A Functionalized Polysaccharide from Sphingomonas sp. HL-1 for High-Performance Flocculation
Abstract
:1. Introduction
2. Methods
2.1. Production and Preparation of Bio-Flocculant HL1
2.2. Extraction and Purification of Exopolysaccharide HL1
2.3. Characterization of HL1-PS
2.3.1. Chemical Analysis of HL1-PS
2.3.2. Monosaccharide Composition Analysis
2.3.3. Potential Analysis
2.4. Flocculating Activity Assay
3. Results and Discussion
3.1. Extraction and Purification of Polysaccharide HL1
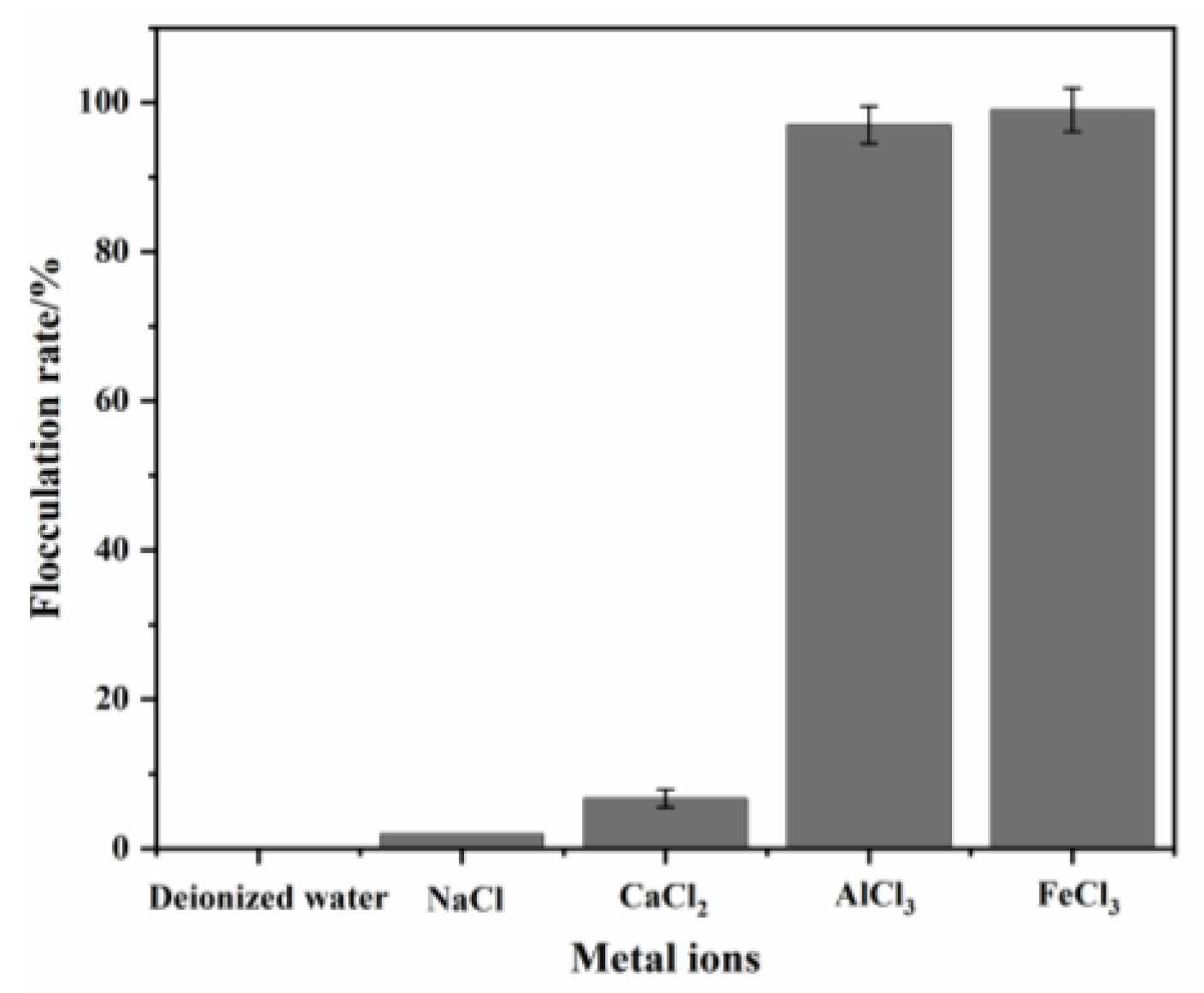
3.2. Effect of Metal Ions on Flocculating Activity of HL1-PS
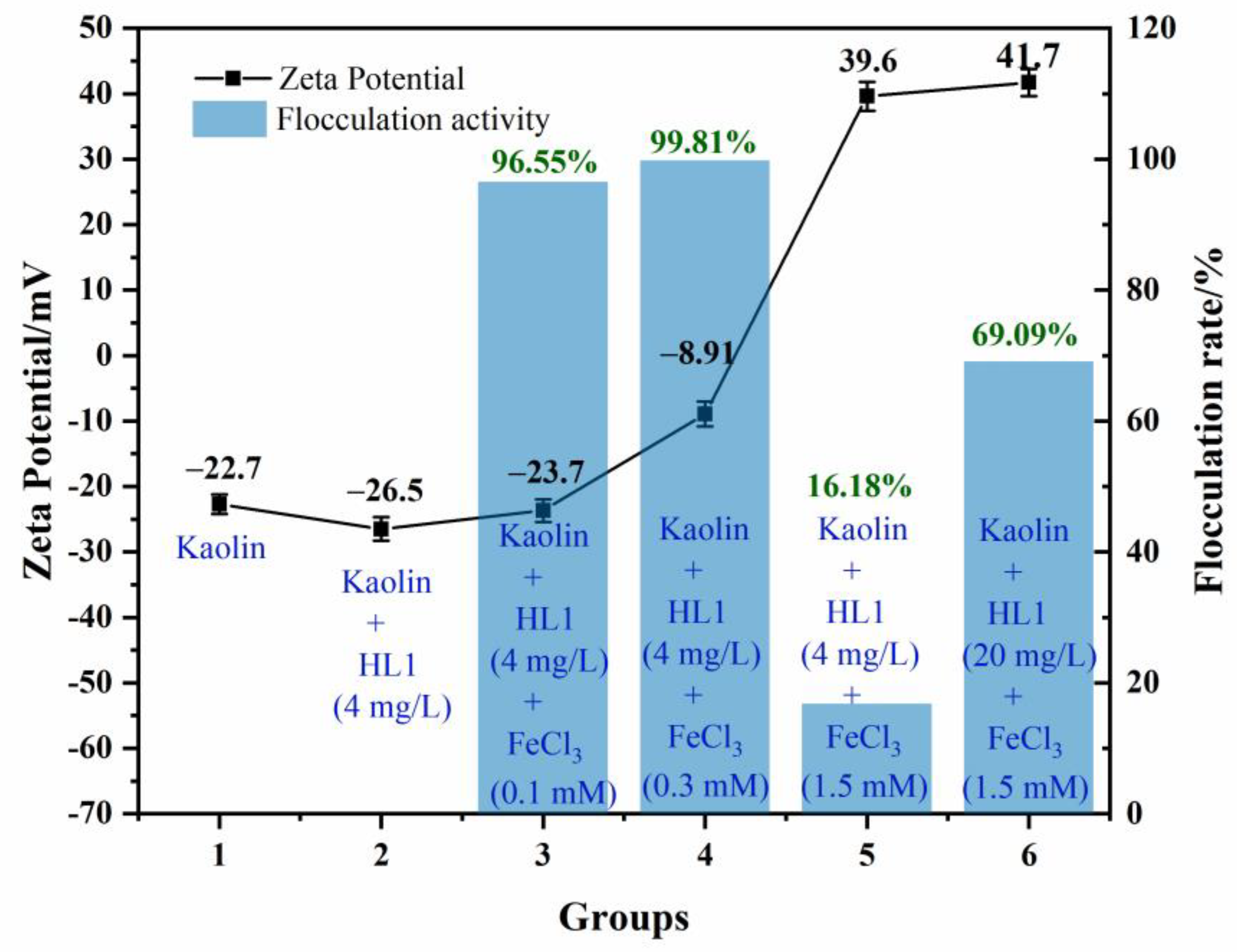
3.3. Flocculation Mechanism of HL1-PS towards Kaolin Clay
3.4. Flocculation Characteristics of Bio-Flocculant HL1
3.4.1. Effect of Dosage on Flocculation Activity
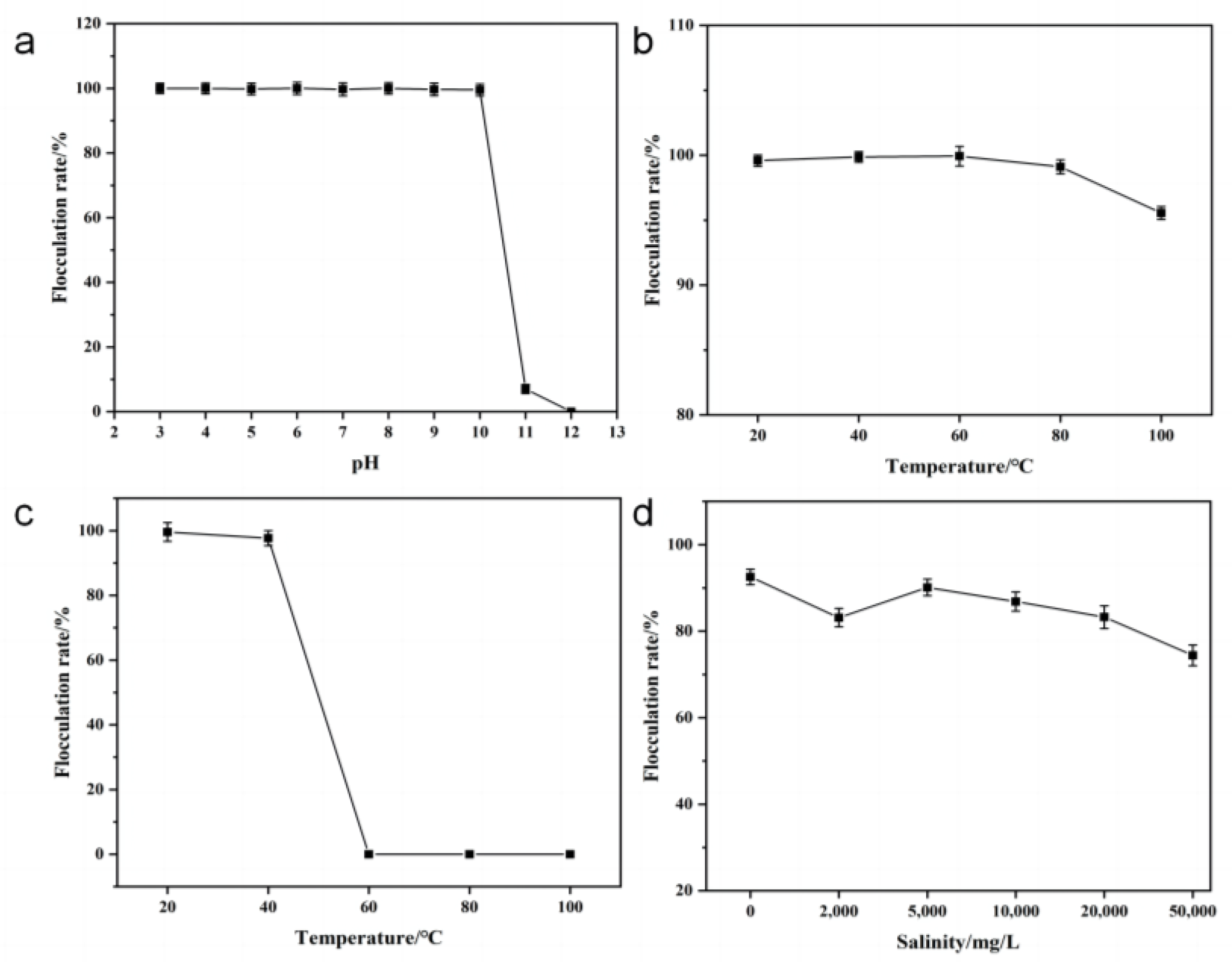
3.4.2. Effect of pH on Flocculating Activity
3.4.3. Effect of Temperature on Flocculating Activity
3.4.4. Effect of Salinity on Flocculating Activity of HL1-FB
3.5. Analysis of Performance, Cost and Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Gunten, U.V.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Poerschmann, J.; Trommler, U.; Nyplova, P.; Morgenstern, P.; Górecki, T. Complexation-flocculation of organic contaminants by the application of oxyhumolite-based humic organic matter. Chemosphere 2008, 70, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Li, Q.; Hao, D.K.; Hu, Z.H.; Song, D.X.; Yang, M. Characterization and flocculation mechanism of an alkali-activated polysaccharide flocculant from Arthrobacter sp. B4. Bioresour. Technol. 2014, 170, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yuan, W.; Cheng, J. Understanding pH and Ionic Strength Effects on Aluminum Sulfate-Induced Microalgae Flocculation. Appl. Biochem. Biotechnol. 