Preparation and Evaluation of Amorphous Solid Dispersions for Enhancing Luteolin’s Solubility in Simulated Saliva
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermophysical Characterization of LUT
2.2.1. Glass Forming Ability (GFA)
2.2.2. Thermal Stability
2.3. Selection of ASD Matrix/Carrier
2.3.1. Film Casting Method
2.3.2. Solvent Shift Measurements
2.4. Miscibility Evaluation
2.4.1. Estimation of the Flory–Huggins (FH) Interaction Parameter
2.4.2. Miscibility Evaluation via Solubility Parameter (δ)
2.4.3. Miscibility Evaluation via Differential Scanning Calorimetry (DSC)
2.5. Preparation of LUT-Loaded ASDs
2.6. Evaluation of LUT-Loaded ASDs
2.6.1. Physical State
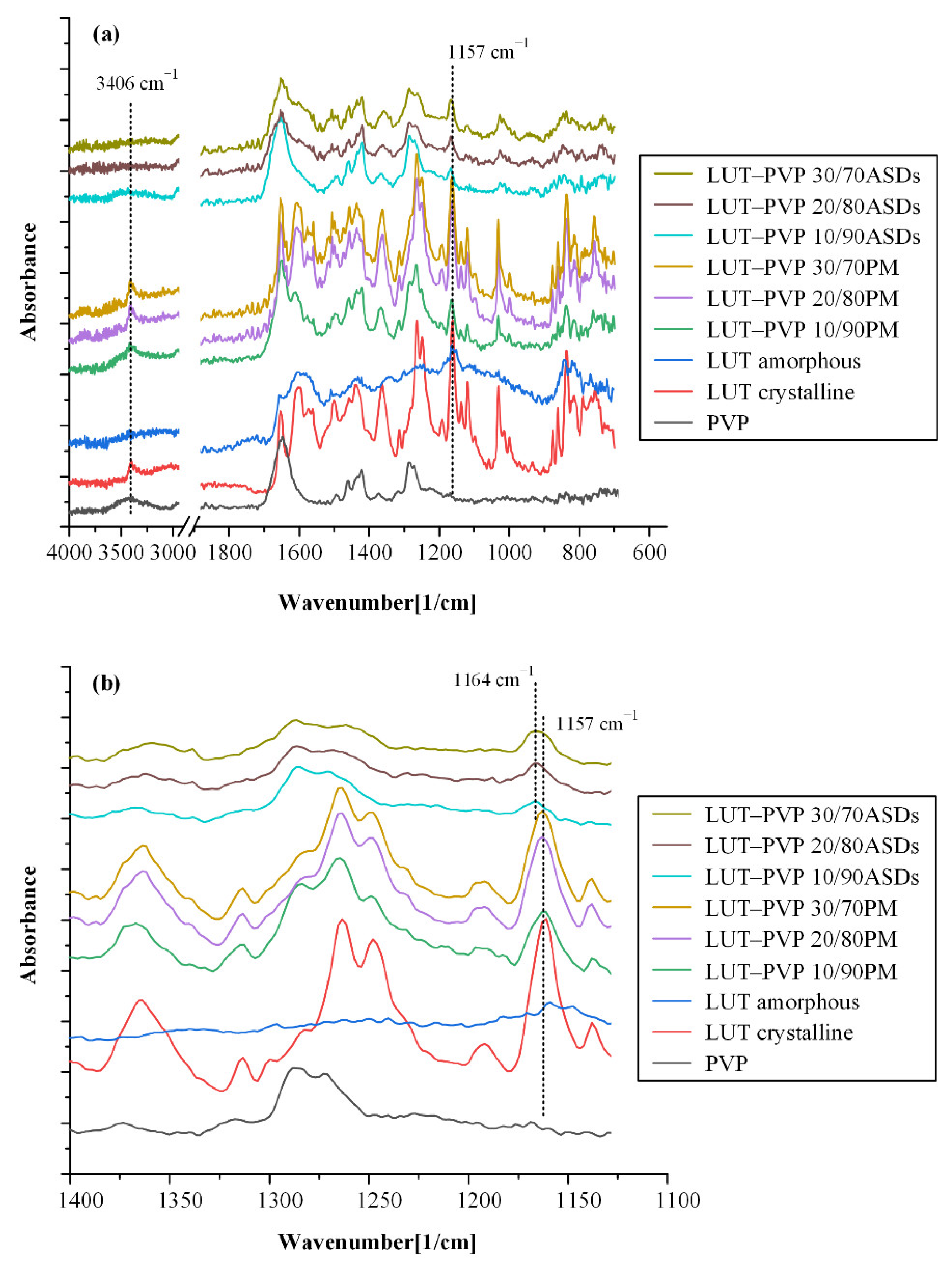
2.6.2. Molecular Interactions
ATR-FTIR Spectroscopy
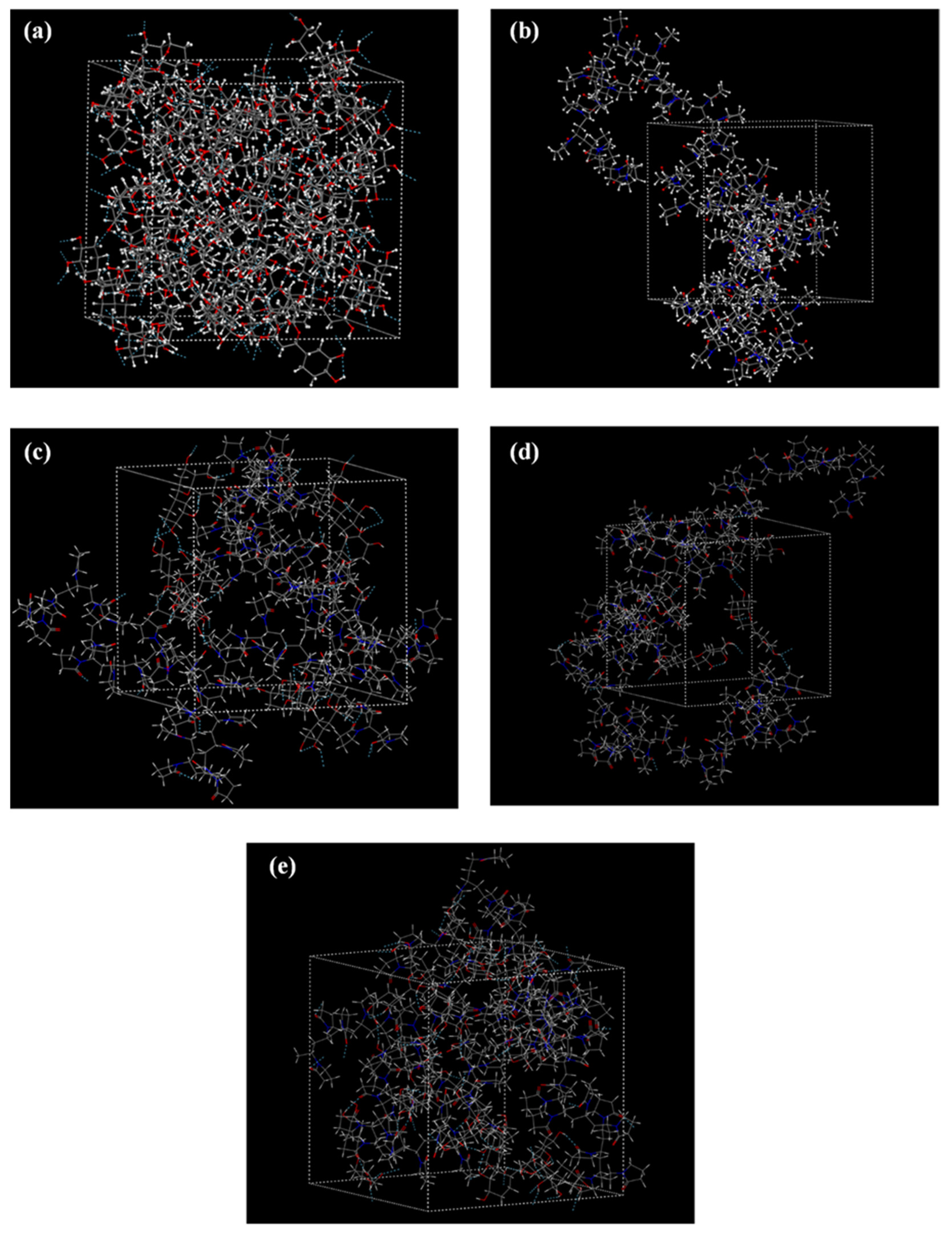
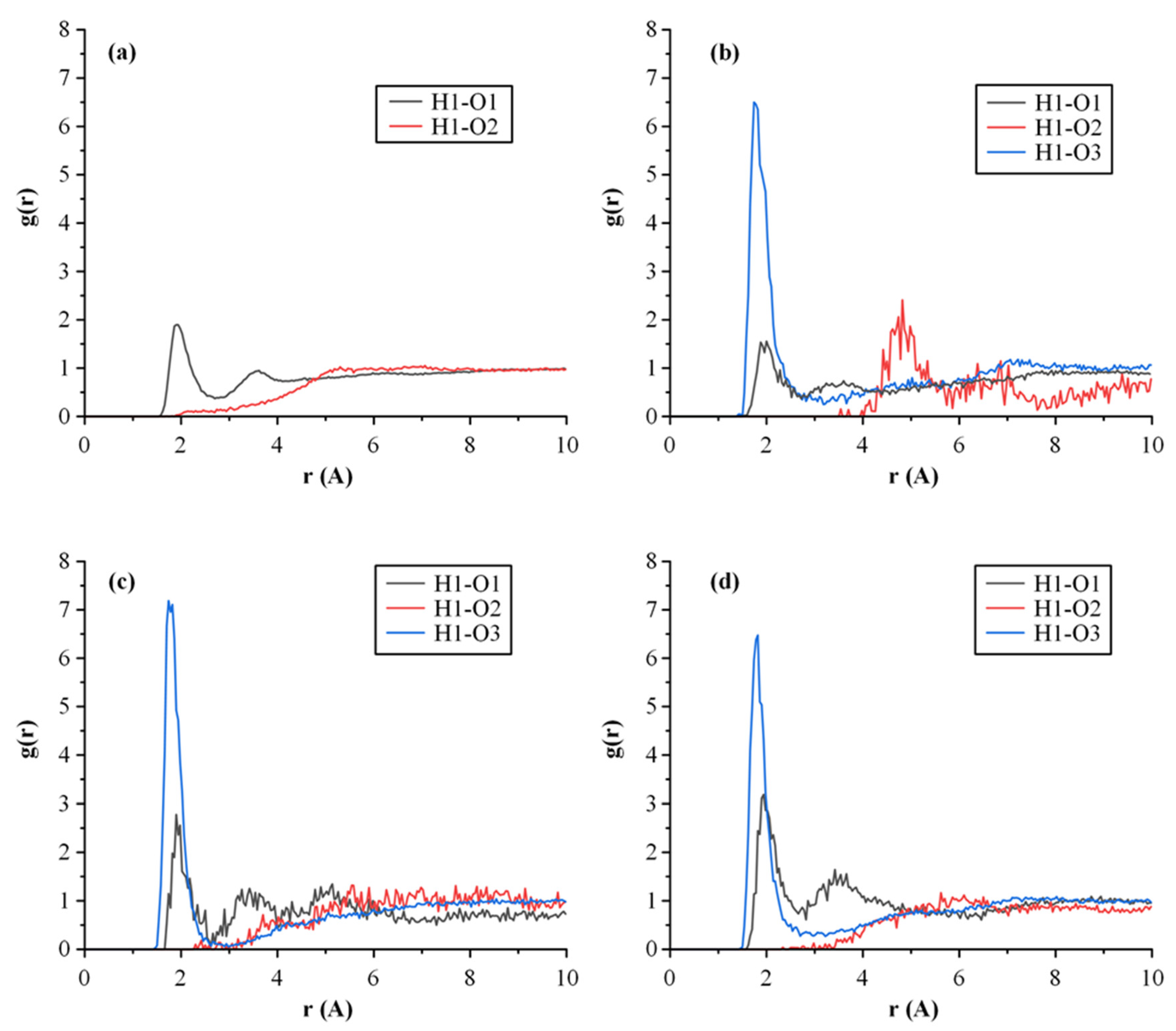
Molecular Dynamics (MD) Simulations
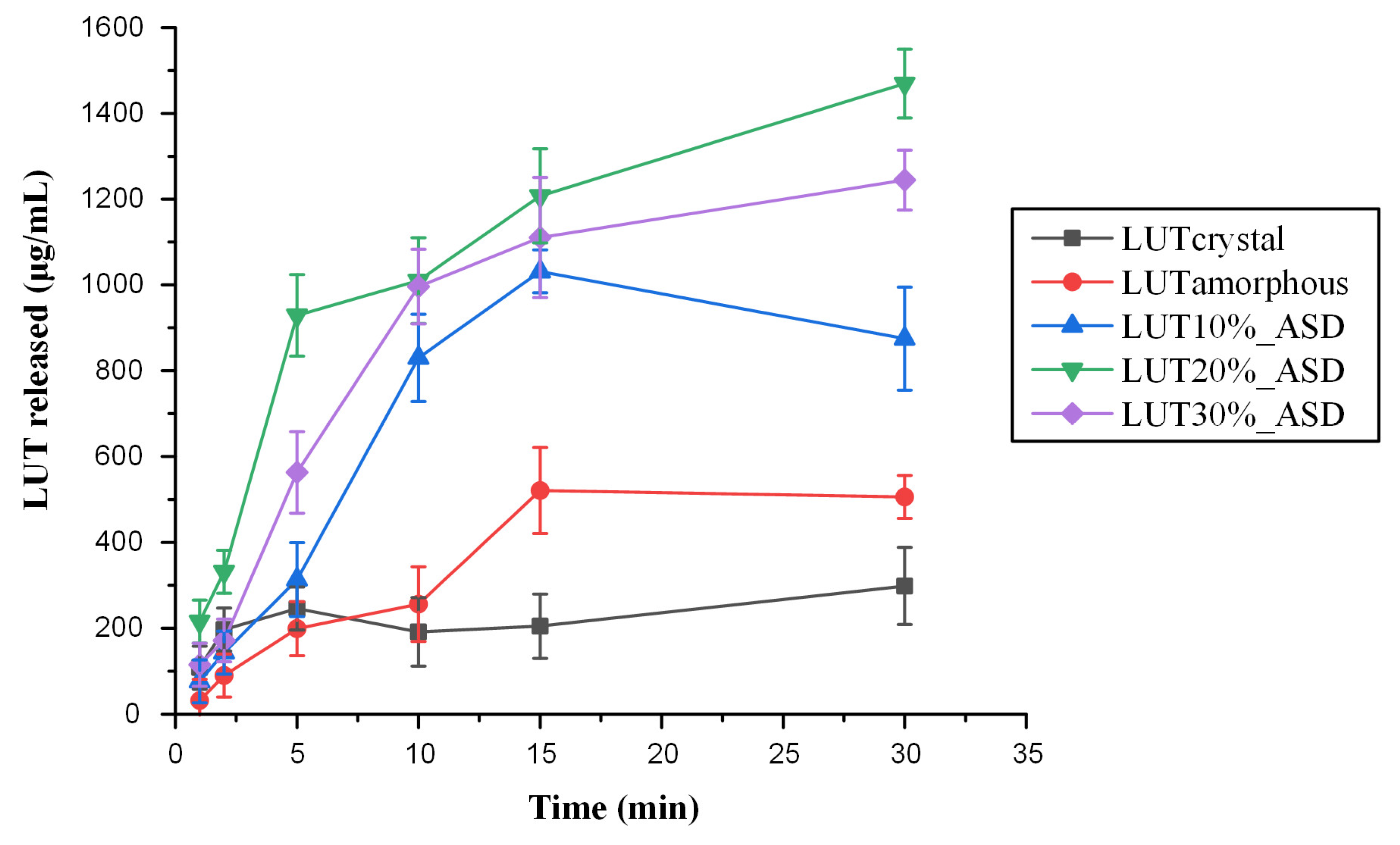
2.7. In Vitro Drug Release Studies in Simulated Saliva
3. Results and Discussion
3.1. Thermophysical Characterization of LUT
3.1.1. GFA of LUT
3.1.2. Thermal Stability of LUT
3.2. Selection of LUT’s ASD Matrix/Carrier
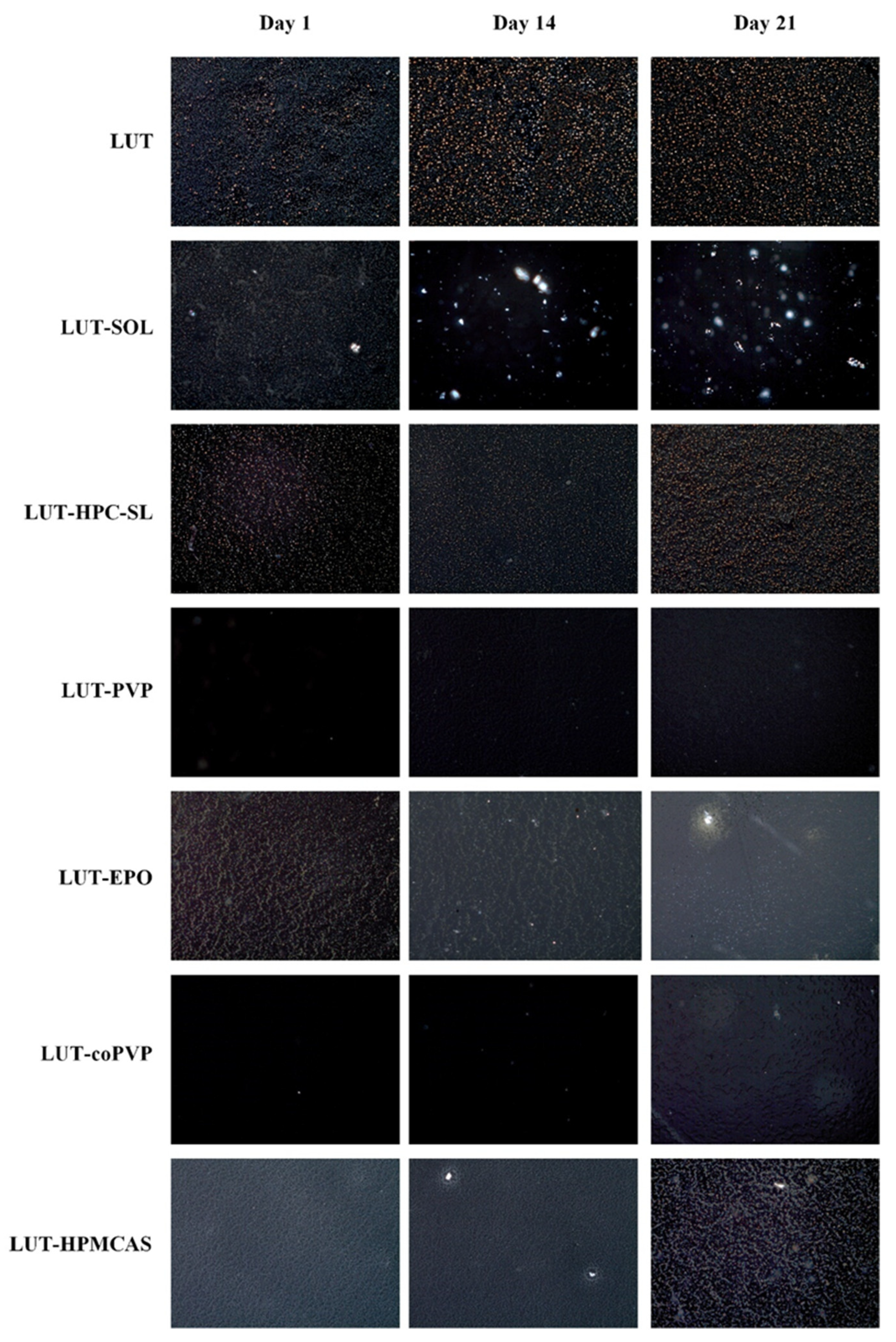
3.2.1. Film Casting Method
3.2.2. Solvent Shift Method
3.3. Miscibility Evaluation
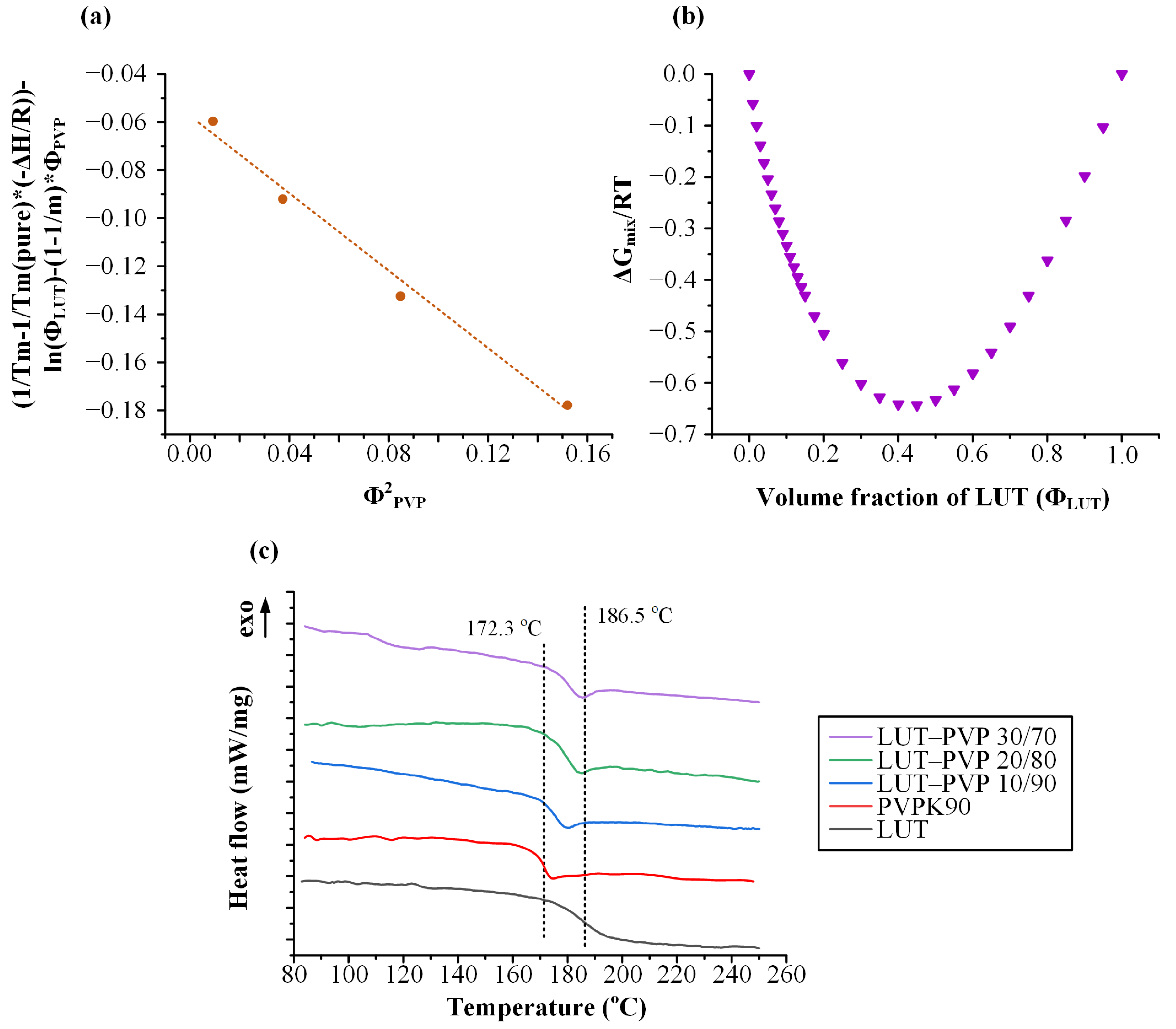
3.3.1. Estimation of the Flory–Huggins (FH) Interaction Parameter
3.3.2. Hansen Solubility Parameters (HSPs)
3.3.3. DSC Measurements
3.4. Evaluation of LUT-PVP ASDs
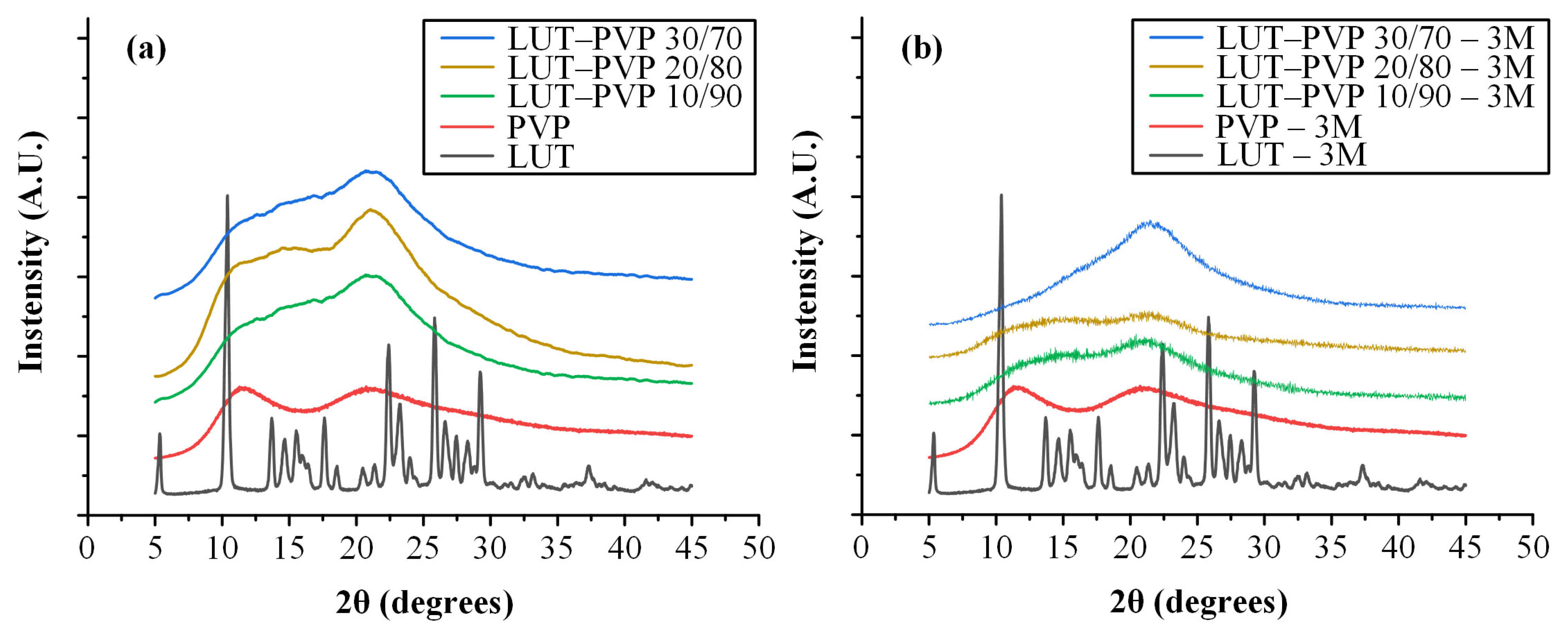
3.4.1. Physical State Evaluation of ASDs
3.4.2. Molecular Interactions
3.4.3. MD Simulations
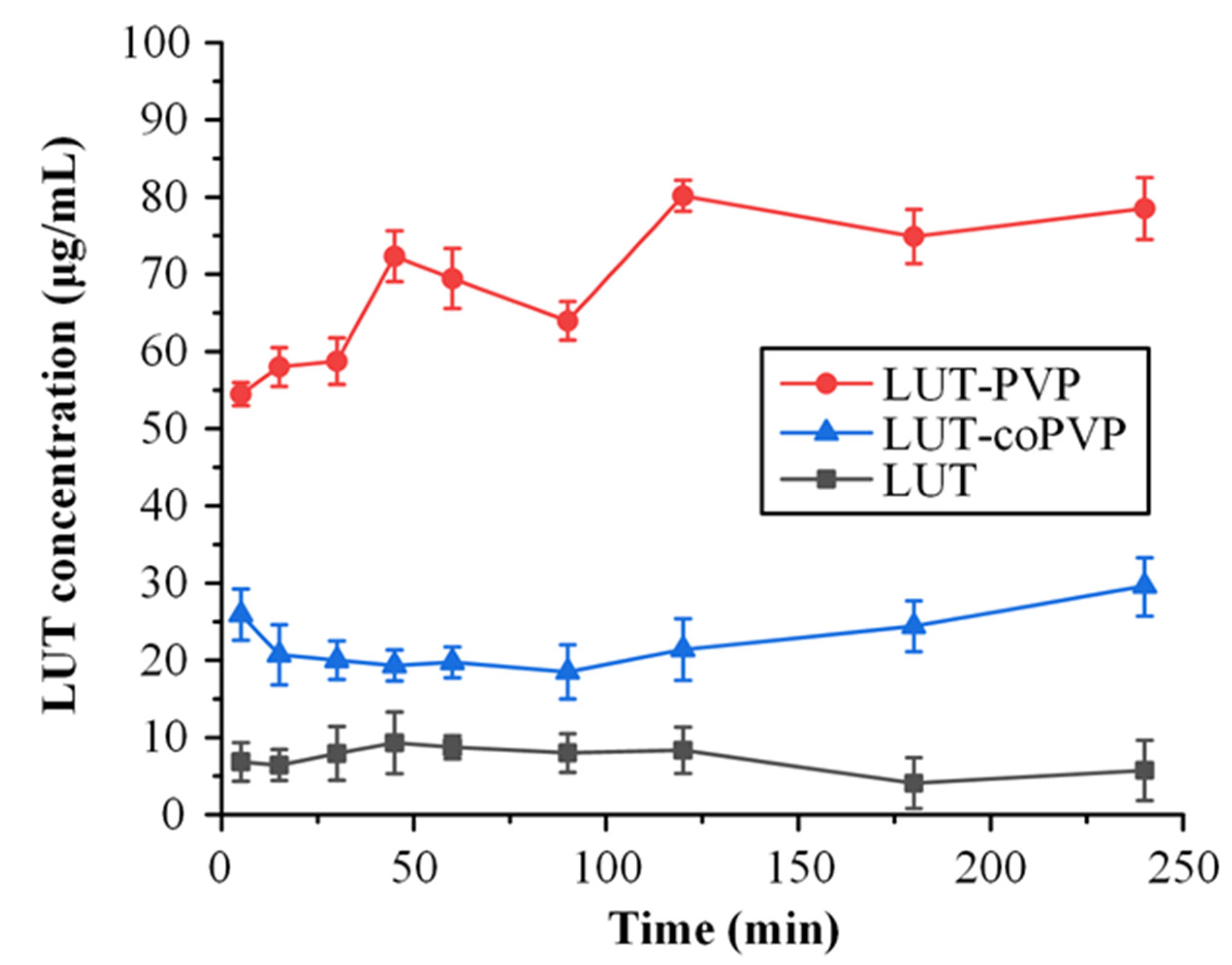
3.5. In Vitro Drug Release Studies in Simulated Saliva
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birt, D.F.; Jeffery, E. Flavonoids. Adv. Nutr. 2013, 4, 576–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karak, P. Biological Activities of Flavonoids: An Overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef]
