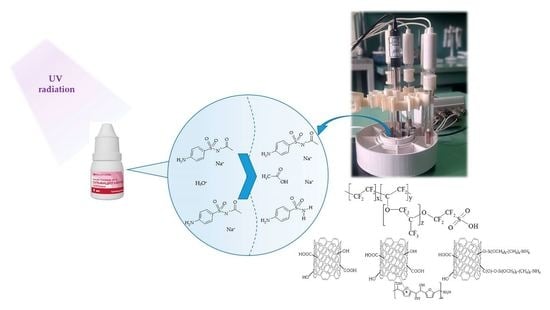
Potentiometric Sensor Arrays Based on Hybrid PFSA/CNTs Membranes for the Analysis of UV-Degraded Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Functionalization of CNTs
2.3. Membrane Synthesis
2.4. Apparatus and Experiment
2.5. Model Solutions and Pharmaceutical Solutions

2.6. Data-Processing Procedures
3. Results and Discussion
3.1. Cross-Sensitivity of the DP-Sensors
3.2. Characteristics of the Multisensory System
3.3. Analysis of the UV-Degraded Drug
3.4. Comparison of the Proposed Sensors with the Reported Ones
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayed, R.A.; Mohamed, A.R.; Hassan, W.S.; Elmasry, M.S. Earth-Friendly-Assessed Chromatographic Platforms for Rapid Analysis of Sulfacetamide Sodium and Prednisolone Acetate in Presence of Sulfanilamide Impurity: Application on Ophthalmic Formulation and Aqueous Humor. Sustain. Chem. Pharm. 2022, 27, 100694. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abdelwahab, M.H.; Hendawy, H.A.M.; Weshahy, S.A.; Abbas, S.S. Selective and Sensitive Chromatographic Methods for Determination of a Co-Formulated Binary Mixture in Antibacterial Eye Drops and Aqueous Humor in the Presence of Their Degradation Products and Potential Impurities. J. Chromatogr. Sci. 2020, 58, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Fang, B.; Wang, S. A Fast and Validated HPLC Method for Simultaneous Determination of Dopamine, Dobutamine, Phentolamine, Furosemide, and Aminophylline in Infusion Samples and Injection Formulations. J. Anal. Methods Chem. 2021, 2021, 8821126. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, N.S.; Abdelrahman, M.M.; Boshra, J.M.; Taha, A.A. Different Stability-indicating Chromatographic Methods for Specific Determination of Paracetamol, Dantrolene Sodium, Their Toxic Impurities and Degradation Products. Biomed. Chromatogr. 2019, 33, e4598. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; El Deeb, S.; Abdellatef, H.E.; Hendawy, H.A.M.; El-Abassy, O.M.; Ibrahim, H. Eco-Friendly and Sensitive HPLC and TLC Methods Validated for the Determination of Betahistine in the Presence of Its Process-Related Impurity. Separations 2022, 9, 49. [Google Scholar] [CrossRef]
- Kamal, A.H.; Marie, A.A.; Hammad, S.F. Stability Indicating RP-HPLC Method for Simultaneous Determination of Omeprazole and Aspirin in the Presence of Salicylic Acid as Degradation Product. Microchem. J. 2020, 152, 104350. [Google Scholar] [CrossRef]
- Walash, M.I.; Abo El Abass Mohamed, S. Two RP-HPLC Assay Methods with Different Chromatographic Approaches for the Simultaneous Estimation of Bambuterol and Its Main Degradation Product, Terbutaline. Anal. Methods 2019, 11, 1680–1688. [Google Scholar] [CrossRef]
- Fajardo, F.A.G.; Tavares, M.F.M.; Rashid, A.; Prado, M.S.A. Novel Eco-Friendly Stability Indicating Capillary Zone Electrophoresis Method for Determination of Aripiprazole in Tablet Dosage Form: DoE Directed Optimization, Development and Method Validation. J. Pharm. Sci. 2022, 111, 3340–3351. [Google Scholar] [CrossRef]
- Al Alamein, A.M.A.; Saad, A.S.; Galal, M.M.; Zaazaa, H.E. A Comparative Study of Different Chromatographic Techniques for Determination of Toxic Impurities of Some Commonly Used Anesthetics. JPC—J. Planar Chromatogr.—Mod. TLC 2018, 31, 280–289. [Google Scholar] [CrossRef]
