Eco-Friendly Epoxy-Terminated Polyurethane-Modified Epoxy Resin with Efficient Enhancement in Toughness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
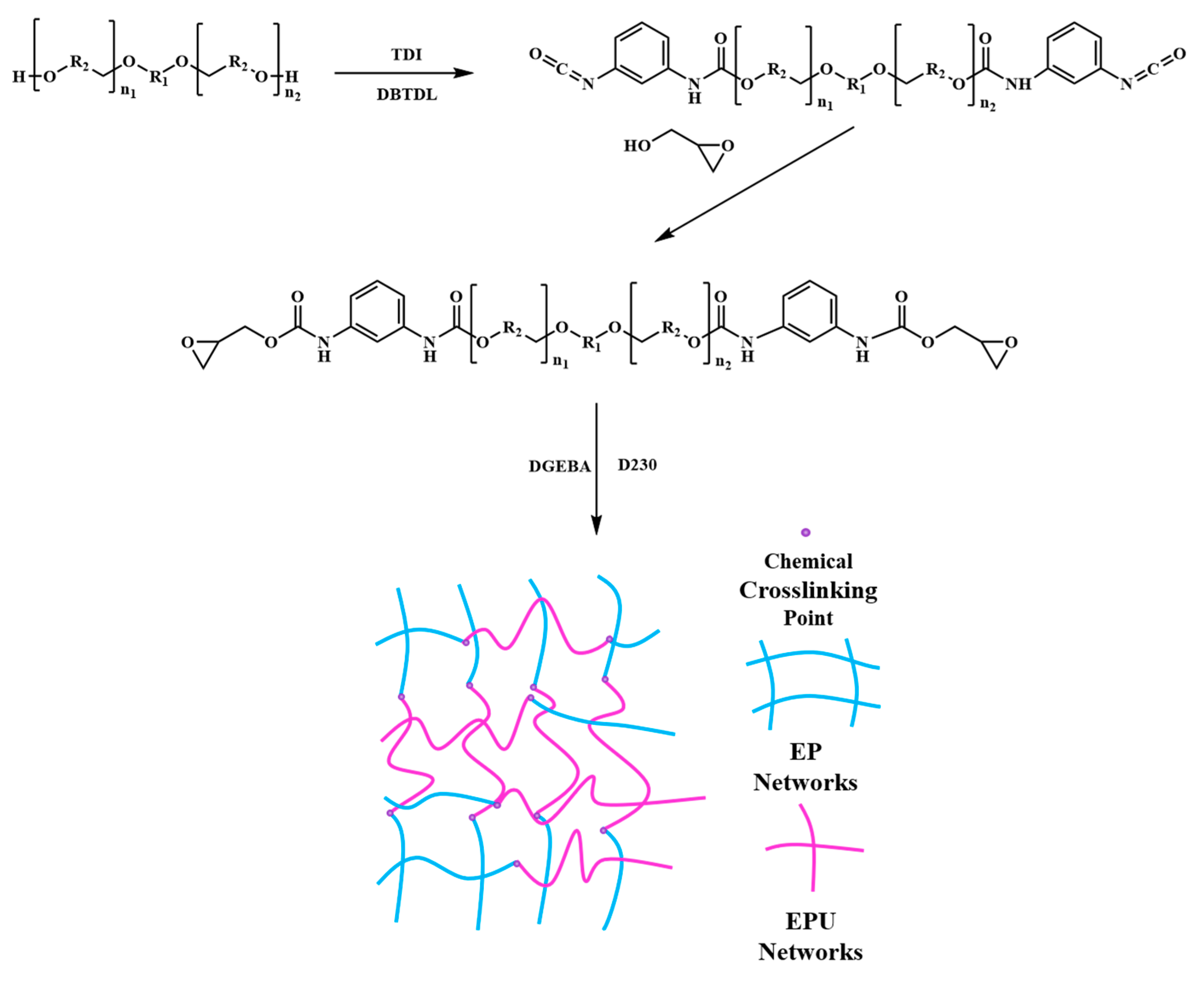
2.2. Synthesis of Epoxy-Terminated Polyurethane (EPU)
2.3. Preparation of Epoxy Resin and the Modified Epoxy Resin Samples
2.4. Characterization
3. Results
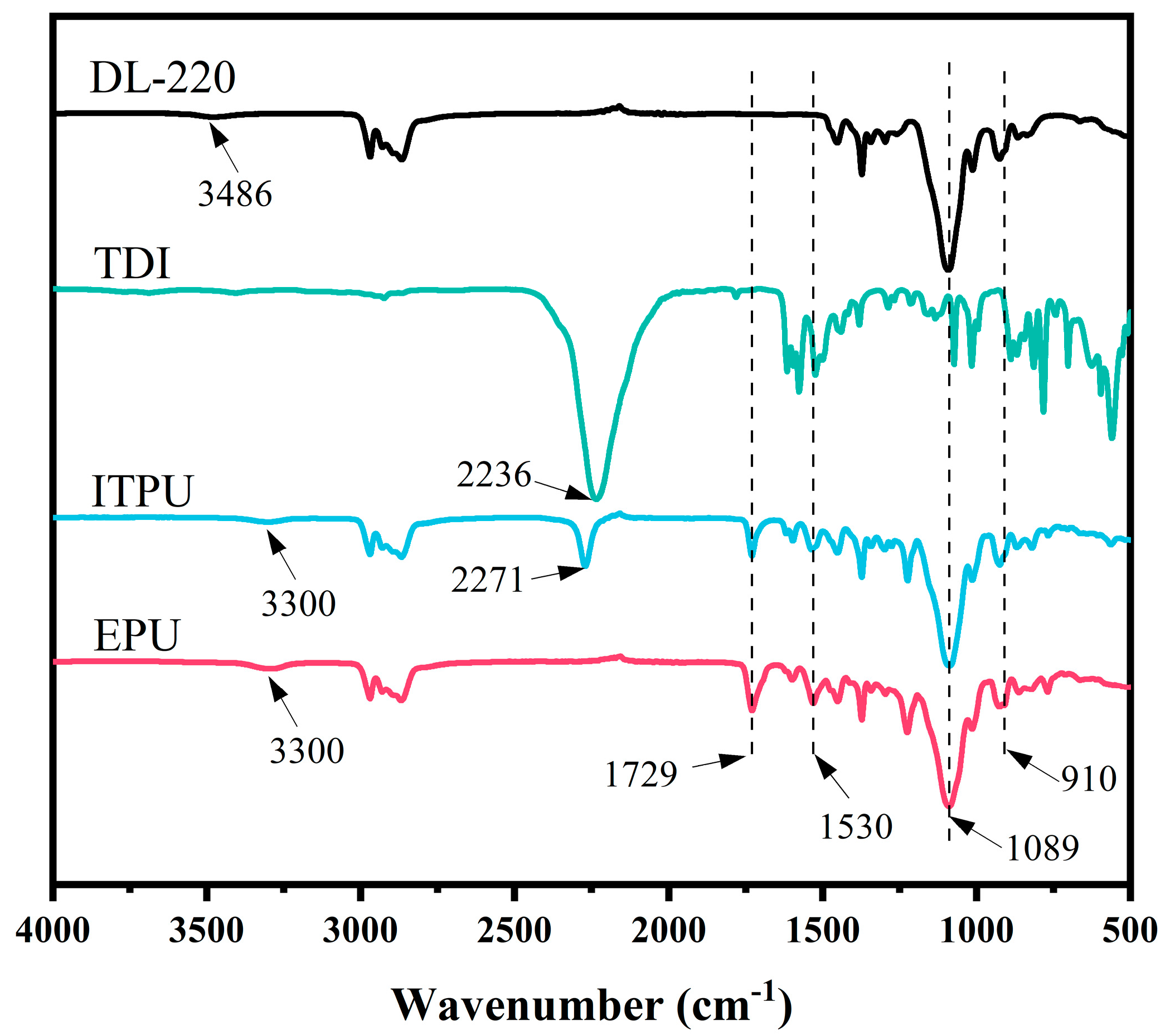
3.1. Synthesis and Structural Characterization of EPU
3.2. Dynamic Mechanical Analysis (DMA)
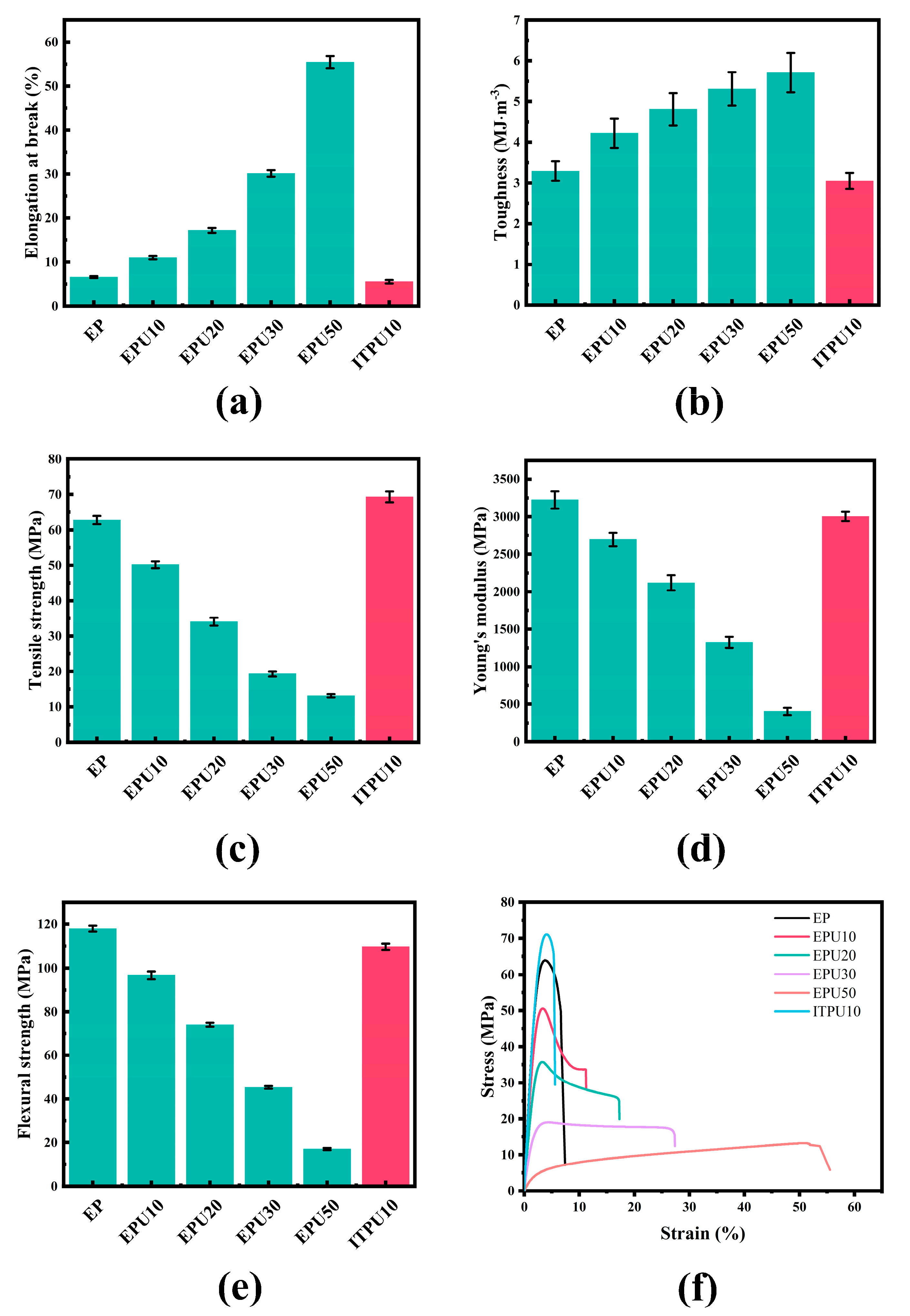
3.3. Mechanical Properties
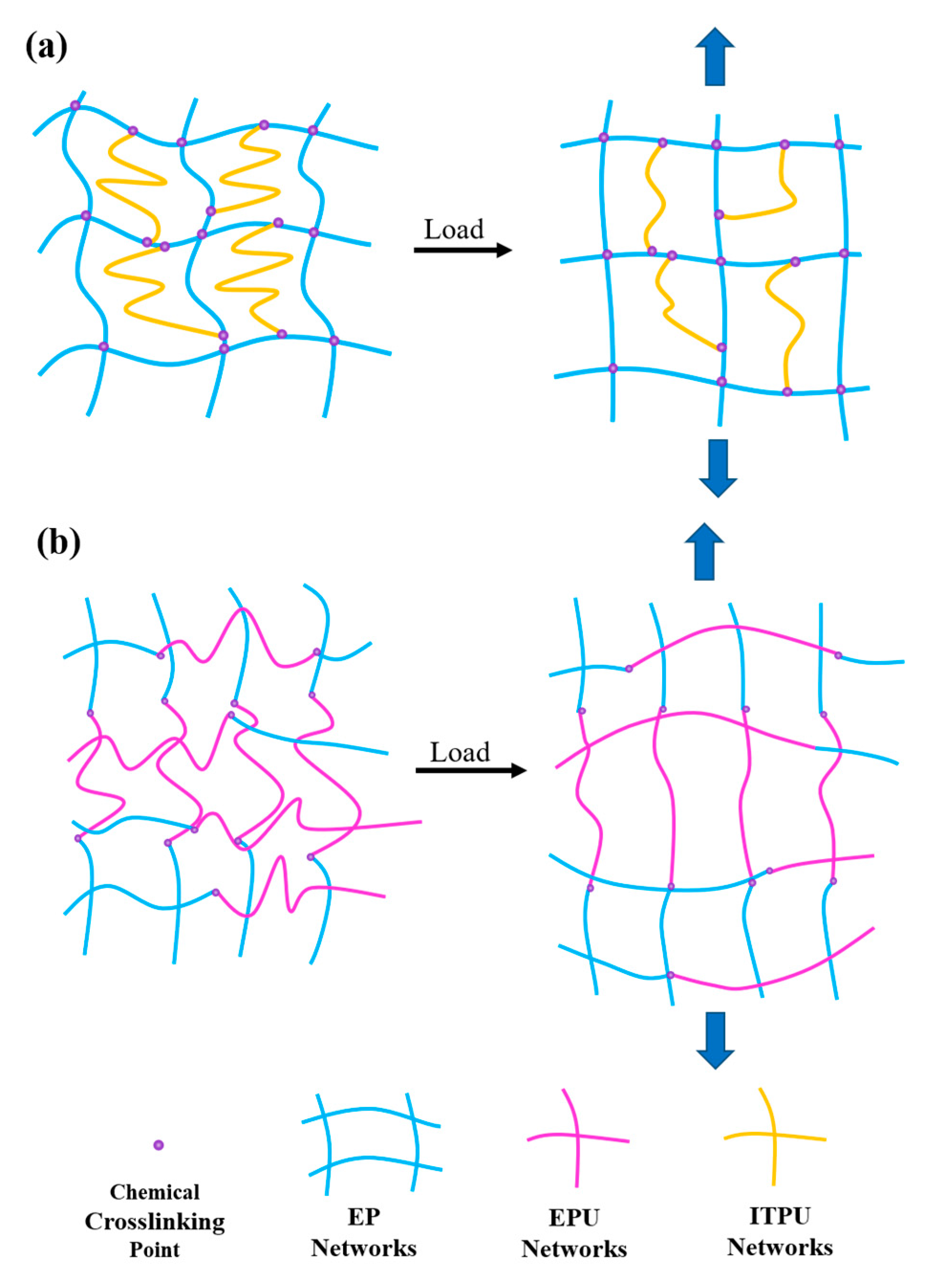
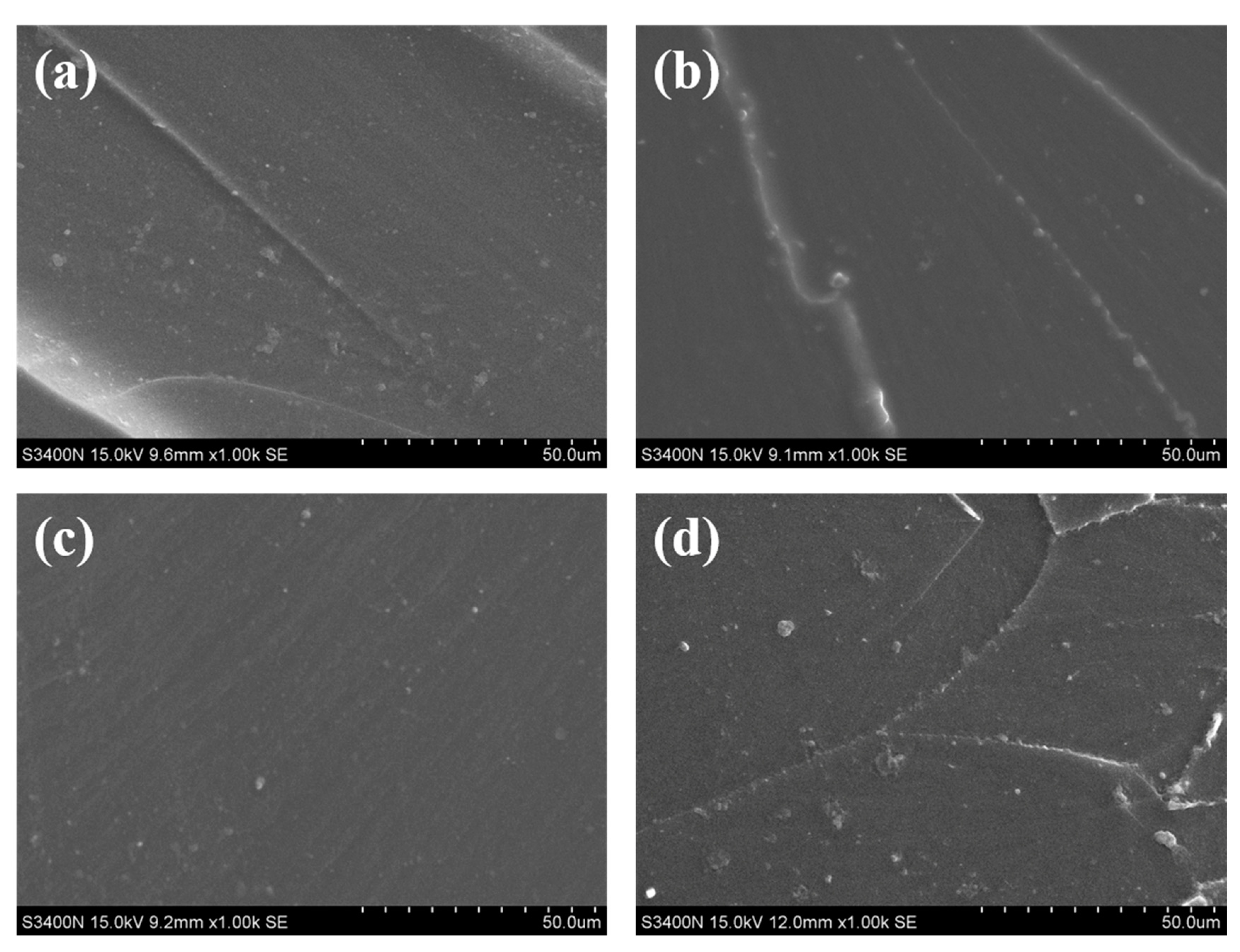
3.4. Phase Morphology
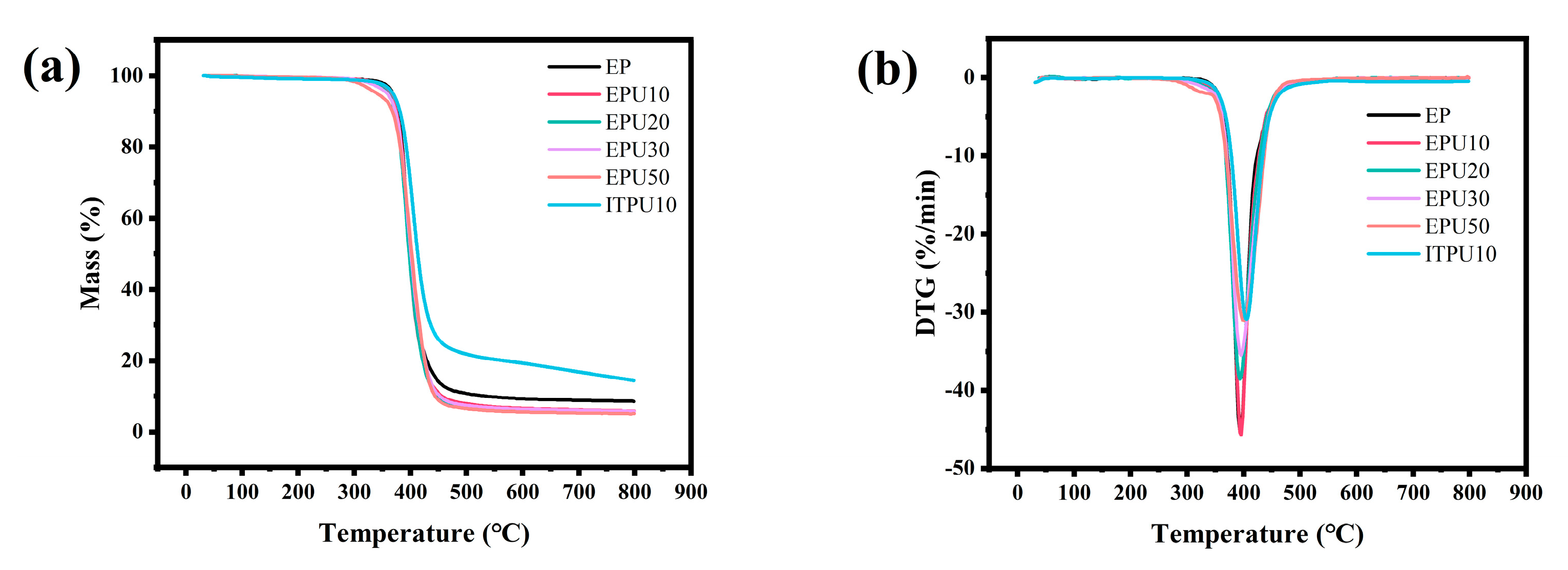
3.5. Thermogravimetric Analysis (TGA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mi, X.; Liang, N.; Xu, H.; Wu, J.; Jiang, Y.; Nie, B.; Zhang, D. Toughness and Its Mechanisms in Epoxy Resins. Prog. Mater. Sci. 2022, 130, 100977. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and Application of Epoxy Resins: A Review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Rybyan, A.A.; Bilichenko, J.V.; Kireev, V.V.; Kolenchenko, A.A.; Chistyakov, E.M. Curing of DER-331 Epoxy Resin with Arylaminocyclotriphosphazenes Based on o-, m-, and p-Methylanilines. Polymers 2022, 14, 5334. [Google Scholar] [CrossRef]
