Dispersion Performances and Fluorescent Behaviors of Naphthalic Anhydride Doped in Poly(acrylic acid) Frameworks for pH-Sensitive Ibuprofen Delivery via Fractal Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. P(NA-AA) Prepared by One-Step Method
2.3. NA-PAA Prepared by Physical Mixed Method
2.4. Swelling Behaviors
2.5. IBU Loading
2.6. In Vitro Release of IBU
2.7. In Vitro Cytotoxicity Experiments
2.8. In Vitro Cellular Uptake Study
2.9. Characterizations
3. Results and Discussion
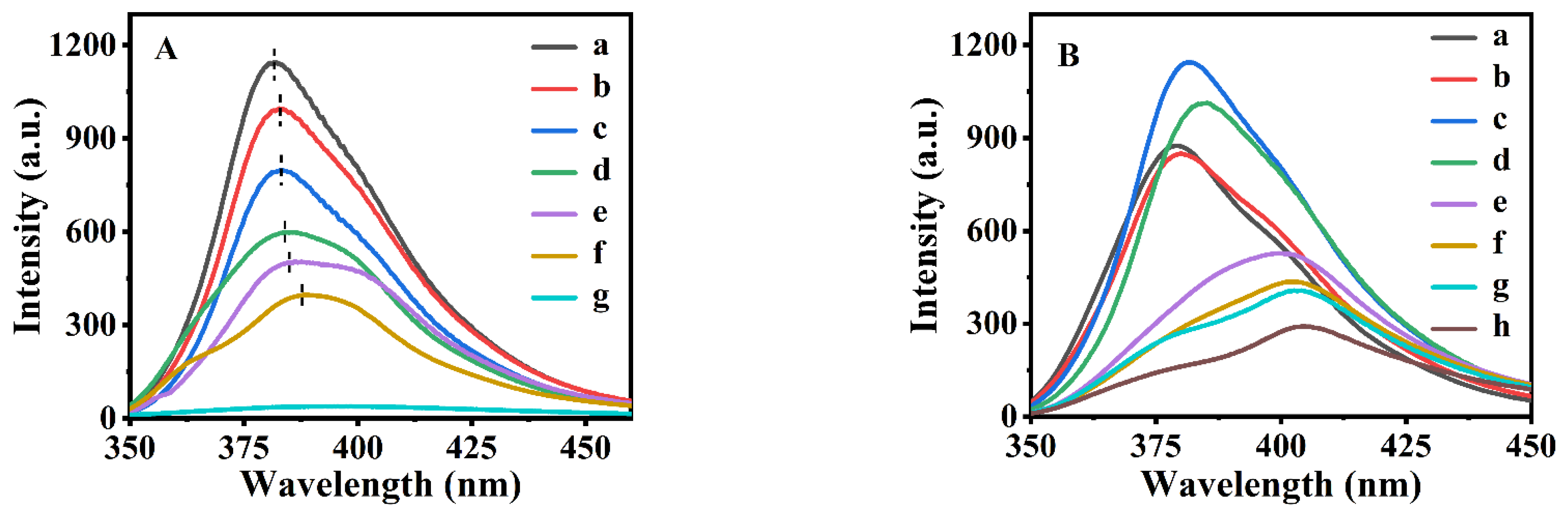
3.1. The Fluorescent Characteristics of the P(NA-AA)
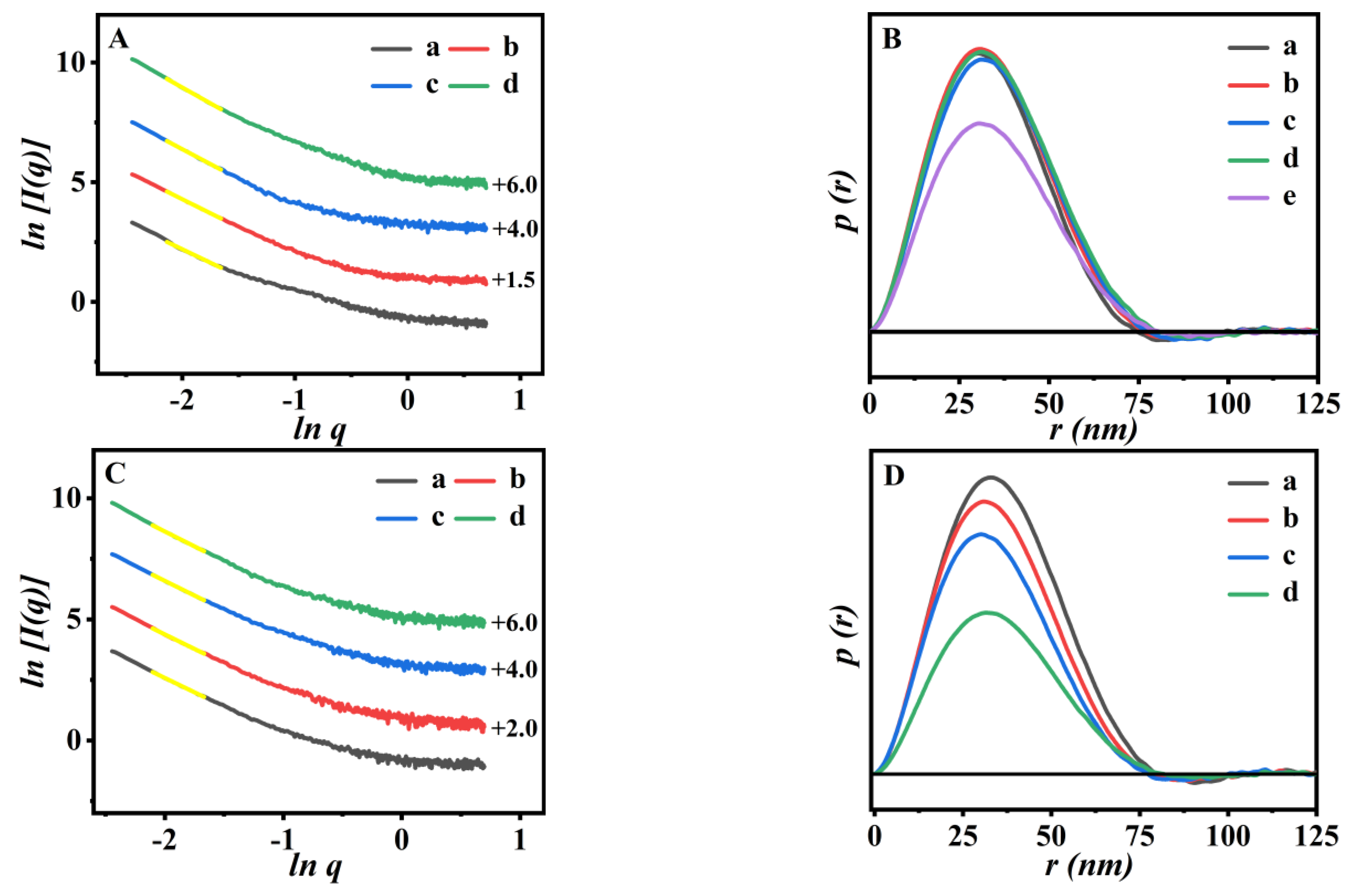
3.2. The Structure Features of the P(NA-AA)
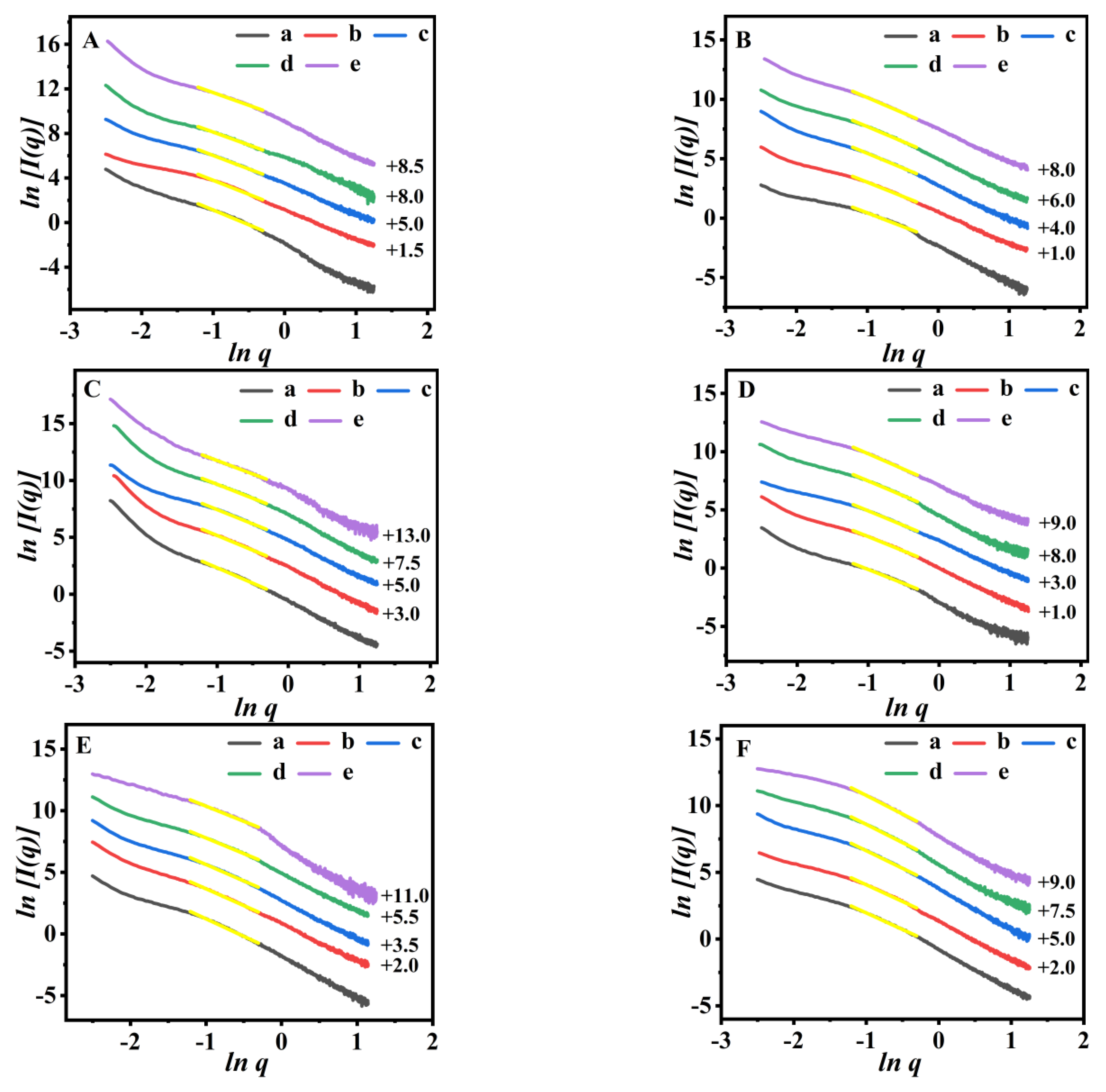
3.3. The Swelling Behaviors
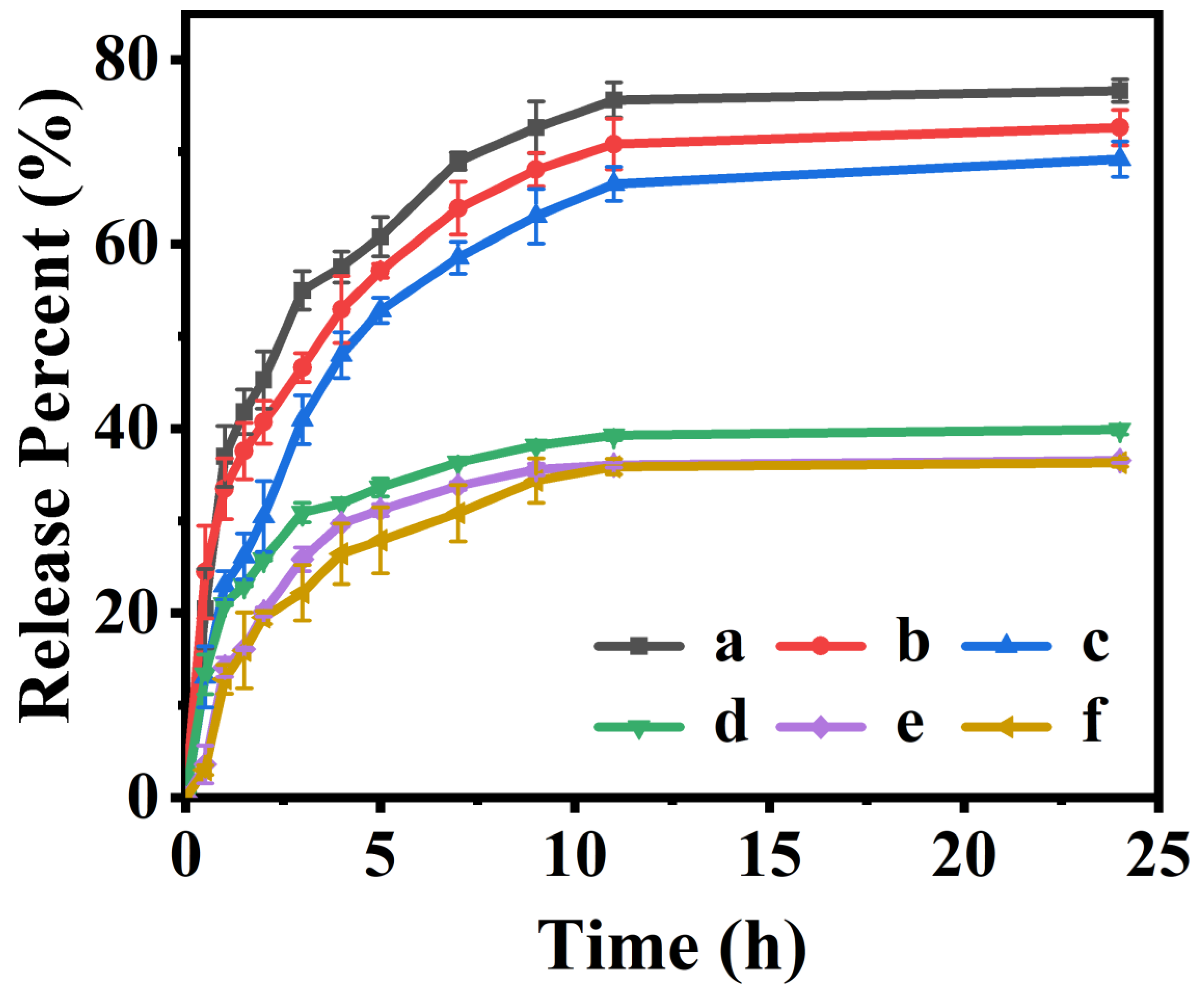
3.4. In Vitro Drug-Releasing Performances
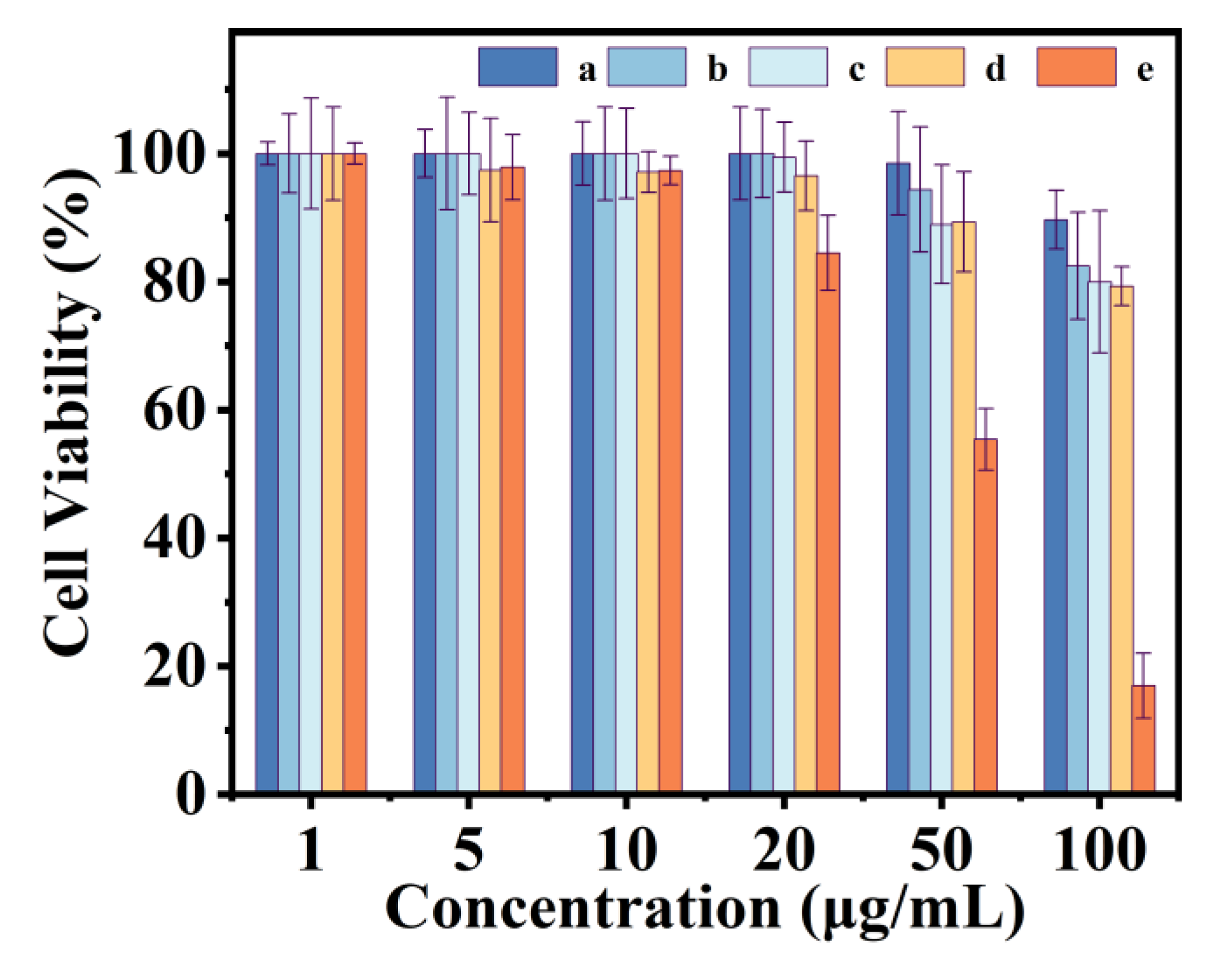
3.5. Cytotoxicity Properties
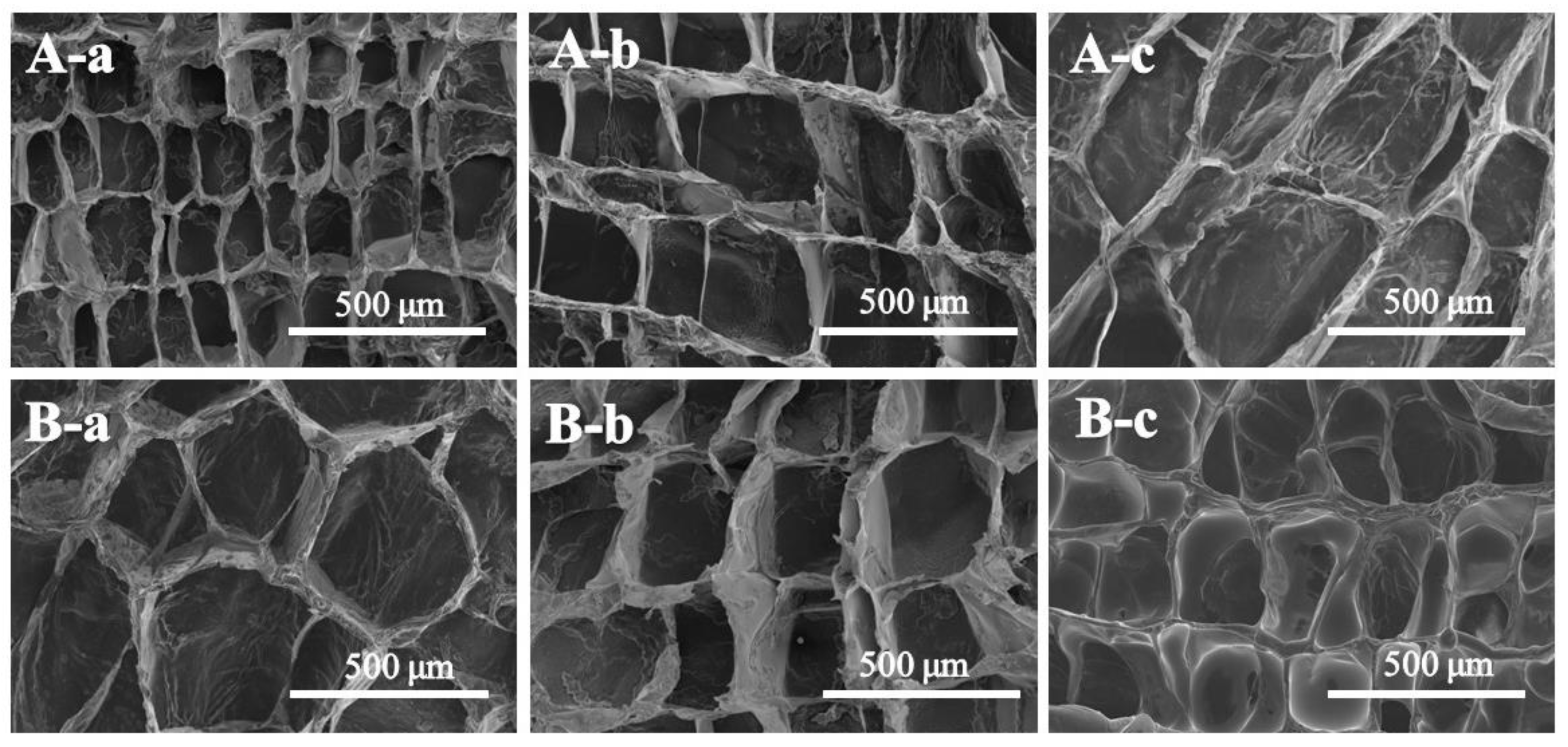
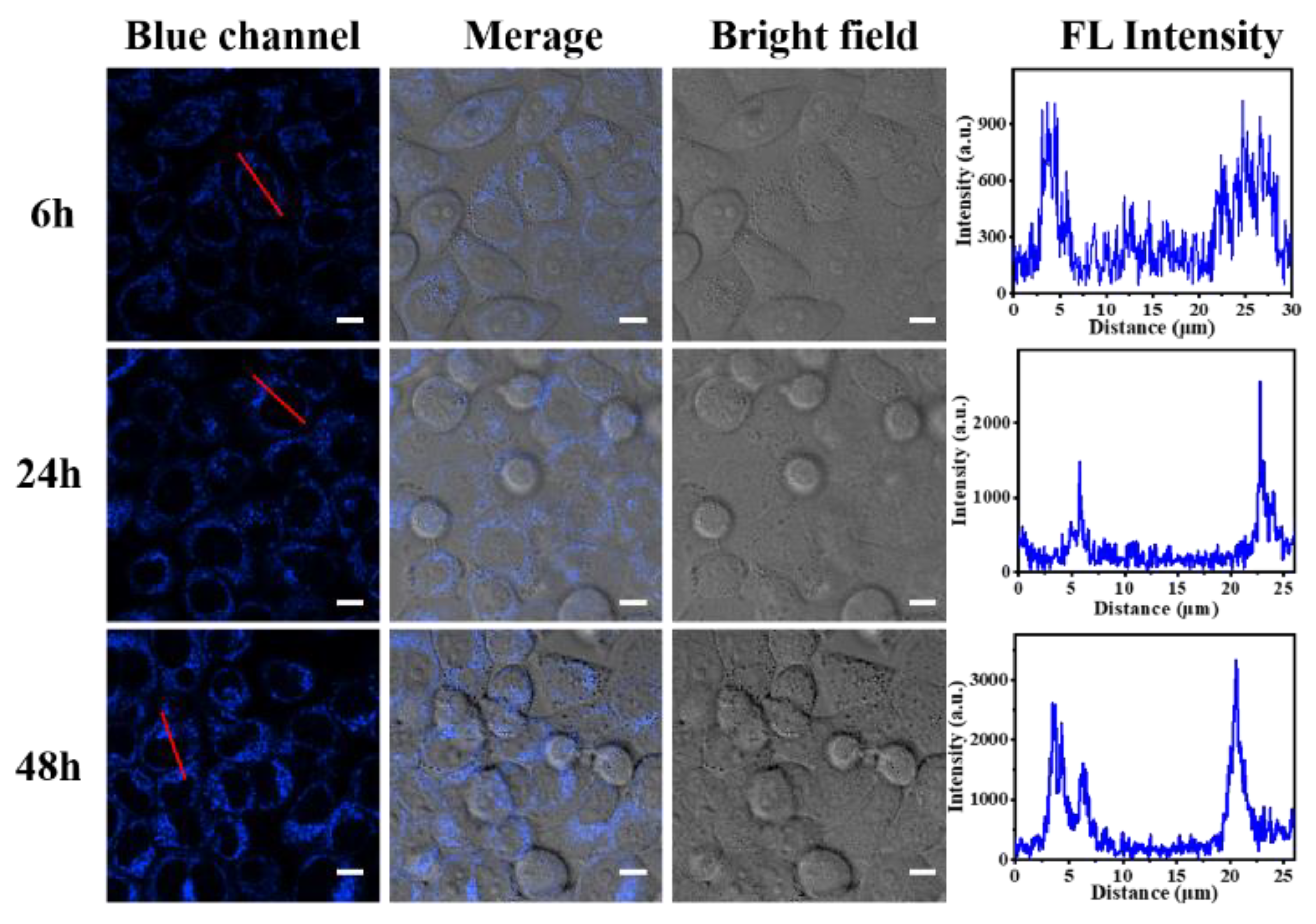
3.6. In Vitro Cellular Uptake Behaviors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. “Intelligent” polymers in medicine and biotechnology. Artif. Organs 1995, 19, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Gutowska, A. Lessons from nature: Stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2002, 20, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.M.; Kang, H.C.; Lee, Y.J.; Bae, Y.H. pH-sensitive polymers for drug delivery. Macromol. Res. 2012, 20, 224–233. [Google Scholar] [CrossRef]
- Na, K.; Bae, Y.H. pH-sensitive polymers for drug delivery. Polym. Drug Delivery Syst. 2005, 148, 129. [Google Scholar]