- Alatawi, H.; Hogan, A.; Albalawi, I.; O’Sullivan-Carroll, E.; Wang, Y.; Moore, E. Fast Determination of Paracetamol and Its Hydrolytic Degradation Product P-aminophenol by Capillary and Microchip Electrophoresis with Contactless Conductivity Detection. Electrophoresis 2022, 43, 857–864. [Google Scholar] [CrossRef]
- Attia, K.A.M.; El-Abasawi, N.M.; El-Olemy, A.; Serag, A. Different Spectrophotometric Methods Applied for the Analysis of Simeprevir in the Presence of Its Oxidative Degradation Product: Acomparative Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Merey, H.A.; Ramadan, N.K.; Diab, S.S.; Moustafa, A.A. Green Spectrophotometric Methods for the Determination of a Binary Mixture of Lidocaine Hydrochloride and Cetylpyridinium Chloride in the Presence of Dimethylaniline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118743. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.A.; Bakr, M.A.; Badawey, A.M.; Abbas, S.S. Univariate and Multivariate Assisted Spectrophotometric Methods for Determination of Rosuvastatin Calcium and Fenofibrate in Bulk Powders and Tablets along with Their Degradation Products. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119163. [Google Scholar] [CrossRef] [PubMed]
- Yehia, A.M.; Sami, I.; Riad, S.M.; El-Saharty, Y.S. Qualitative and Quantitative Chemometry as Stability-Indicating Methods for Determination of Dantrolene Sodium and Paracetamol. CPA 2017, 14, 60–67. [Google Scholar] [CrossRef]
- Darwish, H.W.; Ali, A.N.; Naguib, I.A.; El Ghobashy, M.R.; Al-Hossaini, A.M.; Abdelrahman, M.M. Stability Indicating Spectrophotometric Methods for Quantitative Determination of Bromazepam and Its Degradation Product. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118433. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, Y.A.; Ibrahim, A.E.; El Deeb, S.; Sayed, R.A. Green Chemometric Determination of Cefotaxime Sodium in the Presence of Its Degradation Impurities Using Different Multivariate Data Processing Tools; GAPI and AGREE Greenness Evaluation. Molecules 2023, 28, 2187. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, J.; Yi, Y.; Kingsford, O.J.; Zhu, G. Nitrogen-Doped Hollow Carbon Nanospheres Wrapped with MoS2 Nanosheets for Simultaneous Electrochemical Determination of Acetaminophen and 4-Aminophenol. J. Electroanal. Chem. 2019, 847, 113229. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, S.; Qu, J. Electrochemical Sensor Based on Palladium-Reduced Graphene Oxide Modified with Gold Nanoparticles for Simultaneous Determination of Acetaminophen and 4-Aminophenol. Talanta 2018, 178, 188–194. [Google Scholar] [CrossRef]
- Almandil, N.B.; Ibrahim, M.; Ibrahim, H.; Kawde, A.-N.; Shehatta, I.; Akhtar, S. A Hybrid Nanocomposite of CeO2–ZnO–Chitosan as an Enhanced Sensing Platform for Highly Sensitive Voltammetric Determination of Paracetamol and Its Degradation Product p-Aminophenol. RSC Adv. 2019, 9, 15986–15996. [Google Scholar] [CrossRef] [Green Version]
- Saad, A.S.; Al-Alamein, A.M.A.; Galal, M.M.; Zaazaa, H.E. Voltammetric Determination of Lidocaine and Its Toxic Metabolite in Pharmaceutical Formulation and Milk Using Carbon Paste Electrode Modified with C18 Silica. J. Electrochem. Soc. 2019, 166, B103–B109. [Google Scholar] [CrossRef]
