thyme EOs | Grass carp fillets | 0.25 and 0.5% | Monoterpenes and sesquiterpenes | 2 °C for 16 days | Coatings delayed the increase in TBARs and showed a reduction in TVC from 8.18 to 4.85-5.25 log CFU/g, Pseudomonas (7.45 to 5.46-5.67 log CFU/g), Shewanella putrefaciensa (from 4.53 to 3.29–3.47 log CFU/g), and Enterobacteria (4.25 vs. 3.17–3.39 log CFU/g). | [121] |
| Propolis extract | Nemipterus japonicus fillets | 0.1% | Polyphenolic compounds, vitamins, and minerals | 4 °C for 12 days | Treatments led to a reduction in total mesophilic count (TMC) and total psychrotrophilic count (TPC), and samples showed no increase in the TBARs values during 12 days of storage. | [122] |
| Citrus EO | Pacific mackerel | 1.5% (w/v) | Monoterpenes and sesquiterpenes | −3 °C for 12 days | Coatings were able to significantly reduce biogenic amine, TBARs and TVC values. | [30] |
| Pink pepper residue extract | Salmon fillets | 0.6% (v/v) | Polyphenolic compounds | 2 °C for 28 days | Coated samples displayed a reduction in total psychotropic viable count (TPVC) from 7.32 to 5.26 log CFU/g and LAB from 7.05 to 6.28 log CFU/g as well as reduced TBARs values at 28 days of storage. | [123] |
| Clove EO | Frozen tambaqui fillets | 0.08% and 0.16% | Monoterpenes and sesquiterpenes | −18 °C for 120 days | Fillets coated showed the lowest TBARS values almost every time (mean value of 0.61 mg MDA eq/kg). Although consumer panelists were not accustomed to the taste of clove EO in fish fillets, they still found them acceptable. | [124] |
| Extract added | Product | Bioactive Molecule | Concentration | Effect | Reference |
|---|---|---|---|---|---|
| Rosemary and oregano EOs | Goat cheese | Monoterpenes and sesquiterpenes | Chitosan:EO ratio of 1:0.5 | Coatings delayed or inhibited Penicillium and Mucor growth and prevent weight loss. | [135] |
| Boldo extract | Prato cheese | Polyphenolic compounds | 1% (v/v) | Films exerted protection against oxidation compared to uncoated samples and did not allow psychrotrophic microorganism growth. | [136] |
| Ethanolic extract from Santolina chamaecyparissus | Manchego cheese | Polyphenolic compounds | 1% (w/v) | Coated cheese showed just a few fungal colonies with the untreated samples the most affected by fungal contamination. | [40] |
| Mentha aquatica L. EO | Iranian white cheese | Monoterpenes and sesquiterpenes | 0.5, 1.0 and 1.5% (v/v) | The coating provided a reduction in S. aureus (from 4.80 to 0.55 log CFU/g), L. monocytogenes (5.89 vs. 0.33 log CFU/g), and E. coli was detected with the highest EO concentration. | [137] |
| Extract Added | Product | Bioactive Molecule | Concentration | Effect | Reference |
|---|---|---|---|---|---|
| Olive leaf and olive pomace extracts | Apple and strawberry | Polyphenolic compounds | 10 and 20 g/L | The lowest affected areas by the growth of Penicillium expansum and Rhizopus stolonifera were 7.33 and 8.00 mm in coated apple and strawberry with CH-OLE 20 g/L, respectively. | [143] |
| Lippia sidoides Cham. essential oil and pomegranate peel extract | Italian tomatoes | Polyphenolic compounds, monoterpenes, and sesquiterpenes | EO: 2.5, 5 and 10 mL/L PPE: 5, 10 and 20 mL/L | Coatings delayed the ripening by lowering weight loss and maintaining constant firmness compared to uncoated samples at 12 days of storage. | [144] |
| Procyanidins extracted from grape seeds | Fresh blueberries | Polyphenolic compounds | 0.8% (w/w) | Maintained the overall quality of fresh blueberries during 14 days of storage at 4 °C. | [145] |
| Olive leaves extract | Sweet cherries | Polyphenolic compounds | 1% | Weight loss was significant, and the highest antioxidant activity was recorded lower in samples treated with the chitosan coatings. | [146] |
| Stevia rebaudiana extract | Fresh-cut apple slices | Polyphenolic compounds | 2.5% | Samples coated with the films containing the extract showed higher polyphenoloxidase (PPO) activity and antioxidant capacity values (12.02 μmol TE/g). | [147] |
| Ficus hirta Vahl. fruits extract | Xinyu Tangerines | Polyphenolic compounds | 6.25 g | Edible coating reduced weight loss, respiration rate, and malondialdehyde (MDA) content during storage, and activities of superoxide dismutase (SOD), peroxidase (POD), and phenylalanine ammonia-lyase (PAL) were higher in coated samples. | [148] |
| Byrsonima Crassifolia extract | Bell pepper | Polyphenolic compounds | 2.5 and 5.0% | Edible coatings reduced the microbiological activity by 85% after 21 days of storage and increased secondary metabolites. | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Tebar, N.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Chitosan Edible Films and Coatings with Added Bioactive Compounds: Antibacterial and Antioxidant Properties and Their Application to Food Products: A Review. Polymers 2023, 15, 396. https://doi.org/10.3390/polym15020396
Muñoz-Tebar N, Pérez-Álvarez JA, Fernández-López J, Viuda-Martos M. Chitosan Edible Films and Coatings with Added Bioactive Compounds: Antibacterial and Antioxidant Properties and Their Application to Food Products: A Review. Polymers. 2023; 15(2):396. https://doi.org/10.3390/polym15020396
Chicago/Turabian StyleMuñoz-Tebar, Nuria, José A. Pérez-Álvarez, Juana Fernández-López, and Manuel Viuda-Martos. 2023. "Chitosan Edible Films and Coatings with Added Bioactive Compounds: Antibacterial and Antioxidant Properties and Their Application to Food Products: A Review" Polymers 15, no. 2: 396. https://doi.org/10.3390/polym15020396