- Verma, A.K.; Pratap, R. The biological potential of flavones. Nat. Prod. Rep. 2010, 27, 1571–1593. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Xing, J.-G.; Wang, L.-L.; Jiang, H.-L.; Guo, S.-L.; Liu, R. Luteolin inhibits fibrillary β-Amyloid1–40-induced inflammation in a human blood-brain barrier model by suppressing the p38 MAPK-mediated NF-κB signaling pathways. Molecules 2017, 22, 334. [Google Scholar] [CrossRef]
- Lodhi, S.; Vadnere, G.P.; Patil, K.D.; Patil, T.P. Protective effects of luteolin on injury induced inflammation through reduction of tissue uric acid and pro-inflammatory cytokines in rats. J. Tradit. Complement. Med. 2020, 10, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Hytti, M.; Szabó, D.; Piippo, N.; Korhonen, E.; Honkakoski, P.; Kaarniranta, K.; Petrovski, G.; Kauppinen, A. Two dietary polyphenols, fisetin and luteolin, reduce inflammation but augment DNA damage-induced toxicity in human RPE cells. J. Nutr. Biochem. 2017, 42, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-f.; Xu, N.-n.; Sun, W.; Zhao, Y.; Li, C.-y.; Guo, M.-y. Luteolin reduces inflammation in Staphylococcus aureus -induced mastitis by inhibiting NF-kB activation and MMPs expression. Oncotarget 2017, 8, 28481–28493. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Tian, G.; Tang, W.; Luo, W.; Liu, P.; Ma, Z. Protective effect of luteolin on streptozotocin-induced diabetic renal damage in mice via the regulation of RIP140/NF-κB pathway and insulin signalling pathway. J. Funct. Foods 2016, 22, 93–100. [Google Scholar] [CrossRef]
- Ambasta, R.K.; Gupta, R.; Kumar, D.; Bhattacharya, S.; Sarkar, A.; Kumar, P. Can luteolin be a therapeutic molecule for both colon cancer and diabetes? Brief. Funct. Genom. 2019, 18, 230–239. [Google Scholar] [CrossRef]