- Schmaljohann, D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug Delivery Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Naficy, S.; Razal, J.M.; Whitten, P.G.; Wallace, G.G.; Spinks, G.M. A pH-sensitive, strong double-network hydrogel: Poly (ethylene glycol) methyl ether methacrylates–poly (acrylic acid). J. Polym. Sci. Part B Polym. Phys. 2012, 50, 423–430. [Google Scholar] [CrossRef]
- Ma, H.X.; Niu, Y.S.; Wang, M.; Hu, S.K.; Li, H.C. Synthesis and characterization of pH-sensitive block polymer poly (propylene carbonate)-b-poly (acrylic acid) for sustained chlorpyrifos release. Mater. Today Commun. 2020, 24, 100974. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Dagdelen, S.; Marcisz, K.; Waleka-Bargiel, E.; Stojek, Z.; Karbarz, M. Redox-degradable microgel based on poly (acrylic acid) as drug-carrier with very high drug-loading capacity and decreased toxicity against healthy cells. Polym. Degrad. Stab. 2021, 190, 109652. [Google Scholar] [CrossRef]
- Nikravan, G.; Haddadi-Asl, V.; Salami-Kalajahi, M. Stimuli-responsive DOX release behavior of cross-linked poly (acrylic acid) nanoparticles. e-Polymers 2019, 19, 203–214. [Google Scholar] [CrossRef]
- Wei, W.; Qi, X.; Liu, Y.; Li, J.; Hu, X.; Zuo, G.; Zhang, J.; Dong, W. Synthesis and characterization of a novel pH-thermo dual responsive hydrogel based on salecan and poly (N, N-diethylacrylamide-co-methacrylic acid). Colloids Surf. B 2015, 136, 1182–1192. [Google Scholar] [CrossRef]
- Dadfar, S.M.R.; Pourmahdian, S.; Tehranchi, M.M.; Dadfar, S.M. Novel dual-responsive semi-interpenetrating polymer network hydrogels for controlled release of anticancer drugs. J. Biomed. Mater. Res. Part A 2019, 107, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Liu, Y.B.; Xiang, J.; Meng, Q.; Yu, H.; Yan, G.P. Fabrication of a new fluorescent polymeric nanoparticle containing naphthalimide and investigation on its interaction with