Improvement of Mechanical Properties and Solvent Resistance of Polyurethane Coating by Chemical Grafting of Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
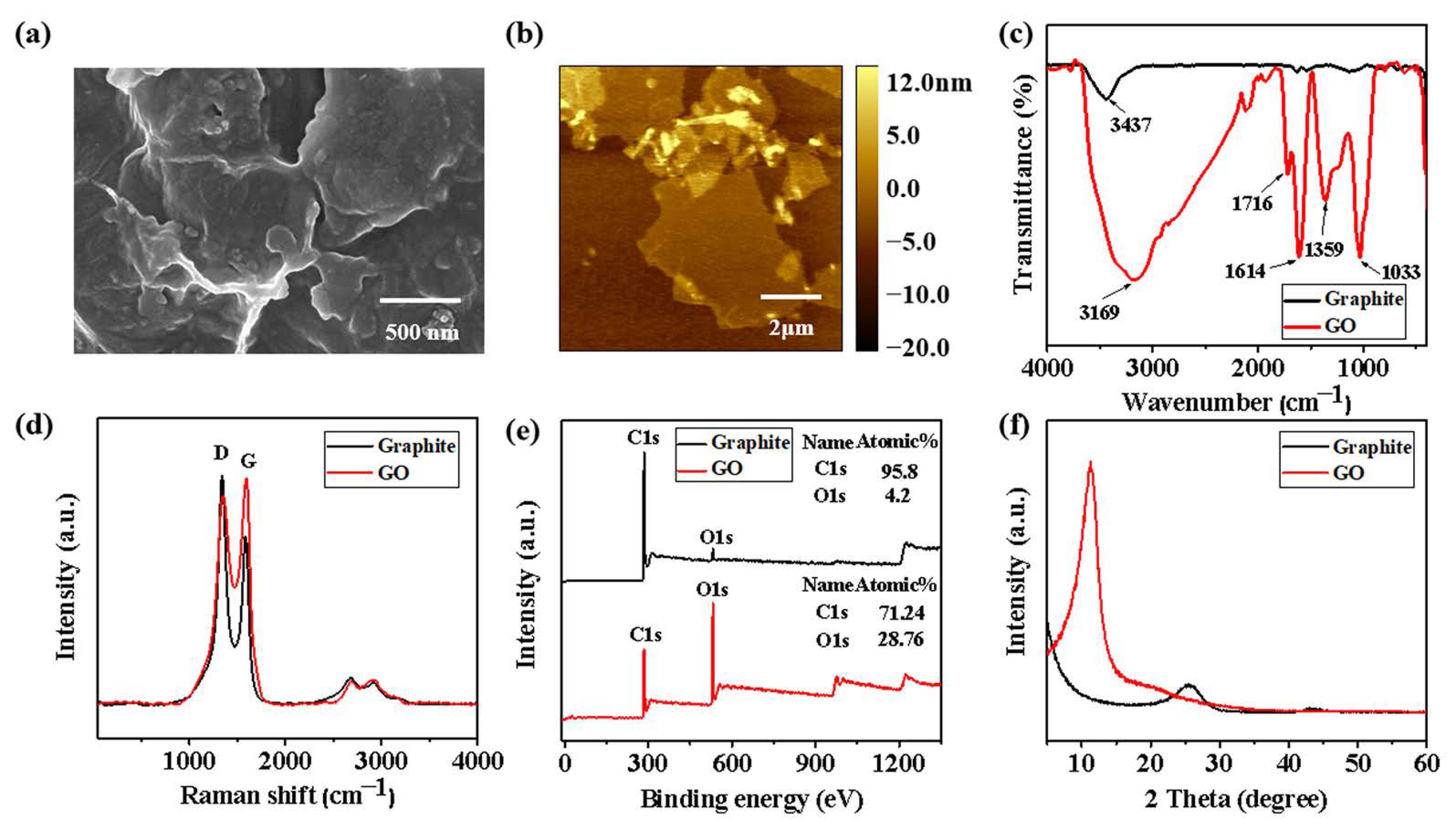
2.2. Preparation of GO
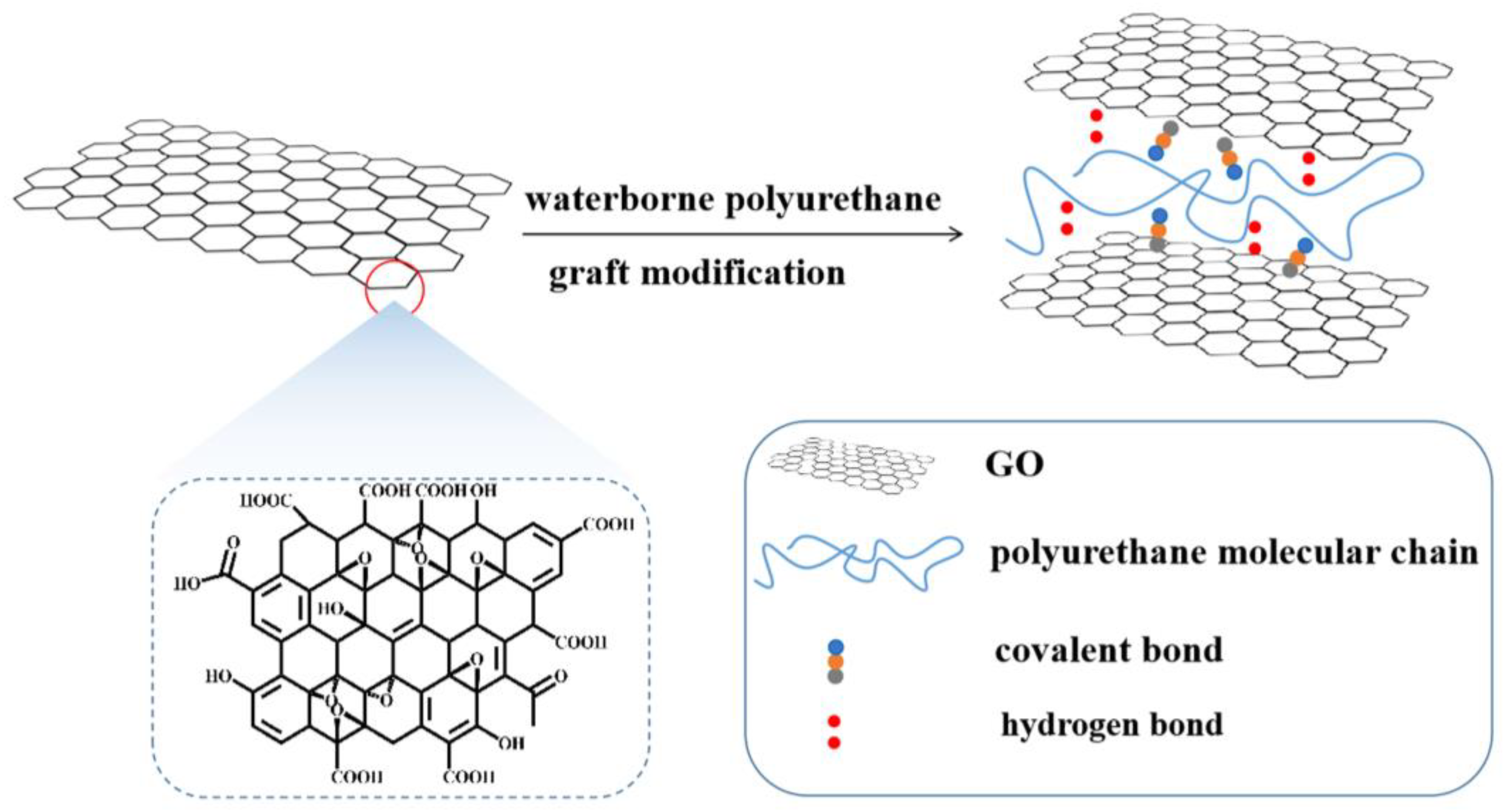
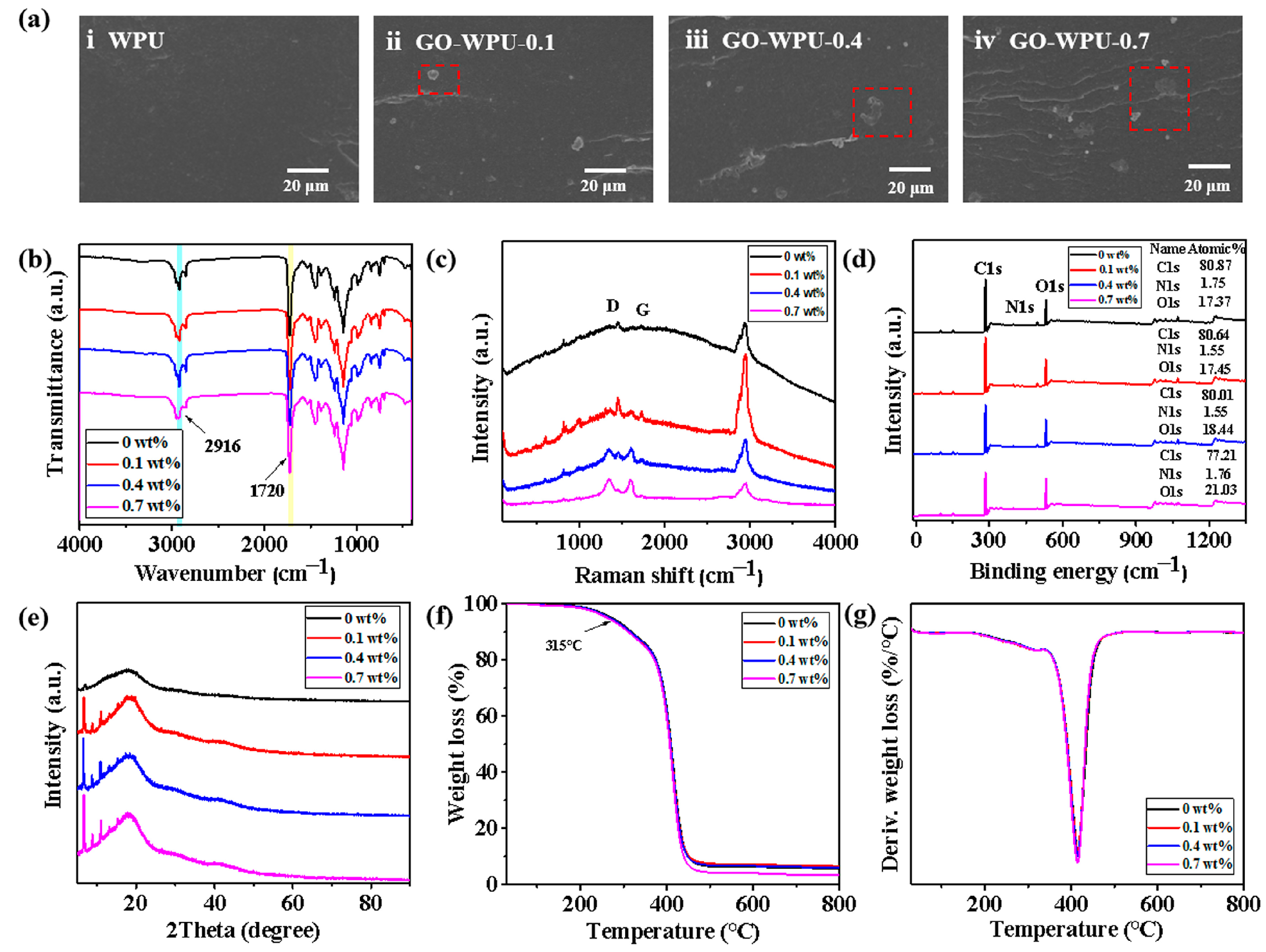
2.3. Preparation of GO-WPU Composite Emulsion
2.4. Preparation of Coating
2.5. Structural Characterization and Performance Testing
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.X.; Kong, D.J. Comparison on electrochemical corrosion performances of arc and laser thermal sprayed Al-Ti-Ni coatings in marine environment. Mater. Chem. Phys. 2020, 251, 123200. [Google Scholar]
- Shi, Z.; Li, A.; Zhang, K.; Zhang, Y.F. Reduced graphene oxide coated polyurethane composite foams as flexible strain sensors for large deformation. Mater. Sci. Eng. B 2021, 272, 115360. [Google Scholar] [CrossRef]
- Wang, W.Q.; Zhang, S.Q.; Xiao, S.; Sato, Y.S.; Wang, D.; Liu, Y.; Liu, D.L.; Hu, Q. Microstructure and properties of multilayer WC-40Co coating on Ti-6Al-4V by electron beam cladding. Mater. Charact. 2022, 183, 111585. [Google Scholar] [CrossRef]
- Chisty, A.H.; Mallik, A.K.; Robel, F.N.; Shahruzzaman, M.; Haque, P.; Hossain, K.S.; Khan, R.A.; Rahman, M.M. Enhanced epoxy/GO composites mechanical and thermal properties by removing air bubbles with shear mixing and ultrasonication. ChemistrySelect 2019, 4, 11417–11425. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.J.; Chen, P.G.; Wang, W.; Cao, J.Z. Enhancing weathering resistance of wood by using bark extractives as natural photostabilizers in polyurethane-acrylate coating. Prog. Org. Coat. 2020, 145, 105665. [Google Scholar] [CrossRef]
- Safarpour, M.; Najjarizad-Peyvasti, S.; Khataee, A.; Karimi, A. Polyethersulfone ultrafiltration membranes incorporated with CeO2/GO nanocomposite for enhanced fouling resistance and dye separation. J. Environ. Chem. Eng. 2022, 10, 107533. [Google Scholar] [CrossRef]