2014, 173, 1692–1702. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Yan, N.; Farnood, R. Recent advances in polysaccharide bio-based flocculants. Biotechnol. Adv. Int. Rev. J. 2018, 36, 92–119. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, L.; Zhuang, X.L.; Shi, J.S.; Wang, Y.P.; He, N.; Chang, Y.I. Flocculation Characterization of a Bioflocculant from Bacillus licheniformis. Ind. Eng. Chem. Res. 2015, 54, 2894–2901. [Google Scholar] [CrossRef]
- Pu, L.; Zeng, Y.J.; Xu, P.; Li, F.Z.; Lou, W.Y. Using a novel polysaccharide BM2 produced by Bacillus megaterium strain PL8 as an efficient bioflocculant for wastewater treatment. Int. J. Biol. Macromol. 2020, 162, 374–384. [Google Scholar] [CrossRef]
- Li, R.H.; Zhang, H.B.; Hu, X.Q.; Gan, W.W.; Li, Q.P. An efficiently sustainable dextran-based flocculant: Synthesis, characterization and flocculation. Chemosphere 2016, 159, 342–350. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Q.M.; Zeng, T.; Yang, J.W.; Hu, X.Q.; Zhang, H.B. Synthesis and characterization of a cationic dextran-based flocculant and its application in bacterial sedimentation. Biochem. Eng. J. 2022, 185, 108535. [Google Scholar] [CrossRef]
- Wang, S.N.; Zhao, L.L.; Li, Q.H.; Liu, C.; Han, J.L.; Zhu, L.J.; Zhu, D.S.; He, Y.T.; Liu, H. Rheological properties and chain conformation of soy hull water-soluble polysaccharide fractions obtained by gradient alcohol precipitation. Food Hydrocoll. 2019, 91, 34–39. [Google Scholar] [CrossRef]