- Orlov, A.; Konstantinova, A.; Korotkov, R.; Yudaev, P.; Mezhuev, Y.; Terekhov, I.; Gurevich, L.; Chistyakov, E. Epoxy Compositions with Reduced Flammability Based on DER-354 Resin and a Curing Agent Containing Aminophosphazenes Synthesized in Bulk Isophoronediamine. Polymers 2022, 14, 3592. [Google Scholar] [CrossRef]
- Wan, J.; Zhao, J.; Zhang, X.; Fan, H.; Zhang, J.; Hu, D.; Jin, P.; Wang, D.-Y. Epoxy Thermosets and Materials Derived from Bio-Based Monomeric Phenols: Transformations and Performances. Prog. Polym. Sci. 2020, 108, 101287. [Google Scholar] [CrossRef]
- Goncalves, F.A.M.M.; Santos, M.; Cernadas, T.; Ferreira, P.; Alves, P. Advances in the Development of Biobased Epoxy Resins: Insight into More Sustainable Materials and Future Applications. Int. Mater. Rev. 2022, 67, 119–149. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Fu, S. Bio-Based Epoxy Resin from Gallic Acid and Its Thermosets Toughened with Renewable Tannic Acid Derivatives. J. Mater. Sci. 2022, 57, 9493–9507. [Google Scholar] [CrossRef]
- Xiao, L.; Li, S.; Wang, Y.; Li, W.; Chen, J.; Huang, J.; Nie, X. Toughening Epoxy Resin by Constructing π-π Interaction between a Tung Oil-Based Modifier and Epoxy. Ind. Crops Prod. 2021, 170, 113723. [Google Scholar] [CrossRef]
- Stajcic, I.; Veljkovic, F.; Petrovic, M.; Veličkovic, S.; Radojevic, V.; Vlahović, B.; Stajcic, A. Impact- and Thermal-Resistant Epoxy Resin Toughened with Acacia Honey. Polymers 2023, 15, 2261. [Google Scholar] [CrossRef]