- Gad, E.M.; Hendawy, H.A.M.; Fouad, M.A.; Khaled, E. Carbonaceous Nanomaterials Integrated Carbon Paste Sensors for Adsorptive Anodic Voltammetric Determination of Butenafine in the Presence of Its Degradation Product. Microchem. J. 2022, 183, 107956. [Google Scholar] [CrossRef]
- Hendawy, H.A.M.; Salem, W.M.; Abd-Elmonem, M.S.; Khaled, E. Nanomaterial-Based Carbon Paste Electrodes for Voltammetric Determination of Naproxen in Presence of Its Degradation Products. J. Anal. Methods Chem. 2019, 2019, 5381031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-nami, S.Y.; Alorabi, A.Q.; Al-Ahmed, Z.A.; Mogharbel, A.T.; Abumelha, H.M.; Hussein, M.A.; El-Metwaly, N.M. Superficial and Inkjet Scalable Printed Sensors Integrated with Iron Oxide and Reduced Graphene Oxide for Sensitive Voltammetric Determination of Lurasidone. ACS Omega 2023, 8, 10449–10458. [Google Scholar] [CrossRef] [PubMed]
- Gadallah, M.I.; Ali, H.R.H.; Askal, H.F.; Saleh, G.A. Poly (Bromocresol Green) Flakes-Decorated Pencil Graphite Electrode for Selective Electrochemical Sensing Applications and Pharmacokinetic Studies. Mater. Sci. Eng. C 2019, 102, 634–645. [Google Scholar] [CrossRef] [PubMed]
- El-Yazbi, A.F.; Sabry, S.M.; Moneeb, M.S.; Elgammal, F.A.H.; Essam, H.M. Economic Electrochemical Sensors for the Determination of Eszopiclone in Pharmaceutical Formulation with Greenness Profile Assessment. Electroanalysis 2023, 35, e202200082. [Google Scholar] [CrossRef]
- Rezk, M.R.; Fayed, A.S.; Marzouk, H.M.; Abbas, S.S. Potentiometric Ion-Selective Electrodes for Determination of Cyclopentolate Hydrochloride and Phenylephrine Hydrochloride in Their Challenging Ophthalmic Formulation. J. Solid State Electrochem. 2018, 22, 3351–3361. [Google Scholar] [CrossRef]
- Abdel-Gawad, S.A.; Altharawi, A. A Comparative Study between Screen-Printed and Solid-Contact Electrodes for the Stability-Indicating Determination of Bromazepam. Molecules 2022, 27, 7616. [Google Scholar] [CrossRef] [PubMed]
- Yaroshenko, I.S.; Alyapyshev, M.Y.; Babain, V.A.; Legin, A.V.; Kirsanov, D.O. Potentiometric Sensors and Multisensor Systems for the Determination of Lanthanides. J. Anal. Chem. 2019, 74, 1003–1018. [Google Scholar] [CrossRef]
- Ashina, J.; Babain, V.; Kirsanov, D.; Legin, A. A Novel Multi-Ionophore Approach for Potentiometric Analysis of Lanthanide Mixtures. Chemosensors 2021, 9, 23. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Crothers, A.R.; Radke, C.J.; Weber, A.Z. Impact of Nano- and Mesoscales on Macroscopic Cation Conductivity in Perfluorinated-Sulfonic-Acid Membranes. J. Phys. Chem. C 2017, 121, 28262–28274. [Google Scholar] [CrossRef] [Green Version]
- Mabuchi, T.; Tokumasu, T. Relationship between Proton Transport and Morphology of Perfluorosulfonic Acid Membranes: A Reactive Molecular Dynamics Approach. J. Phys. Chem. B 2018, 122, 5922–5932. [Google Scholar] [CrossRef]
- Welch, C.; Labouriau, A.; Hjelm, R.; Orler, B.; Johnston, C.; Kim, Y.S. Nafion in Dilute Solvent Systems: Dispersion or Solution? ACS Macro Lett. 2012, 1, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Loppinet, B.; Gebel, G.; Williams, C.E. Small-Angle Scattering Study of Perfluorosulfonated Ionomer Solutions. J. Phys. Chem. B 1997, 101, 1884–1892. [Google Scholar] [CrossRef]