- Dubois, M.K.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F.A. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Chen, A.J.; Ge, X.Y.; Li, S.; Zhang, T.; Xu, H. Chain conformation and physicochemical properties of polysaccharide (glucuronoxylomannan) from Fruit Bodies of Tremella fuciformis. Carbohydr. Polym. 2020, 245, 116354. [Google Scholar] [CrossRef] [PubMed]
- Li, O.; Lu, C.; Liu, A.; Zhu, L.; Wu, X.C. Optimization and characterization of polysaccharide-based bioflocculant produced by Paenibacillus elgii B69 and its application in wastewater treatment. Bioresour. Technol. 2013, 134, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Lin, J.Z.; Wang, W.D.; Li, S. Biopolymers Produced by Sphingomonas Strains and Their Potential Applications in Petroleum Production. Polymers 2022, 14, 1920. [Google Scholar] [CrossRef]
- Liu, W.J.; Cong, L.; Yuan, H.L.; Yang, J.S. The mechanism of kaolin clay flocculation by a cation-independent bioflocculant produced by Chryseobacterium daeguense W6. Aims Environ. Sci. 2015, 2, 169–179. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoh, A.I. Bacillus toyonensis Strain AEMREG6, a Bacterium Isolated from South African Marine Environment Sediment Samples Produces a Glycoprotein Bioflocculant. Molecules 2015, 20, 5239–5259. [Google Scholar] [CrossRef]
- Yin, Y.J.; Tian, Z.M.; Tang, W.; Li, L.; Song, L.Y.; Mcelmurry, S.P. Production and characterization of high efficiency bioflocculant isolated from Klebsiella sp. ZZ-3. Bioresour. Technol. 2014, 171, 336–342. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoh, A.I. Evaluation of the flocculation potential and characterization of bioflocculant produced by Micrococcus sp. Leo. Appl. Biochem. Microbiol. 2014, 50, 601–608. [Google Scholar] [CrossRef]
- Pu, S.Y.; Qin, L.L.; Che, J.P.; Zhang, B.R.; Xu, M. Preparation and application of a novel bioflocculant by two strains of Rhizopus sp. using potato starch wastewater as nutrilite. Bioresour. Technol. 2014, 162, 184–191. [Google Scholar] [CrossRef]