- Khan, A.A.; De Vera, M.A.T.; Mohamed, B.A.; Javed, R.; Al-Kheraif, A.A. Enhancing the physical properties of acrylic resilient denture liner using graphene oxide nanosheets. J. Vinyl. Addit. Techn. 2022, 28, 487–493. [Google Scholar] [CrossRef]
- Jing, L.C.; Wang, T.; Cao, W.W.; Wen, J.G.; Zhao, H.; Ning, Y.J.; Yuan, X.T.; Tian, Y.; Teng, L.H.; Geng, H.Z. Water-based polyurethane composite anticorrosive barrier coating via enhanced dispersion of functionalized graphene oxide in the presence of acidified multi-walled carbon nanotubes. Prog. Org. Coat. 2020, 146, 105734. [Google Scholar] [CrossRef]
- Mohammadi, A.; Barikani, M.; Doctorsafaei, A.H.; Isfahani, A.P.; Shams, E.; Ghalei, B. Aqueous dispersion of polyurethane nanocomposites based on calix arenes modified graphene oxide nanosheets: Preparation, characterization, and anti-corrosion properties. Chem. Eng. J. 2018, 349, 466–480. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Shao, L.S.; Liu, B.; Wang, F.; Wang, Y.H. Effect of molecular weight of liquid polysulfide on water and organic solvent resistances of waterborne polyurethane/polysulfide copolymer. Prog. Org. Coat. 2017, 112, 219–224. [Google Scholar] [CrossRef]
- Fu, X.K.; Cao, H.B.; An, Y.L.; Zhou, H.D.; Shi, Y.P.; Hou, G.L.; Ha, W. Bioinspired hydroxyapatite coating infiltrated with a graphene oxide hybrid supramolecular hydrogel orchestrates antibacterial and self-lubricating performance. ACS Appl. Mater. Interfaces 2022, 14, 31702–31714. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Pan, M.W.; Yuan, J.F.; Wang, J.X.; Zhu, L.; Jia, Z.Y.; Song, S.F. Facile fabrication of dual-crosslinked single network heterostructural polyurethane hydrogels with superior mechanical and fluorescent performance. React. Funct. Polym. 2020, 146, 10443. [Google Scholar] [CrossRef]