- Zang, Y.; Igarashi, K.; Li, Y. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-A y mice. Biosci. Biotechnol. Biochem. 2016, 80, 1580–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, M.T. Mechanism of metastasis suppression by luteolin in breast cancer. Breast Cancer 2018, 10, 89–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganai, S.A.; Sheikh, F.A.; Baba, Z.A.; Mir, M.A.; Mantoo, M.A.; Yatoo, M.A. Anticancer activity of the plant flavonoid luteolin against preclinical models of various cancers and insights on different signalling mechanisms modulated. Phytother. Res. 2021, 35, 3509–3532. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Tsai, P.-H.; Kandaswami, C.C.; Cheng, C.-H.; Ke, F.-C.; Lee, P.-P.; Hwang, J.-J.; Lee, M.-T. Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial–mesenchymal transition in A431 epidermal cancer cells. Cancer Sci. 2011, 102, 1829–1839. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, K.; Yuan, C.; Xing, R.; Ni, J.; Hu, G.; Chen, F.; Wang, X. Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int. J. Mol. Med. 2017, 39, 113–125. [Google Scholar] [CrossRef]
- Kooti, W.; Daraei, N. A Review of the Antioxidant Activity of Celery (Apium graveolens L.). J. Evid. Based Complement. Altern. Med. 2017, 22, 1029–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özgen, U.; Mavi, A.; Terzi, Z.; Kazaz, C.; Asçi, A.; Kaya, Y.; Seçen, H. Relationship between chemical structure and antioxidant activity of luteolin and its glycosides isolated from T. sipyleus subsp. sipyleus var. sipyleus. Rec. Nat. Prod. 2011, 5, 12. [Google Scholar]
- Jeon, I.H.; Kim, H.S.; Kang, H.J.; Lee, H.-S.; Jeong, S.I.; Kim, S.J.; Jang, S.I. Anti-Inflammatory and Antipruritic Effects of Luteolin from Perilla (P. frutescens L.) Leaves. Molecules 2014, 19, 6941–6951. [Google Scholar] [CrossRef] [Green Version]