bovine serum albumin. Colloids Surf. B 2014, 116, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Liu, Y.M.; Fu, T.; Yang, F.; Chen, X.H.; Yan, G.P. A water-soluble and biocompatible polymeric nanolabel based on naphthalimide grafted poly (acrylic acid) for the two-photon fluorescence imaging of living cells and C. elegans. Colloids Surf. B 2016, 148, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.P.; He, H.H.; Ding, H.Y.; Chen, X.B.; Wei, T.H.; Song, T.M.; You, S.J.; Xie, H.P.; Min, C.Y. Preparation of polyacrylic acid surface-crosslinked strong fluorescent polymer nanoparticles and their sensitive in vitro imaging of cancer cells and long-life in vivo imaging of in situ tumors. Anal. Methods 2017, 9, 4797–4807. [Google Scholar] [CrossRef]
- Wei, T.T.; Sheng, M.D.; Liu, C.; Sun, J.H.; Wu, X.; Bai, S.Y. Fluorescent pH-Responsive Mesoporous Silica Nanoparticles with Core-Shell Feature as a Traceable Delivery Carrier for Ibuprofen. ChemistrySelect 2020, 5, 6123–6130. [Google Scholar] [CrossRef]
- Ghosh, S.; Biswas, S.; Mondal, M.; Basu, S. Photophysical properties of 1, 8-naphthalic anhydride in aprotic solvents: An electron acceptor in excited state. J. Lumin. 2014, 145, 410–419. [Google Scholar] [CrossRef]
- Tarai, M.; Mishra, A.K. Inner filter effect and the onset of concentration dependent red shift of synchronous fluorescence spectra. Anal. Chim. Acta 2016, 940, 113–119. [Google Scholar] [CrossRef]
- Yu, Y.; Xing, H.; Zhou, Z.C.; Liu, J.K.; Sung, H.H.Y.; Williams, I.D.; Halpert, J.H.; Zhao, Z.; Tang, B.Z. How do molecular interactions affect fluorescence behavior of AIEgens in solution and aggregate states? Sci. China Chem. 2022, 65, 135–144. [Google Scholar] [CrossRef]