- Lei, Y.; Liu, Z.M.; Wu, B.; Jiang, L.; Lei, J.X. Preparation and properties of cross-linked waterborne polyurethane based on solvent-free route. Polym. Bull. 2020, 77, 3263–3275. [Google Scholar] [CrossRef]
- Brzeska, J.; Tercjak, A.; Sikorska, W.; Kowalczuk, M.; Rutkowsk, M. Predicted studies of branched and cross-linked polyurethanes based on polyhydroxybutyrate with polycaprolactone triol in soft segments. Polymers 2020, 12, 1068. [Google Scholar] [CrossRef]
- Si, H.Y.; Liu, H.; Shang, S.B.; Song, J.; Liao, S.L.; Wang, D.; Song, Z.Q. Preparation and properties of maleopimaric acid-based polyester polyol dispersion for two-component waterborne polyurethane coating. Prog. Org. Coat. 2016, 90, 309–316. [Google Scholar] [CrossRef]
- Okamoto, Y.; Hasegawa, Y.; Yoshino, F. Urethane/acrylic composite polymer emulsions. Prog. Org. Coat. 2006, 29, 175–182. [Google Scholar] [CrossRef]
- Ma, L.; Song, L.; Li, F.; Wang, H.; Liu, B.H. Preparation and properties of poly (propylene carbonate)-based waterborne polyurethane-acrylate composite emulsion. Colloid Polym. Sci. 2017, 295, 2299–2307. [Google Scholar] [CrossRef]
- Zhu, X.B.; Yan, Q.Q.; Cheng, L.; Wu, H.; Zhao, H.C.; Wang, L.P. Self-alignment of cationic graphene oxide nanosheets for anticorrosive reinforcement of epoxy coatings. Chem. Eng. J. 2020, 389, 124435. [Google Scholar] [CrossRef]
- Yue, S.J.; Zhang, Z.Y.; Fan, X.J.; Liu, P.; Xiao, C.F. Effect of 3-aminopropyltriethoxysilane on solvent resistance, thermal stability, and mechanical propertiesof two-component waterborne polyurethane. Int. J. Polym. Anal. Charact. 2015, 20, 285–297. [Google Scholar] [CrossRef]
- Mohanty, D.; Kanny, M.K.; Mohanty, S. Highly transparent castor oil-derived polyurethane/silica nanocomposite coating synthesized by in situ polymerization. Polym. Adv. Technol. 2022, 10, 3605–3618. [Google Scholar] [CrossRef]
- Wei, Z.K.; Liu, Z.M.; Fu, X.W.; Wang, Y.C.; Yuan, A.Q.; Lei, J.X. Effect of crystalline structure on water resistance of waterborne polyurethane. Eur. Polym. J. 2021, 157, 110647. [Google Scholar] [CrossRef]