- Jang, T.Y.; Jung, A.-Y.; Kyung, T.-S.; Kim, D.-Y.; Hwang, J.-H.; Kim, Y.H. Anti-allergic effect of luteolin in mice with allergic asthma and rhinitis. Cent. Eur. J. Immunol. 2017, 42, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özkütük, A.S. Antimicrobial effects of carnosic acid, kaempferol and luteolin on biogenic amine production by spoilage and food-borne pathogenic bacteria. Food Biosci. 2022, 46, 101588. [Google Scholar] [CrossRef]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.B.; Sun, X.; Wang, M.; Ye, J.X.; Si, J.Y.; Xu, H.B.; Meng, X.B.; Qin, M.; Sun, J.; Wang, H.W.; et al. Oxidative stress suppression by luteolin-induced heme oxygenase-1 expression. Toxicol. Appl. Pharmacol. 2012, 265, 229–240. [Google Scholar] [CrossRef]
- Tsilioni, I.; Taliou, A.; Francis, K.; Theoharides, T.C. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl. Psychiatry 2015, 5, e647. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y. Luteolin as a potential preventive and therapeutic candidate for Alzheimer’s disease. Exp. Gerontol. 2017, 95, 39–43. [Google Scholar] [CrossRef]
- Balci Yuce, H.; Toker, H.; Yildirim, A.; Tekin, M.B.; Gevrek, F.; Altunbas, N. The effect of luteolin in prevention of periodontal disease in Wistar rats. J. Periodontol. 2019, 90, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Casili, G.; Ardizzone, A.; Lanza, M.; Gugliandolo, E.; Portelli, M.; Militi, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Treatment with Luteolin Improves Lipopolysaccharide-Induced Periodontal Diseases in Rats. Biomedicines 2020, 8, 442. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Jin, J.-Y.; Choi, J.-I.; Choi, I.S.; Kim, S.-J. Effects of Luteolin on the Release of Nitric Oxide and Interleukin-6 by Macrophages Stimulated with Lipopolysaccharide from Prevotella intermedia. J. Periodontol. 