- Wu, J.; Li, C.; Hailatihan, B.; Mi, L.; Baheti, Y.; Yan, Y. Effect of the Addition of Thermoplastic Resin and Composite on Mechanical and Thermal Properties of Epoxy Resin. Polymers 2022, 14, 1087. [Google Scholar] [CrossRef]
- Shi, Q.; Huo, S.; Wang, C.; Ye, G.; Yu, L.; Fang, Z.; Wang, H.; Liu, Z. A Phosphorus/Silicon-Based, Hyperbranched Polymer for High-Performance, Fire-Safe, Transparent Epoxy Resins. Polym. Degrad. Stab. 2022, 203, 110065. [Google Scholar] [CrossRef]
- Liu, S.; Fan, X.; He, C. Improving the Fracture Toughness of Epoxy with Nanosilica-Rubber Core-Shell Nanoparticles. Compos. Sci. Technol. 2016, 125, 132–140. [Google Scholar] [CrossRef]
- Ricciardi, M.R.; Papa, I.; Langella, A.; Langella, T.; Lopresto, V.; Antonucci, V. Mechanical Properties of Glass Fibre Composites Based on Nitrile Rubber Toughened Modified Epoxy Resin. Compos. Part B Eng. 2018, 139, 259–267. [Google Scholar] [CrossRef]
- Huang, J.; Li, N.; Xiao, L.; Liu, H.; Wang, Y.; Chen, J.; Nie, X.; Zhu, Y. Fabrication of a Highly Tough, Strong, and Stiff Carbon Nanotube/Epoxy Conductive Composite with an Ultralow Percolation Threshold via Self-Assembly. J. Mater. Chem. A 2019, 7, 15731–15740. [Google Scholar] [CrossRef]