- Feng, C.; Zhu, L.J.; Cao, K.Y.; Yu, Z.X.; Song, Y.C. Difunctional silicon dioxide combined with graphene oxide nanocomposite to enhance the anticorrosion performance of epoxy coatings. ACS Omega. 2022, 7, 24134–24144. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.H.; Mahajan, M.S.; Sreeharsha, N.; Gite, V.V.; Al-Dhubiab, B.E.; Kaliyadan, F.; Nanjappa, S.H.; Meravanige, G.; Aleyadhy, D.M. Enhancement of anticorrosive performance of cardanol based polyurethane coatings by incorporating magnetic hydroxyapatite nanoparticles. Materials 2022, 15, 2308. [Google Scholar] [CrossRef]
- Kong, L.L.; Xu, D.D.; He, Z.X.; Wang, F.Q.; Gui, S.H.; Fan, J.L.; Pan, X.Y.; Dai, X.H.; Dong, X.Y.; Liu, B.X.; et al. Nanocellulose-reinforced polyurethane for waterborne wood coating. Molecules 2019, 24, 3151. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.N.; Hiraoka, T.H.; Honda, T.; Hsu, U.I.; Asoh, T.A.; Uyama, H. Oligoether grafting on cellulose microfibers for dispersion in poly (propylene glycol) and fabrication of reinforced polyurethane composite. Compos. Sci. Technol. 2021, 202, 108595. [Google Scholar] [CrossRef]
- Tayebia, P.; Asefnejada, A.; Khonakdar, H.A. Water-based polyurethane/functionalized chitosan/zinc oxide nanoparticles nanocomposites: Physical, mechanical and biocompatibility properties. Polym. Plast. Technol. Mater. 2021, 13, 1474–1489. [Google Scholar] [CrossRef]
- Du, W.N.; Jin, Y.; Lai, S.Q.; Shi, L.J.; Shen, Y.C.; Yang, H. Multifunctional light-responsive graphene-based polyurethane composites with shape memory, self-healing, and flame retardancy properties. Composites Part A 2020, 128, 105686. [Google Scholar] [CrossRef]
- Zhang, W.G.; Zou, X.B.; Liu, X.L.; Liang, Z.; Ge, Z.; Luo, Y.J. Preparation and properties of waterborne polyurethane modified by aminoethylaminopropyl polydimethylsiloxane for fluorine-free water repellents. Prog. Org. Coat. 2020, 139, 105407. [Google Scholar] [CrossRef]