- Xia, S.Q.; Zhang, Z.Q.; Wang, X.J.; Yang, A.; Chen, L.; Zhao, J.F.; Leonard, D.; Jaffrezic-Renault, N. Production and characterization of a bioflocculant by Proteus mirabilis TJ-1. Bioresour. Technol. 2008, 99, 6520–6527. [Google Scholar] [CrossRef] [PubMed]
- Shojaosadati, H.; Shojaosadati, S.A. Isolation and characterisation of a bioflocculant produced by Bacillus firmus. Biotechnol. Lett. 2002, 24, 35–40. [Google Scholar]
- Wu, J.Y.; Ye, H.F. Characterization and flocculating properties of an extracellular biopolymer produced from a Bacillus subtilis DYU1 isolate. Process Biochem. 2007, 42, 1114–1123. [Google Scholar] [CrossRef]
- Sobeck, D.C.; Higgins, M.J. Examination of three theories for mechanisms of cation-induced bioflocculation. Water Res. 2002, 36, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Fard, M.B.; Hamidi, D.; Yetilmezsoy, K.; Alavi, J.; Hosseinpour, F. Utilization of Alyssum mucilage as a natural coagulant in oily-saline wastewater treatment. J. Water Process Eng. 2020, 40, 101763. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Imron, M.F.; Slugocki, L.; Nowakowski, K.; Ahmad, A.; Najiya, D.; Abdullah, S.R.S.; Othman, A.R.; Purwanti, I.F.; Hasan, H.A. Assessing the effect of multiple variables on the production of bioflocculant by Serratia marcescens: Flocculating activity, kinetics, toxicity, and flocculation mechanism. Sci. Total Environ. 2022, 836, 155564. [Google Scholar] [CrossRef]
- Ma, X.L.; Duan, D.M.; Chen, X.; Feng, X.M.; Ma, Y.H. A polysaccharide-based bioflocculant BP50-2 from banana peel waste: Purification, structure and flocculation performance. Int. J. Biol. Macromol. 2022, 205, 604–614. [Google Scholar] [CrossRef]