- Lin, J.; Wu, X.; Zheng, C.; Zhang, P.; Li, Q.; Wang, W.; Yang, Z. A Novolac Epoxy Resin Modified Polyurethane Acylates Polymer Grafted Network with Enhanced Thermal and Mechanical Properties. J. Polym. Res. 2014, 21, 435. [Google Scholar] [CrossRef]
- Tang, B.; Liu, X.; Zhao, X.; Zhang, J. Highly Efficient in Situ Toughening of Epoxy Thermosets with Reactive Hyperbranched Polyurethane. J. Appl. Polym. Sci. 2014, 131, 40614. [Google Scholar] [CrossRef]
- Farooq, U.; Teuwen, J.; Dransfeld, C. Toughening of Epoxy Systems with Interpenetrating Polymer Network (IPN): A Review. Polymers 2020, 12, 1908. [Google Scholar] [CrossRef]
- Jena, R.K.; Yue, C.Y. Development of Nanocomposite for Rigid Riser Application: Diallyl Bisphenol A Modified Bismaleimide/Epoxy Interpenetrating Network and Its Nanocomposite (NH 2 -MWCNT). Compos. Sci. Technol. 2016, 124, 27–35. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Hao, M.; Zhi, J.; Qian, X. Synergistically Toughened Epoxy Resin Based on Modified-POSS Triggered Interpenetrating Network. Polymer 2023, 268, 125719. [Google Scholar] [CrossRef]
- Jia, Q.; Zheng, M.; Chen, H.; Shen, R. Morphologies and Properties of Polyurethane/Epoxy Resin Interpenetrating Network Nanocomposites Modified with Organoclay. Mater. Lett. 2006, 60, 1306–1309. [Google Scholar] [CrossRef]
- Luo, X.; Liu, X.-F.; Ding, X.-M.; Chen, L.; Chen, S.-C.; Wang, Y.-Z. Effects of Curing Temperature on the Structure and Properties of Epoxy Resin-Poly(ε-Caprolactam) Blends. Polymer 2021, 228, 123940. [Google Scholar] [CrossRef]