- Cong, H.; Zheng, S.X. Poly (N-isopropylacrylamide)-block-poly (acrylic acid) hydrogels: Synthesis and rapid thermoresponsive properties. Colloid Polym. Sci. 2014, 292, 2633–2645. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Liang, J.; Shan, G.R.; Pan, P.J. High strength of hybrid double-network hydrogels imparted by inter-network ionic bonds. J. Mater. Chem. B 2019, 7, 324–333. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Mo, G.; Xing, X.; Liu, P. A small-angle X-ray scattering station at Beijing synchrotron radiation facility. Instrum. Sci. Technol. 2014, 42, 128–141. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Z. Calibration of instrument and sample parameters for small angle X-ray scattering. Instrum. Sci. Technol. 2016, 44, 521–536. [Google Scholar] [CrossRef]
- Li, Z. A program for SAXS data processing and analysis. Chin. Phys. C 2013, 37, 108002. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, D.W. Polymers, fractals, and ceramic materials. Science 1989, 243, 1023–1027. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Zhang, L.; Gao, L.; Wu, X. Preparation of hybrid bimodal mesoporous silicas loaded with various capacity of 1, 8-naphthalic anhydride and their luminescent properties. Appl. Surf. Sci. 2012, 258, 3333–3339. [Google Scholar] [CrossRef]
- Liu, C.; Sheng, M.; Wei, T.; Sun, J.; Bai, S.; Wu, X. Core-shell structured assembly strategy of naphthalene anhydride derivatives and MPS-modified mesoporous SiO2 with temperature-responsive property for controlled drug delivery with strong fluorescence. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 903–915. [Google Scholar] [CrossRef]
- Hu, W.; Guo, L.; Bai, L.; Miao, X.; Ni, Y.; Wang, Q.; Zhao, H.; Xie, M.; Li, L.; Lu, X.; et al. Maximizing Aggregation of Organic Fluorophores to Prolong Fluorescence Lifetime for Two-Photon Fluorescence Lifetime Imaging. Adv. Healthc. Mater. 2018, 7, 1800299. [Google Scholar] [CrossRef]