- Xiang, X.; Liang, Y.J.; Lan, S.H.; Li, X.D.; Xie, Y.F.; Yuan, W. Production and flocculating properties of a compound biopolymer flocculant from corn ethanol wastewater. Bioresour. Technol. 2017, 247, 924–929. [Google Scholar]
- More, T.T.; Yadav, J.S.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances of bacteria and their potential environmental applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef]
- Zhong, C.Y.; Xu, A.H.; Wang, B.Y.; Yang, X.H.; Zhou, J.G. Production of a value added compound from the H-acid waste water—Bioflocculants by Klebsiella pneumoniae. Colloids Surf. B Biointerfaces 2014, 122, 583–590. [Google Scholar] [CrossRef]
- Luo, Z.S.; Chen, L.; Chen, C.H.; Zhang, W.; Liu, M.; Han, Y.; Zhou, J.G. Production and Characteristics of a Bioflocculant by Klebsiella pneumoniae YZ-6 Isolated from Human Saliva. Appl. Biochem. Biotechnol. 2014, 172, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Salehizadeh, H.; Yan, N. Recent advances in extracellular biopolymer flocculants. Biotechnol. Adv. 2014, 32, 1506–1522. [Google Scholar] [CrossRef]
- Wang, B.; Shui, Y.Y.; Liu, P.Z.; He, M. Preparation, characterization and flocculation performance of an inorganic-organic composite coagulant by polyferric chloride and polydimethyldiallylammonium chloride. J. Chem. Technol. Biotechnol. 2017, 92, 884–892. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhang, J.F.; Zhang, Q.H.; Shen, X.T.; Chen, T.Q. Effects of organic matter and salinity on the flocculation of kaolinites in a settling column. J. Hydrodyn. 2021, 33, 150–156. [Google Scholar] [CrossRef]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef]