- Frisch, K.C.; Klempner, D.; Mukherjee, S.K.; Frisch, H.L. Stress–Strain Properties and Thermal Resistance of Polyurethane–Polyepoxide Interpenetrating Polymer Networks. J. Appl. Polym. Sci. 1974, 18, 689–698. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Li, N.; Huang, G.-W.; Qu, C.-B.; Xiao, H.-M. Cryogenic Mechanical Properties of Epoxy Resin Toughened by Hydroxyl-Terminated Polyurethane. Polym. Test. 2019, 74, 45–56. [Google Scholar] [CrossRef]
- Triwulandari, E.; Fatriasari, W.; Iswanto, A.H.; Septiyanti, M.; Umam, E.F.; Ghozali, M. Effect of Reaction Time on the Molecular Weight Distribution of Polyurethane Modified Epoxy and Its Properties. J. Mater. Res. Technol. 2022, 19, 2204–2214. [Google Scholar] [CrossRef]
- Yu, P.; Li, G.; Zhang, L.; Zhao, F.; Chen, S.; Dmitriev, A.I.; Zhang, G. Regulating Microstructures of Interpenetrating Polyurethane-Epoxy Networks towards High-Performance Water-Lubricated Bearing Materials. Tribol. Int. 2019, 131, 454–464. [Google Scholar] [CrossRef]
- Qu, C.-B.; Wu, T.; Huang, G.-W.; Li, N.; Li, M.; Ma, J.-L.; Liu, Y.; Xiao, H.-M. Improving Cryogenic Mechanical Properties of Carbon Fiber Reinforced Composites Based on Epoxy Resin Toughened by Hydroxyl-Terminated Polyurethane. Compos. Part B Eng. 2021, 210, 108569. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Q.; Yuan, A.; Liu, Z.; Zhou, S.; Fu, X.; Lei, J.; Jiang, L. Intrinsic Toughened Conductive Thermosetting Epoxy Resins: Utilizing Dynamic Bond and Electrical Conductivity to Access Electric and Thermal Dual-Driven Shape Memory. Mater. Chem. Front. 2022, 6, 1989–1999. [Google Scholar] [CrossRef]
- Peng, Y.-J.; He, X.; Wu, Q.; Sun, P.-C.; Wang, C.-J.; Liu, X.-Z. A New Recyclable Crosslinked Polymer Combined Polyurethane and Epoxy Resin. Polymer 2018, 149, 154–163. [Google Scholar] [CrossRef]
- Lv, X.; Huang, Z.; Huang, C.; Shi, M.; Gao, G.; Gao, Q. Damping Properties and the Morphology Analysis of the Polyurethane/Epoxy Continuous Gradient IPN Materials. Compos. Part B Eng. 2016, 88, 139–149. [Google Scholar] [CrossRef]
- Sivanesan, D.; Kim, S.; Jang, T.W.; Kim, H.J.; Song, J.; Seo, B.; Lim, C.-S.; Kim, H.-G. Effects of Flexible and Rigid Parts of ε-Caprolactone and Tricyclodecanediol Derived Polyurethane on the Polymer Properties of Epoxy Resin. Polymer 2021, 237, 124374. [Google Scholar] [CrossRef]
- Zou, Z.-P.; Liu, X.-B.; Wu, Y.-P.; Tang, B.; Chen, M.; Zhao, X.-L. Hyperbranched Polyurethane as a Highly Efficient Toughener in Epoxy Thermosets with Reaction-Induced Microphase Separation. RSC Adv. 2016, 6, 18060–18070. [Google Scholar] [CrossRef]