- Svergun, D.I.; Koch, M.H.J. Small-angle scattering studies of biological macromolecules in solution. Rep. Prog. Phys. 2003, 66, 1735–1782. [Google Scholar] [CrossRef]
- Chalal, M.; Ehrburger-Dolle, F.; Morfin, I.; de Armas, M.R.A.; Lopez, M.L.; Bley, F. Small angle X-ray scattering study of poly(N-isopropyl acrylamide) based cryogels near the volume-phase transition temperature. J. Phys. Conf. Ser. 2010, 247, 012041. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Liu, R.; Zhang, H.; Shang, Y.; Song, Y.U.; Liu, C.; Wang, T.; Gong, Y.; Li, Z. Structure evolution of aluminosilicate sol and its structure-directing effect on the synthesis of NaY zeolite. J. Appl. Crystallogr. 2017, 50, 231–239. [Google Scholar] [CrossRef]
- Cheng, J.J.; Shan, G.R.; Pan, P.J. Temperature and pH-dependent swelling and copper(ii) adsorption of poly(N-isopropylacrylamide) copolymer hydrogel. RSC Adv. 2015, 5, 62091–62100. [Google Scholar] [CrossRef]
- Xu, X.; Sun, J.; Bing, L.; Cui, X.; Jia, B.; Bai, S. Fractal features of dual temperature/pH-sensitive poly (N-isopropylacrylamide-co-acrylic acid) hydrogels and resultant effects on the controlled drug delivery performances. Eur. Polym. J. 2022, 171, 111203. [Google Scholar] [CrossRef]
- McNeill, I.C.; Sadeghi, S.M.T. Thermal stability and degradation mechanisms of poly (acrylic acid) and its salts: Part 1-Poly (acrylic acid). Polym. Degrad. Stab. 1990, 29, 233–246. [Google Scholar] [CrossRef]
- Sovizi, M.R.; Hajimirsadeghi, S.S.; Naderizadeh, B. Effect of particle size on thermal decomposition of nitrocellulose. J. Hazard. Mater. 2009, 168, 1134–1139. [Google Scholar] [CrossRef]