| Component | Content |
|---|---|
| Total sugar (w/w %) | 64.7 |
| Uronic acid (w/w %) | 14.2 |
| Protein (w/w %) | 4.6 |
| Monosaccharide content (molar ratio, %) | |
| Glucose | 89.3 |
| Galacturonic acid | 5.5 |
| Guluronic acid | 2.5 |
| Mannose | 1.9 |
| Galactose | 0.4 |
| Arabinose | 0.2 |
| Glucosamine hydrochloride | 0.2 |
| Strain | Promoted Metal Ions | Inhibited Metal Ions | Optimal FR | Ref. |
|---|---|---|---|---|
| Bacillus toyonensis AEMREG6 | Na+, K+, Ca2+, Mg2+ and Al3+ | Fe3+ | 87% | [17] |
| Klebsiella sp. ZZ-3 | Fe3+ and Al3+ | Monovalents (Na+ and K+) and divalents | 94.5% | [18] |
| Micrococcus sp. Leo | K+, Ca2+, Mn2+, Ba2+, Fe3+ and Al3+ | Na+, Li+ and Fe3+ | 85.2% | [19] |
| Rhizopus sp. M9 and M17 | Mg2+ and Ca2+ | Na+, K+, Al3+ and Fe3+ | 92.71% | [20] |
| Flocculant | Dosage | Zeta Potential | Fe3+ Content | FR | Zeta Potential |
|---|---|---|---|---|---|
| HL1-PS | 4 mg/L | −26.5 mV | 0.1 mM | 96.55% | −23.7 mV |
| 0.3 mM | 99.81% | −8.9 mV | |||
| HL1-FB | 1/5000 (v/v) | −28.9 mV | 0.1 mM | 92.54% | −28.0 mV |
| 0.3 mM | 99.59% | −19.4 mV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Li, J.; Tao, W.; Li, S. A Functionalized Polysaccharide from Sphingomonas sp. HL-1 for High-Performance Flocculation. Polymers 2023, 15, 56. https://doi.org/10.3390/polym15010056
Huang H, Li J, Tao W, Li S. A Functionalized Polysaccharide from Sphingomonas sp. HL-1 for High-Performance Flocculation. Polymers. 2023; 15(1):56. https://doi.org/10.3390/polym15010056
Chicago/Turabian StyleHuang, Haolin, Jingsong Li, Weiyi Tao, and Shuang Li. 2023. "A Functionalized Polysaccharide from Sphingomonas sp. HL-1 for High-Performance Flocculation" Polymers 15, no. 1: 56. https://doi.org/10.3390/polym15010056