- Berlinger, S.A.; Dudenas, P.J.; Bird, A.; Chen, X.; Freychet, G.; McCloskey, B.D.; Kusoglu, A.; Weber, A.Z. Impact of Dispersion Solvent on Ionomer Thin Films and Membranes. ACS Appl. Polym. Mater. 2020, 2, 5824–5834. [Google Scholar] [CrossRef]
- Collette, F.M.; Thominette, F.; Mendil-Jakani, H.; Gebel, G. Structure and Transport Properties of Solution-Cast Nafion® Membranes Subjected to Hygrothermal Aging. J. Membr. Sci. 2013, 435, 242–252. [Google Scholar] [CrossRef]
- Safronova, E.; Safronov, D.; Lysova, A.; Parshina, A.; Bobreshova, O.; Pourcelly, G.; Yaroslavtsev, A. Sensitivity of Potentiometric Sensors Based on Nafion®-Type Membranes and Effect of the Membranes Mechanical, Thermal, and Hydrothermal Treatments on the on Their Properties. Sens. Actuators B Chem. 2017, 240, 1016–1023. [Google Scholar] [CrossRef]
- Xiao, R.; Guo, J.; Nguyen, T.D. Modeling the Multiple Shape Memory Effect and Temperature Memory Effect in Amorphous Polymers. RSC Adv. 2015, 5, 416–423. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Ionic Mobility in Ion-Exchange Membranes. Membranes 2021, 11, 198. [Google Scholar] [CrossRef]
- Prykhodko, Y.; Fatyeyeva, K.; Hespel, L.; Marais, S. Progress in Hybrid Composite Nafion®-Based Membranes for Proton Exchange Fuel Cell Application. Chem. Eng. J. 2021, 409, 127329. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Ryu, S.K.; Yoo, D.J. Structurally Modulated and Functionalized Carbon Nanotubes as Potential Filler for Nafion Matrix toward Improved Power Output and Durability in Proton Exchange Membrane Fuel Cells Operating at Reduced Relative Humidity. J. Membr. Sci. 2022, 649, 120393. [Google Scholar] [CrossRef]
- Asgari, M.S.; Nikazar, M.; Molla-abbasi, P.; Hasani-Sadrabadi, M.M. Nafion®/Histidine Functionalized Carbon Nanotube: High-Performance Fuel Cell Membranes. Int. J. Hydrogen Energy 2013, 38, 5894–5902. [Google Scholar] [CrossRef]
- Maiti, T.K.; Singh, J.; Dixit, P.; Majhi, J.; Bhushan, S.; Bandyopadhyay, A.; Chattopadhyay, S. Advances in Perfluorosulfonic Acid-Based Proton Exchange Membranes for Fuel Cell Applications: A Review. Chem. Eng. J. Adv. 2022, 12, 100372. [Google Scholar] [CrossRef]
- Voropaeva, D.Y.; Safronova, E.Y.; Novikova, S.A.; Yaroslavtsev, A.B. Recent Progress in Lithium-Ion and Lithium Metal Batteries. Mendeleev Commun. 2022, 32, 287–297. [Google Scholar] [CrossRef]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of Material Research and Development for Vanadium Redox Flow Battery Applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Falina, I.; Loza, N.; Loza, S.; Titskaya, E.; Romanyuk, N. Permselectivity of Cation Exchange Membranes Modified by Polyaniline. Membranes 2021, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Wenninger, N.; Bračič, U.; Kollau, A.; Pungjunun, K.; Leitinger, G.; Kalcher, K.; Ortner, A. Development of an Electrochemical Sensor for Nitric Oxide Based on Carbon Paste Electrode Modified with Nafion, Gold Nanoparticles and Graphene Nanoribbons. Sens. Actuators B Chem. 2021, 346, 130532. [Google Scholar] [CrossRef]