- Zhu, M.J.; Li, S.Y.; Sun, Q.Y.; Shi, B. Enhanced mechanical property, chemical resistance and abrasion durability of waterborne polyurethane based coating by incorporating highly dispersed polyacrylic acid modified graphene oxide. Prog. Org. Coat. 2022, 170, 106949. [Google Scholar] [CrossRef]
- Liu, C.; Yan, H.X.; Lv, Q.; Li, S.; Niu, S. Enhanced tribological properties of aligned reduced graphene oxide-Fe3O4@polyphosphazene/bismaleimides composites. Carbon 2016, 102, 145e153. [Google Scholar] [CrossRef]
- Akhan, S.; Oktay, B.; Özdemir, O.K.; Madakbaş, S.; Apohan, N.K. Polyurethane graphene nanocomposites with self-healing properties by azide-alkyne click reaction. Mater. Chem. Phys. 2020, 254, 123315. [Google Scholar] [CrossRef]
- Zhu, Q.S.; Li, E.; Liu, X.H.; Song, W.Q.; Li, Y.L.; Wang, X.C.; Liu, C.T. Epoxy coating with in-situ synthesis of polypyrrole functionalized graphene oxide for enhanced anticorrosive performance. Prog. Org. Coat. 2020, 140, 105488. [Google Scholar] [CrossRef]
- Xu, D.D.; Liang, G.T.; Qi, Y.R.; Gong, R.Z.; Zhang, X.Q.; Zhang, Y.M.; Liu, B.X.; Kong, L.L.; Dong, X.Y.; Li, Y.F. Enhancing the Mechanical Properties of Waterborne Polyurethane Paint by Graphene Oxide for Wood Products. Polymers 2022, 14, 5456. [Google Scholar] [CrossRef]
- Tian, X.X.; Sun, Y.L.; Xie, H.P.; Shi, B.R.; Zhong, J.H.; Sheng, D.K.; Yang, Y.M. Preparation of graphene oxide/waterborne polyurethane via boric acid cross-linked dopamine enhanced barrier and mechanical properties. Front. Mater. 2022, 9, 1046125. [Google Scholar] [CrossRef]
- Zhuo, Y.G.; Liu, J.; Li, Q.S.; Qiu, B.; Xing, G.Z. Preparation and characterization of WPU/CNT/GO nanocomposites. Integr. Ferroelectr. 2016, 171, 52–58. [Google Scholar] [CrossRef]