- Dong, J.; Ozaki, Y.; Nakashima, K. FTIR studies of conformational energies of poly (acrylic acid) in cast films. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 507–515. [Google Scholar] [CrossRef]
- Peng, H.; Dong, R.; Wang, S.; Zhong, Z.; Luo, M.; Bai, C.; Zhao, Q.; Li, J.; Chen, L.; Xiong, H. A pH-responsive nano-carrier with mesoporous silica nanoparticles cores and poly (acrylic acid) shell-layers: Fabrication, characterization and properties for controlled release of salidroside. Int. J. Pharm. 2013, 446, 153–159. [Google Scholar] [CrossRef]
- Orgill, M.; Baker, B.L.; Owen, N.L. FTIR studies of conformational isomerism in acrylates and acrylic acids. Spectrochim. Acta Part A 1999, 55, 1021–1024. [Google Scholar] [CrossRef]
- Aroca, R.; Corrent, S.; Menendez, J.R. Vibrational spectra and structure of 1, 8-naphthalic anhydride. Vib. Spectrosc. 1997, 13, 221–226. [Google Scholar] [CrossRef]
- Lim, L.S.; Rosli, N.A.; Ahmad, I.; Lazim, M.T.; Amin, M.C.I.M. Synthesis and swelling behavior of pH-sensitive semi-IPN superabsorbent hydrogels based on poly (acrylic acid) reinforced with cellulose nanocrystals. Nanomaterials 2017, 7, 399. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Yu, R.; Wang, L.; Zheng, S.; Li, X. Poly(N-vinylpyrrolidone)-grafted poly(N-isopropylacrylamide) copolymers: Synthesis, characterization and rapid deswelling and reswelling behavior of Hydrogels. Polymer 2011, 52, 2340–2350. [Google Scholar] [CrossRef]
- Oshiro, A.; da Silva, D.C.; de Mello, J.C.; de Moraes, V.W.R.; Cavalcanti, L.P.; Franco, M.K.K.D.; Alkschbirs, M.I.; Fraceto, L.F.; Yokaichiya, F.; Rodrigues, T.; et al. Pluronics F-127/L-81 binary hydrogels as drug-delivery systems: Influence of physicochemical aspects on release kinetics and cytotoxicity. Langmuir 2014, 30, 13689–13698. [Google Scholar] [CrossRef]