2011, 82, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Dai, X.; Liu, M.; Wu, C.; Wang, D. Luteolin supports osteogenic differentiation of human periodontal ligament cells. BMC Oral Health 2019, 19, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeel, F.; Haq, N.; Alshehri, S.; Ibrahim, M.; Elzayat, E.; Altamimi, M.; Kazi, M.; Alanazi, F.; Alsarra, I. Solubility, thermodynamic properties and solute-solvent molecular interactions of luteolin in various pure solvents. J. Mol. Liq. 2018, 255, 43–50. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S.; Altamimi, M.A.; Jafar, M.; Hassan, M.Z.; Hussain, A.; Ahad, A.; Mahdi, W. Host-guest complex of β-cyclodextrin and pluronic F127 with Luteolin: Physicochemical characterization, anti-oxidant activity and molecular modeling studies. J. Drug Deliv. Sci. Technol. 2020, 55, 101356. [Google Scholar] [CrossRef]
- Dang, H.; Meng, M.H.W.; Zhao, H.; Iqbal, J.; Dai, R.; Deng, Y.; Lv, F. Luteolin-loaded solid lipid nanoparticles synthesis, characterization, & improvement of bioavailability, pharmacokinetics in vitro and vivo studies. J. Nanopart Res. 2014, 16, 2347. [Google Scholar]
- Khan, J.; Alexander, A.; Saraf, S.; Saraf, S. Luteolin–phospholipid complex: Preparation, characterization and biological evaluation. J. Pharm. Pharmacol. 2014, 66, 1451–1462. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.; Chen, Y.; He, W.; Ni, L.; Xiong, P.; Wei, M. Vitamin E TPGS modified liposomes enhance cellular uptake and targeted delivery of luteolin: An in vivo/in vitro evaluation. Int. J. Pharm. 2016, 512, 262–272. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Cheng, M.; Liu, Q.; Liu, W.; Gao, C.; Feng, J.; Jin, Y.; Tu, L. Improving Oral Bioavailability of Luteolin Nanocrystals by Surface Modification of Sodium Dodecyl Sulfate. AAPS PharmSciTech 2021, 22, 133. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, S.; Zhou, J.; Chen, J.; Tian, L.; Gao, W.; Zhang, Y.; Ma, A.; Li, L.; Zhou, Z. Luteolin cocrystals: Characterization, evaluation of solubility, oral bioavailability and theoretical calculation. J. Drug Deliv. Sci. Technol. 2019, 50, 248–254. [Google Scholar] [CrossRef]