- Hou, L.T.; Zhou, M.; Gu, Y.H.; Chen, Y.P. WPU/CB/GO nanocomposites: In situ polymerization preparation, thermal, and anticorrosion performance evaluation. J. Appl. Polym. Sci. 2019, 137, 48716. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Wang, S.; Liu, W.Q.; Shi, H.Y.; Liang, L.Y.; Liu, C.H.; Pi, K.; Zhang, W.C.; Zeng, J.J. Design on the corrosion protection of eco-friendly and multifunctional polyhedral oligomeric silsesquioxane functionalized graphene oxide reinforced waterborne polyurethane. Colloids Surf. A 2020, 640, 127718. [Google Scholar] [CrossRef]
- Bai, T.; Liu, Z.; Pei, Z.G.; Fang, W.Q.; Ma, Y. Tribological Performance Studies of Waterborne Polyurethane Coatings with Aligned Modified Graphene Oxide@Fe3O4. ACS Omega 2021, 6, 9243–9253. [Google Scholar] [CrossRef]
- Zheng, F.L.; Jiang, P.P.; Hu, L.; Bao, Y.M.; Xia, J. Functionalization of graphene oxide with different diisocyanates and their use as a reinforcement in waterborne polyurethane composites. J. Macromol. Sci., Part A Pure Appl. Chem. 2019, 56, 1071–1081. [Google Scholar] [CrossRef]
- Chen, C.; Wei, S.C.; Xiang, B.; Wang, B.; Wang, Y.J.; Liang, Y.; Yuan, Y. Synthesis of Silane Functionalized Graphene Oxide and Its Application in Anti-Corrosion Waterborne Polyurethane Composite Coatings. Coatings 2019, 9, 587. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Liu, W.Q.; Wang, S.; Jiang, C.; Xie, Y.K.; Yang, M.P.; Shi, H.Y. A novel and feasible approach for polymer amine modified graphene oxide to improve water resistance, thermal, and mechanical ability of waterborne polyurethane. Appl. Surf. Sci. 2019, 491, 301–312. [Google Scholar] [CrossRef]
- Li, R.; Shan, Z.H. 3-Aminopropyltriethoxysilane functionalized graphite oxide/waterborne polyurethane composites: Structural, thermal conductivity and physic-chemical properties. Diamond Relat. Mater. 2019, 98, 107481. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Razieh Chaharmahali, R. Enhancing corrosion and wear performance of PEO coatings on Mg alloys using graphene and graphene oxide additions: A review. FlatChem 2021, 27, 100241. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Perumbilavil, S.; Sankar, P.; Rose, T.P.; Philip, R. White light z-scan measurements of ultrafast optical nonlinearity in reduced graphene oxide nanosheets in the 400–700nm region. Appl. Phys. Lett. 2015, 114, 12517–12650. [Google Scholar]