- Karthika, A.; Rosaline, D.R.; Inbanathan, S.S.R.; Suganthi, A.; Rajarajan, M. Fabrication of Cupric Oxide Decorated β-Cyclodextrin Nanocomposite Solubilized Nafion as a High Performance Electrochemical Sensor for l-Tyrosine Detection. J. Phys. Chem. Solids 2020, 136, 109145. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Rahman, M.M.; Sheikh, T.A.; Nadeem Arshad, M.; Al-Zahrani, F.A.M.; Asiri, A.M. A New Cr3+ Electrochemical Sensor Based on ATNA/Nafion/Glassy Carbon Electrode. Materials 2020, 13, 2695. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Y.; Yang, H.; Xu, Z. Preparation of Ultra-Stable PFSA-Ag Colloid and Its Application as Electrochemical Sensor for 4-Nitrophenol Detection. Synth. Met. 2020, 260, 116269. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Al-Ghamdi, H.A.; Alam, M.M.; Al-amshany, Z.M.; Asiri, A.M.; Rahman, M.M. Development of Cd2+ Sensor Based on BZNA/Nafion/Glassy Carbon Electrode by Electrochemical Approach. Chem. Eng. J. 2018, 352, 225–231. [Google Scholar] [CrossRef]
- Shahzad, F.; Iqbal, A.; Zaidi, S.A.; Hwang, S.-W.; Koo, C.M. Nafion-Stabilized Two-Dimensional Transition Metal Carbide (Ti3C2Tx MXene) as a High-Performance Electrochemical Sensor for Neurotransmitter. J. Ind. Eng. Chem. 2019, 79, 338–344. [Google Scholar] [CrossRef]
- Chen, Z.; Patel, R.; Berry, J.; Keyes, C.; Satterfield, C.; Simmons, C.; Neeson, A.; Cao, X.; Wu, Q. Development of Screen-Printable Nafion Dispersion for Electrochemical Sensor. Appl. Sci. 2022, 12, 6533. [Google Scholar] [CrossRef]
- Olejnik, A.; Siuzdak, K.; Karczewski, J.; Grochowska, K. A Flexible Nafion Coated Enzyme-free Glucose Sensor Based on Au-dimpled Ti Structures. Electroanalysis 2020, 32, 323–332. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Q.; Yang, B.; Xu, Q.; Xu, Q.; Hu, X. Electrochemical Sensor Construction Based on Nafion/Calcium Lignosulphonate Functionalized Porous Graphene Nanocomposite and Its Application for Simultaneous Detection of Trace Pb2+ and Cd2+. Sens. Actuators B Chem. 2018, 259, 540–551. [Google Scholar] [CrossRef]
- Prikhno, I.A.; Safronova, E.Y.; Yaroslavtsev, A.B. Hybrid Materials Based on Perfluorosulfonic Acid Membrane and Functionalized Carbon Nanotubes: Synthesis, Investigation and Transport Properties. Int. J. Hydrogen Energy 2016, 41, 15585–15592. [Google Scholar] [CrossRef]
- Prikhno, I.A.; Safronova, E.Y.; Il’in, A.B.; Yaroslavtsev, A.B. MF-4SC Hybrid Membranes Doped with Carbon Nanotubes Functionalized with Proton-Acceptor Groups. Nanotechnol. Russ. 2017, 12, 236–242. [Google Scholar] [CrossRef]
- Liu, J.; Cao, Y. An Electrochemical Sensor Based on an Anti-Fouling Membrane for the Determination of Histamine in Fish Samples. Anal. Methods 2021, 13, 685–694. [Google Scholar] [CrossRef]
- Kuyumcu Savan, E. Electrochemical Determination of N-Acetyl Cysteine in the Presence of Acetaminophen at Multi-Walled Carbon Nanotubes and Nafion Modified Sensor. Sens. Actuators B Chem. 2019, 282, 500–506. [Google Scholar] [CrossRef]
- Parshina, A.; Yelnikova, A.; Safronova, E.; Kolganova, T.; Kuleshova, V.; Bobreshova, O.; Yaroslavtsev, A. Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Functionalized CNTs for Determination of Sulfamethoxazole and Trimethoprim in Pharmaceuticals. Membranes 2022, 12, 1091. [Google Scholar] [CrossRef]