- Qing, W.; Wang, Y.; Li, H.; Ma, F.; Zhu, J.; Liu, X. Preparation and characterization of copolymer micelles for the solubilization and in vitro release of luteolin and luteoloside. AAPS PharmSciTech 2017, 18, 2095–2101. [Google Scholar] [CrossRef]
- Shin, K.; Choi, H.; Song, S.K.; Yu, J.W.; Lee, J.Y.; Choi, E.J.; Lee, D.H.; Do, S.H.; Kim, J.W. Nanoemulsion vehicles as carriers for follicular delivery of luteolin. ACS Biomater. Sci. Eng. 2018, 4, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Shinde, P.; Agraval, H.; Singh, A.; Yadav, U.C.; Kumar, U. Synthesis of luteolin loaded zein nanoparticles for targeted cancer therapy improving bioavailability and efficacy. J. Drug Deliv. Sci. Technol. 2019, 52, 369–378. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S.; Altamimi, M.A.; Hussain, A.; Shakeel, F.; Elzayat, E.; Mohsin, K.; Ibrahim, M.; Alanazi, F. Enhanced dissolution of luteolin by solid dispersion prepared by different methods: Physicochemical characterization and antioxidant activity. ACS Omega 2020, 5, 6461–6471. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Chen, J.; Zhang, Z.-x.; Wang, F.-b.; Wang, L.; Lin, Y.; Zhang, X.; Liu, J. Solubilization of luteolin in PVP40 solid dispersion improves inflammation-induced insulin resistance in mice. Eur. J. Pharm. Sci. 2022, 106188. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Sandhu, H.; Choi, D.S.; Chokshi, H.; Malick, A.W. Amorphous solid dispersions. In Advances in Delivery Science and Technology; Springer: New York, NY, USA, 2014. [Google Scholar]