- Wang, L.; Shan, G.; Pan, P. A strong and tough interpenetrating network hydrogel with ultrahigh compression resistance. Soft Matter 2014, 10, 3850–3856. [Google Scholar] [CrossRef]
- Wei, H.; Lv, X.; Zhao, Y.; Li, C.; Yan, H.; Sun, R.; Kang, M. Quantitative description of filler dispersion in composite materials by fractal analysis and fluorescent labeling-LSCM visualization technology. Polym. Compos. 2022, 43, 3598–3608. [Google Scholar] [CrossRef]
- Lv, X.; Kang, M.; Yuan, L.; Shen, S.; Sun, R.; Song, L.; Zeng, S.; Yin, M. Quantitative evaluation of fillers dispersion state in CaCO3/polypropylene composites through visualization and fractal analysis. Polym. Compos. 2020, 41, 1605–1613. [Google Scholar] [CrossRef]
- Pérez, J.; Vachette, P.; Russo, D.; Desmadril, M.; Durand, D. Heat-induced unfolding of Neocarzinostatin, a small all-β protein investigated by small-angle X-ray scattering. J. Mol. Biol. 2001, 308, 721–743. [Google Scholar] [CrossRef]
- Mertens, H.D.T.; Svergun, D.I. Structural characterization of proteins and complexes using small-angle X-ray solution scattering. J. Struct. Biol. 2010, 172, 128–141. [Google Scholar] [CrossRef]
- Doniach, S. Changes in Biomolecular Conformation Seen by Small Angle X-ray Scattering. Chem. Rev. 2001, 101, 1763–1778. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, H.; Qian, S.; Zhai, H. Effects of different lignins on absorption properties and pore structure of polyacrylic acid resin. Wood Sci. Technol. 2019, 53, 1001–1014. [Google Scholar] [CrossRef]