- Cesarino, I.; Cesarino, V.; Lanza, M.R.V. Carbon Nanotubes Modified with Antimony Nanoparticles in a Paraffin Composite Electrode: Simultaneous Determination of Sulfamethoxazole and Trimethoprim. Sens. Actuators B Chem. 2013, 188, 1293–1299. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Pourcelly, G.; Yaroslavtsev, A.B. The Transformation and Degradation of Nafion® Solutions under Ultrasonic Treatment. The Effect on Transport and Mechanical Properties of the Resultant Membranes. Polym. Degrad. Stab. 2020, 178, 109229. [Google Scholar] [CrossRef]
- Safronova, E.; Parshina, A.; Kolganova, T.; Yelnikova, A.; Bobreshova, O.; Pourcelly, G.; Yaroslavtsev, A. Potentiometric Multisensory System Based on Perfluorosulfonic Acid Membranes and Carbon Nanotubes for Sulfacetamide Determination in Pharmaceuticals. J. Electroanal. Chem. 2020, 873, 114435. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Sobczak, A.; Makowski, A. Photocatalytic Degradation of Sulfa Drugs with TiO2, Fe Salts and TiO2/FeCl3 in Aquatic Environment—Kinetics and Degradation Pathway. Appl. Catal. B Environ. 2009, 90, 516–525. [Google Scholar] [CrossRef]
- Moravcová, D.; Planeta, J.; Wiedmer, S.K. Silica-Based Monolithic Capillary Columns Modified by Liposomes for Characterization of Analyte–Liposome Interactions by Capillary Liquid Chromatography. J. Chromatogr. A 2013, 1317, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Anwar, N.; Sheraz, M.A.; Ahmad, I. Validation of a Stability-Indicating Spectrometric Method for the Determination of Sulfacetamide Sodium in Pure Form and Ophthalmic Preparations. J. Pharm. Bioallied Sci. 2017, 9, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Parshina, A.; Yelnikova, A.; Titova, T.; Kolganova, T.; Yurova, P.; Stenina, I.; Bobreshova, O.; Yaroslavtsev, A. Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Polyaniline and PEDOT for Multicomponent Analysis of Sulfacetamide Pharmaceuticals. Polymers 2022, 14, 2545. [Google Scholar] [CrossRef] [PubMed]
- Parshina, A.; Yelnikova, A.; Kolganova, T.; Titova, T.; Yurova, P.; Stenina, I.; Bobreshova, O.; Yaroslavtsev, A. Perfluorosulfonic Acid Membranes Modified with Polyaniline and Hydrothermally Treated for Potentiometric Sensor Arrays for the Analysis of Combination Drugs. Membranes 2023, 13, 311. [Google Scholar] [CrossRef]
- El-Ragehy, N.A.; Hegazy, M.A.; AbdElHamid, G.; Tawfik, S.A. Validated Potentiometric Method for the Determination of Sulfacetamide Sodium; Application to Its Pharmaceutical Formulations and Spiked Rabbit Aqueous Humor. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 207–212. [Google Scholar] [CrossRef]
- Kamel, A.H.; Almeida, S.A.A.; Goreti, M.; Sales, F.; Moreira, F.T.C. Sulfadiazine-Potentiometric Sensors for Flow and Batch Determinations of Sulfadiazine in Drugs and Biological Fluids. Anal. Sci. 2009, 25, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Almeida, S.A.A.; Moreira, F.T.C.; Heitor, A.M.; Montenegro, M.C.B.S.M.; Aguilar, G.G.; Sales, M.G.F. Sulphonamide-Imprinted Sol–Gel Materials as Ionophores in Potentiometric Transduction. Mater. Sci. Eng. C 2011, 31, 1784–1790. [Google Scholar] [CrossRef] [Green Version]
- Soleymanpour, A.; Rezvani, S.A. Development of a Novel Carbon Paste Sensor for Determination of Micromolar Amounts of Sulfaquinoxaline in Pharmaceutical and Biological Samples. Mater. Sci. Eng. C 2016, 58, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Arvand, M.; Alirezanejad, F. Sulfamethoxazole-Imprinted Polymeric Receptor as Ionophore for Potentiometric Transduction. Electroanalysis 2011, 23, 1948–1957. [Google Scholar] [CrossRef]