- Song, B.; Liu, Z.D.; Chen, L.; Ma, L.C.; Huang, Y.D. Poly(ρ-phenylene benzobisoxazole) fiber/epoxy composites reinforced with carbon nanotubes and graphene oxide for enhanced interfacial adhesion and mechanical strength. ACS Appl. Nano Mater. 2021, 4, 12158–12169. [Google Scholar] [CrossRef]
- Fang, Z.; Huang, L.J.; Fu, J.J. Research status of graphene polyurethane composite coating. Coatings 2022, 12, 264. [Google Scholar] [CrossRef]
- Piotr, K.; Bozena, K.; Kinga, P.; Milena, Š. Composites prepared from the waterborne polyurethane cationomers-modified graphene. Part II. Electrical properties of the polyurethane films. Colloid Polym. Sci. 2015, 293, 2941–2947. [Google Scholar]
- Cheng, L.S.; Ren, S.B.; Lu, X.N. Application of eco-friendly waterborne polyurethane composite coating incorporated with nano cellulose crystalline and silver nano particles on wood antibacterial board. Polymers 2020, 12, 407. [Google Scholar] [CrossRef]
- Corcione, A.; Corcione, C.E.; Frigione, M.; Pegoretti, A. Photocurable resin/nanocellulose composite coatings for wood protection. Prog. Org. Coat. 2017, 106, 128–136. [Google Scholar]
- Kluge, M.; Veigel, S.; Pink, S.; Henniges, U.; Zollfrank, C.; Rossler, A.; Gindl-Altmutter, W. Nanocellulosic fillers for waterborne wood coatings:reinforcement effect on free-standing coating films. Wood Sci. Technol. 2017, 51, 601–613. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Li, Z.F. Preparation and characterization of a novel polyurethane/polyurethane modified graphene oxide composites. Colloid Polym. Sci. 2021, 299, 1767–1776. [Google Scholar] [CrossRef]