- Varga, N.; Benkő, M.; Sebők, D.; Bohus, G.; Janovak, L.; Dekany, I. Mesoporous silica core–shell composite functionalized with polyelectrolytes for drug delivery. Microporous Mesoporous Mater. 2015, 213, 134–141. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, X.; Sun, J.; Bai, S.; Wu, X. PAA-grafted surface and fractal feature of dense nanosilica spheres for ibuprofen delivery. Mater. Chem. Phys. 2017, 195, 213–223. [Google Scholar] [CrossRef]
- Ma, J.; Sun, J.; Fan, L.; Bai, S.; Panezai, H.; Jiao, Y. Fractal evolution of dual pH-and temperature-responsive P(NIPAM-co-AA)@BMMs with bimodal mesoporous silica core and coated-copolymer shell during drug delivery procedure via SAXS characterization. Arabian J. Chem. 2020, 13, 4147–4161. [Google Scholar] [CrossRef]
- Tian, B.; Liu, S.; Wu, S.; Lu, W.; Wang, D.; Jin, L.; Hu, B.; Li, K.; Wang, Z.; Quan, Z. pH-responsive poly (acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin. Colloids Surf. B 2017, 154, 287–291. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Wang, X.; Xu, X.; Xu, B.; Sun, J.; Bai, S. Dispersion Performances and Fluorescent Behaviors of Naphthalic Anhydride Doped in Poly(acrylic acid) Frameworks for pH-Sensitive Ibuprofen Delivery via Fractal Evolution. Polymers 2023, 15, 596. https://doi.org/10.3390/polym15030596
Cui X, Wang X, Xu X, Xu B, Sun J, Bai S. Dispersion Performances and Fluorescent Behaviors of Naphthalic Anhydride Doped in Poly(acrylic acid) Frameworks for pH-Sensitive Ibuprofen Delivery via Fractal Evolution. Polymers. 2023; 15(3):596. https://doi.org/10.3390/polym15030596
Chicago/Turabian StyleCui, Xueqing, Xiaoli Wang, Xiaohuan Xu, Bang Xu, Jihong Sun, and Shiyang Bai. 2023. "Dispersion Performances and Fluorescent Behaviors of Naphthalic Anhydride Doped in Poly(acrylic acid) Frameworks for pH-Sensitive Ibuprofen Delivery via Fractal Evolution" Polymers 15, no. 3: 596. https://doi.org/10.3390/polym15030596