| Designation | Precursor | Treatment | Drying |
|---|---|---|---|
| CNTs-COO− | 30 wt% HNO3 aqueous solution | CNTs:HNO3 (1:8 by weight), 90 °C (1 h), washing with deionized water | in air, 90 °C (24 h) |
| CNTs-SO3− | p-toluenesulfonic acid, D-glucose | CNTs-COO−:p-toluenesulfonic acid:D-glucose (1:1.25:1.25 by weight), hydrothermal treatment at 180 °C (24 h), washing with deionized water and ethanol | in air, 110 °C (24 h) |
| CNTs-NH3+ | 6 M HNO3 aqueous solution | CNTs-COO−:HNO3 (1:8 by weight), 90 °C (1 h), washed with deionized water | in air, 90 °C (24 h) |
| 5 wt% solution of (3-aminopropyl)trimethoxy- silane in acetone | CNTs-COO−:(3-aminopropyl)trimethoxysilane (10:1 by weight), 80 °C (0.5 h), washing with deionized water | in air, 90 °C (24 h) |
| Designation | CNTs-COO− | CNTs-SO3− | CNTs-NH3+ | |||
|---|---|---|---|---|---|---|
| Surface fragments |  |  |  | |||
| Functionalization | covalent | non-covalent | covalent | |||
| Outer diameter, nm | 20–40 | 20–40 | 20–40 | |||
| IEC, mmol/g | 0.014 | 0.27 | 0.64 | |||
| FTIR spectra characteristics | 1364 cm−1 | -COOH | 986 cm−1 | -SO3H | 1056, 899 cm−1 | -NH2 |
| 3700–3500 cm−1 | -OH | 1178 cm−1 | 1196 cm−1 | Si-O | ||
| 3700–3500 cm−1 | -OH | 3700–3500 cm−1 | -OH | |||
| Designation | Precursor | Treatment, Viscosity | Film Formation | Drying, Pressing | Conditioning |
|---|---|---|---|---|---|
| PFSA | 10 wt% DMF dispersion of the PFSA polymer in the Li+ form | -, 82.5 ± 0.4 mPa·s | Casting on a glass surface | Drying in vacuum at 60 °C (4 h), 80 °C (12 h), 110 °C (4 h), hot-pressing at 5 MPa, 110 °C (3 min) | 5 wt% HCl solution at room temperature (1.5 h), washing with deionized water, 2 M KCl solution (72 h), washing with deionized water |
| PFSA-US | US, 35 kHz, ≤50 °C (45 min), 64.6 ± 0.6 mPa·s | ||||
| PFSA/CNTs-X | DMF dispersion of CNTs-X and PFSA polymer (10 wt%) in the Li+ form | US, 35 kHz, ≤50 °C (45 min), 58–62 mPa·s | Casting two dispersions on a glass surface toward each other | ||
| 10 wt% DMF dispersion of the PFSA polymer in the Li+ form | -, 82.5 ± 0.4 mPa·s |
| Characteristics | SA | SAA |
|---|---|---|
| Dissociation constant | pKa1 = 2.40, pKa2 = 10.43 [64] | pKa1 = 1.78, pKa2 = 5.38 [64] |
| Log P | −0.67 [65] | −0.96 [65] |
| Log DpH=7 | −0.67 [65] | −2.24 [65] |
| Mole fractions at pH = 7 | 1.00 SA | 0.98 SAA− 0.02 SAA |
| Characteristic | Membrane Composition | ||
|---|---|---|---|
| PFSA-US | PFSA/1.0 wt% CNTs-COO− | PFSA/1.0 wt% CNTs-SO3− | |
| ε, mV | 6 | 7 | 6 |
| D, mV2 | 17 | 16 | 18 |
| Drift, mV/h | 4 | insignificant | insignificant |
| Response time, min | <1 | ||
| pH (working range) | 4.68–10.56 | ||
| c, M (working range) | 1.0 × 10−5–1.0 × 10−3 | ||
| Stability, month | ≥12 | ||
| LOD, M | 1.8 × 10−7/5.8 × 10−7/1.8 × 10−7 (SAA−/SA/Na+) | ||
| RSD, % (n = 4, p = 0.95) | 5–18/4–13/4–20 (SAA−/SA/Na+) | ||
| Relative error, % | 5–10/6–17/1.7–9 (SAA−/SA/Na+) | ||
| Membrane Composition | b0, mV | b1, mV/pSAA | b2, mV/pSA | b3, mV/pNa | b4, mV/pH | t-Test, f = 8, p = 0.95 | F-Test, f1 = 3, f2 = 5, p = 0.95 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t | F | t | F | t | F | t | F | |||||