- Sinha, S.; Ali, M.; Baboota, S.; Ahuja, A.; Kumar, A.; Ali, J. Solid dispersion as an approach for bioavailability enhancement of poorly water-soluble drug ritonavir. AAPS PharmSciTech 2010, 11, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, I.; Doshi, A.; Joshi, B.; Wankhede, V.; Doshi, J. Solid dispersions: An approach to enhance solubility of poorly water soluble drug. J. Sci. Innov. Res. 2013, 2, 685–694. [Google Scholar]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef]
- Lin, L.-C.; Pai, Y.-F.; Tsai, T.-H. Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. J. Agric. Food Chem. 2015, 63, 7700–7706. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Thakur, A.; Deshmukh, K.; Jha, A.; Verma, S. Routes of drug administration. Int. J. Pharm. Stud. Res. 2010, 1, 54–59. [Google Scholar]
- Baird, J.A.; Van Eerdenbrugh, B.; Taylor, L.S. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.-T.; Tran, C.-S.; Nguyen, T.-L.; Chi, S.-C.; Nguyen, H.-A.; Bui, Q.-D.; Bui, D.-N.; Tran, T.-Q. Effect of surfactant on the in vitro dissolution and the oral bioavailability of a weakly basic drug from an amorphous solid dispersion. Eur. J. Pharm. Sci. 2021, 162, 105836. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Greenhalgh, D.J.; Williams, A.C.; Timmins, P.; York, P. Solubility parameters as predictors of miscibility in solid dispersions. J. Pharm. Sci. 1999, 88, 1182–1190. [Google Scholar] [CrossRef]
- Cox, P.J.; Kumarasamy, Y.; Nahar, L.; Sarker, S.D.; Shoeb, M. Luteolin. Acta Crystallogr. Sect. E 2003, 59, o975–o977. [Google Scholar] [CrossRef]
- Li, D.; Panchal, K.; Mafi, R.; Xi, L. An Atomistic Evaluation of the Compatibility and Plasticization Efficacy of Phthalates in Poly(vinyl chloride). Macromolecules 2018, 51, 6997–7012. [Google Scholar] [CrossRef]
- Gunsteren, W.F.v.; Mark, A.E. Validation of molecular dynamics simulation. J. Chem. Phys. 1998, 108, 6109–6116. [Google Scholar] [CrossRef] [Green Version]
- Ali, J.; Lee, J.B.; Gittings, S.; Iachelini, A.; Bennett, J.; Cram, A.; Garnett, M.; Roberts, C.J.; Gershkovich, P. Development and optimisation of simulated salivary fluid for biorelevant oral cavity dissolution. Eur. J. Pharm. Biopharm. 2021, 160, 125–133. [Google Scholar] [CrossRef]