- Zhi, L.; Zhang, C.Q.; Liu, Z.Z.; Liu, T.; Dou, X.Y.; Chen, Y.Q.; Ou, R.X.; Wang, Q.W. Flexible decorative wood veneer with high strength, wearability and moisture penetrability enabled by infiltrating castor oil-based waterborne polyurethanes. Composites Part B 2022, 230, 109502. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, S.B.; Wang, D.; Li, Y.L.; Zheng, P.; Gao, L.; Huo, D.; Cheng, L.; Wei, S.Y. Study on antibacterial wood coatings with soybean protein isolate nano-silver hydrosol. Prog. Org. Coat. 2022, 165, 106766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.W.; Ding, J.J.; Ye, L. Structure and solvent-resistant property of fluorinated polyurethane elastomer. J. Fluorine Chem. 2014, 159, 38–47. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, Z.; Jing, X.; Li, X.D.; Wang, J.W.; Kang, M.Q.; Zhao, Y.H.; Li, Q.F. Construction and effect of intramolecular hydrogen bond on solvent resistance of polymeric membranes and their application in impermeable membranes. J. Ind. Eng. Chem. 2022, 107, 302–312. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, G.; Yao, F.; Qi, Y.; Gong, R.; Li, R.; Liu, B.; Zhao, Y.; Lian, C.; Li, L.; Dong, X.; et al. Improvement of Mechanical Properties and Solvent Resistance of Polyurethane Coating by Chemical Grafting of Graphene Oxide. Polymers 2023, 15, 882. https://doi.org/10.3390/polym15040882
Liang G, Yao F, Qi Y, Gong R, Li R, Liu B, Zhao Y, Lian C, Li L, Dong X, et al. Improvement of Mechanical Properties and Solvent Resistance of Polyurethane Coating by Chemical Grafting of Graphene Oxide. Polymers. 2023; 15(4):882. https://doi.org/10.3390/polym15040882
Chicago/Turabian StyleLiang, Guotao, Fengbiao Yao, Yanran Qi, Ruizhi Gong, Rui Li, Baoxuan Liu, Yueying Zhao, Chenglong Lian, Luming Li, Xiaoying Dong, and et al. 2023. "Improvement of Mechanical Properties and Solvent Resistance of Polyurethane Coating by Chemical Grafting of Graphene Oxide" Polymers 15, no. 4: 882. https://doi.org/10.3390/polym15040882