| PFSA-US | 1.71 | 1.08 | 0.55 | 1.09 | 0.38 | 1.11 | 1.61 | 1.10 | 1.11 | 1.08 | 2.31 | 8.91 |
| PFSA/1.0 wt% CNTs-COO− | 0.33 | 1.06 | 0.53 | 1.04 | 0.09 | 1.10 | 0.39 | 1.07 | 0.44 | 1.03 | ||
| PFSA/1.0 wt% CNTs-SO3− | 0.42 | 1.13 | 0.49 | 1.11 | 0.23 | 1.13 | 0.87 | 1.11 | 0.34 | 1.14 | ||
| Drug Pretreatment | c, M (Pharmaceutical Solution) | RSD, % (n = 4, p = 0.95) | c, mg/mL (Drug) | |||
|---|---|---|---|---|---|---|
| SAA | SA | SAA | SA | SAANa | SA | |
| Dilution 1/25,000, acetate buffer (pH = 4.0) | (3.40 ± 0.02) × 10−5 | - | 0.3 | - | 200.6 ± 1.2 | - |
| UV treatment, dilution 1/25,000, acetate buffer (pH = 4.0) | (3.11 ± 0.04) × 10−5 | insignificant | 1.5 | - | 184.5 ± 1.7 | - |
| Drug Pretreatment | c, M (Pharmaceutical Solution) | RSD, % (n = 4, p = 0.95) | c, mg/mL (Drug) | Relative Error, % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAA− | SA | Na+ | SAA− | SA | Na+ | SAANa | SA | SAANa | SA | |
| UV treatment, dilution 1/1000 | (8.1 ± 0.8) × 10−4 | (6.7 ± 0.8) × 10−5 | (8.9 ± 0.8) × 10−4 | 6 | 8 | 6 | 190 ± 18 | 11.5 ± 1.4 | 3 | 2 |
| Analyte * | Sensor Composition | Linear Range, M; LOD, M | Relative Error, %; RSD, % | Stability | Ref. |
|---|---|---|---|---|---|
| SAANa | PVC membrane with tetradodecylammonium SAA | 1.0 × 10−4.5–1.0 × 10−2; 2.23 × 10−5 | 0.16–1.99; - | 4 weeks | [69] |
| SAANa, SA | PFSA/PANI, PFSA/PEDOT | 1.0 × 10−4–1.0 × 10−2; (4.1–7.2) × 10−6 (SAA−), 1.0 × 10−5 (SA) | 1.2–1.4 (SAANa), 1.7–4 (SA); 6–7 (SAANa), 8–9 (SA) | ≥12 months | [67] |
| PFSA/CNTs–X | 1.0 × 10−5–1.0 × 10−3; 1.8 × 10−7 (SAA−), 5.8 × 10−7 (SA) | 3 (SAANa), 2 (SA); 6 (SAANa), 8 (SA) | This work | ||
| SDZ | PVC membrane with bis(triphenylphosphoranilidene)- ammonium SDZ | 1.0 × 10−5–1.0 × 10−2; 4.36 × 10−6 | 0.7–2.6; 4.6–4.7 | - | [70] |
| PVC membrane with MIPs | 9.0 × 10−6–1.0 × 10−4; 2.7 × 10−6 | 0.3–3.7; 0.6–1.4 | 2 months | [71] | |
| SQX | Carbon paste electrode with 2,3,5-triphenyltetrazolium SQX | 5.0 × 10−6–1.0 × 10−2; 3.0 × 10−6 | -; - | - | [72] |
| SMX | PVC membrane with MIPs | 1.0 × 10−7–1.0 × 10−3; 6.3 × 10−8 | -; 0.25–0.36 | 3 months | [73] |
| SMX, TMP | PFSA/PANI with hydrothermal treatment | 1.0 × 10−5–1.0 × 10−3; 1.4 × 10−6 (SMX−), 8.5 × 10−8 (TMP+) | 4 (SMX), 5 (TMP); 5 (SMX), 6 (TMP) | ≥12 months | [68] |
| PFSA/CNTs–X | 1.0 × 10−5–1.0 × 10−3; 3.5 × 10−7 (SMX−), 1.3 × 10−7 (TMP+) | 4 (SMX), 5 (TMP); 6 (SMX), 7 (TMP) | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parshina, A.; Yelnikova, A.; Safronova, E.; Kolganova, T.; Bobreshova, O.; Yaroslavtsev, A. Potentiometric Sensor Arrays Based on Hybrid PFSA/CNTs Membranes for the Analysis of UV-Degraded Drugs. Polymers 2023, 15, 2682. https://doi.org/10.3390/polym15122682
Parshina A, Yelnikova A, Safronova E, Kolganova T, Bobreshova O, Yaroslavtsev A. Potentiometric Sensor Arrays Based on Hybrid PFSA/CNTs Membranes for the Analysis of UV-Degraded Drugs. Polymers. 2023; 15(12):2682. https://doi.org/10.3390/polym15122682
Chicago/Turabian StyleParshina, Anna, Anastasia Yelnikova, Ekaterina Safronova, Tatyana Kolganova, Olga Bobreshova, and Andrey Yaroslavtsev. 2023. "Potentiometric Sensor Arrays Based on Hybrid PFSA/CNTs Membranes for the Analysis of UV-Degraded Drugs" Polymers 15, no. 12: 2682. https://doi.org/10.3390/polym15122682