- Kapourani, A.; Vardaka, E.; Katopodis, K.; Kachrimanis, K.; Barmpalexis, P. Crystallization tendency of APIs possessing different thermal and glass related properties in amorphous solid dispersions. Int. J. Pharm. 2020, 579, 119149. [Google Scholar] [CrossRef]


| Structural Group | N | N·Fdi (J1/2 cm3/2 mol−1/2) | N·Fpi 2 (J cm3 mol−2) | N·Ehi (J mol−1) |
|---|---|---|---|---|
| LUT | ||||
| –OH | 4 | 840 | 1,000,000 | 80,000 |
| =CH– | 3 | 600 | 0 | 0 |
| –CO– | 1 | 290 | 592,900 | 2000 |
| –O– | 1 | 100 | 160,000 | 3000 |
| =C< | 5 | 350 | 0 | 0 |
| Substituted Benzyl Ring | 1 | 1270 | 12,100 | 0 |
| Sum | 3450 | 1,765,000 | 85,000 | |
| δt (MPa1/2) | δd(MPa1/2) | δp(MPa1/2) | δh(MPa1/2) | |
| 28.65 | 18.08 | 7.00 | 21.11 | |
| PVP | ||||
| –CH2– | 4 | 1080 | 0 | 0 |
| >CH– | 1 | 80 | 0 | 0 |
| >N– | 1 | 20 | 640,000 | 5000 |
| –CO– | 1 | 290 | 592,900 | 2000 |
| Sum | 1470 | 1,232,900 | 7000 | |
| δt (MPa1/2) | δd(MPa1/2) | δp(MPa1/2) | δh(MPa1/2) | |
| 22.37 | 16.40 | 12.38 | 8.83 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koromili, M.; Kapourani, A.; Barmpalexis, P. Preparation and Evaluation of Amorphous Solid Dispersions for Enhancing Luteolin’s Solubility in Simulated Saliva. Polymers 2023, 15, 169. https://doi.org/10.3390/polym15010169
Koromili M, Kapourani A, Barmpalexis P. Preparation and Evaluation of Amorphous Solid Dispersions for Enhancing Luteolin’s Solubility in Simulated Saliva. Polymers. 2023; 15(1):169. https://doi.org/10.3390/polym15010169
Chicago/Turabian StyleKoromili, Maria, Afroditi Kapourani, and Panagiotis Barmpalexis. 2023. "Preparation and Evaluation of Amorphous Solid Dispersions for Enhancing Luteolin’s Solubility in Simulated Saliva" Polymers 15, no. 1: 169. https://doi.org/10.3390/polym15010169