- Feng, L.; He, X.; Zhang, Y.; Qu, D.; Chai, C. Triple Roles of Thermoplastic Polyurethane in Toughening, Accelerating and Enhancing Self-Healing Performance of Thermo-Reversible Epoxy Resins. J. Polym. Environ. 2021, 29, 829–836. [Google Scholar] [CrossRef]
- Chen, D.-S.; Ma, C.-C.M.; Hsia, H.-C.; Wang, W.-N.; Lin, S.-R. Preparation and Characterization of Cryogenic Adhesives. I. Glycidyl-Terminated Polyurethane Resins. J. Appl. Polym. Sci. 1994, 51, 1199–1206. [Google Scholar] [CrossRef]
- Li, Y.-S.; Li, M.-S.; Ma, C.-C.M.; Hsia, H.-C.; Chen, D.-S. Polyether and Polyester Polyol Based Glycidyl-Terminated Polyurethane Modified Epoxy Resins: Mechanical Properties and Morphology. Polym. Int. 1994, 35, 371–378. [Google Scholar] [CrossRef]
- Yeganeh, H.; Lakouraj, M.M.; Jamshidi, S. Synthesis and Properties of Biodegradable Elastomeric Epoxy Modified Polyurethanes Based on Poly(ε-Caprolactone) and Poly(Ethylene Glycol). Eur. Polym. J. 2005, 41, 2370–2379. [Google Scholar] [CrossRef]
- Huang, J.; Nie, X. A Simple and Novel Method to Design Flexible and Transparent Epoxy Resin with Tunable Mechanical Properties. Polym. Int. 2016, 65, 835–840. [Google Scholar] [CrossRef]
- Wu, T.; Guo, F.-L.; Hu, J.-M.; Li, Y.-Q.; Fu, Y.-T.; Fu, S.-Y. Investigation of Polyurethane Toughened Epoxy Resins for Composite Cryotank Applications. Part I: Phase Separation Phenomenon and Cryogenic Mechanical Behaviors. Compos. Commun. 2022, 35, 101278. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, C.; Yu, M.; Huang, Y.; Tan, J.; Zhang, M.; Zhu, X. Multifunctional Tannin Extract-Based Epoxy Derived from Waste Bark as a Highly Toughening and Strengthening Agent for Epoxy Resin. Ind. Crops Prod. 2022, 176, 114255. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, W.; Zhu, Y.; Luo, J.; Liu, X. Noncovalent Functionalization of Carbon Nanotubes via Co-Deposition of Tannic Acid and Polyethyleneimine for Reinforcement and Conductivity Improvement in Epoxy Composite. Compos. Sci. Technol. 2019, 170, 25–33. [Google Scholar] [CrossRef]
- Li, N.; Huang, J.; Wang, Y.; Xiao, L.; Fu, P.; Yu, H.; Nie, X.; Jiang, J.; Zhu, Y.; Guo, Z. Simultaneously Strengthening, Toughening, and Conductivity Improving for Epoxy at Ultralow Carbonaceous Filler Content by Constructing 3D Nanostructures and Sacrificial Bonds. Compos. Part A Appl. Sci. Manuf. 2020, 137, 106014. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wu, X.; Wang, D.; Zhou, S.; Han, S.; Wang, H.; Sun, F. Simultaneously Reinforcing and Toughening of Shape-Memory Epoxy Resin with Carboxylated Lignosulfonate: Facile Preparation and Effect Mechanism. Int. J. Biol. Macromol. 2022, 217, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Lawn, B. Fracture of Brittle Solids, 2nd ed.; Cambridge Solid State Science Series; Cambridge University Press: Cambridge, UK, 1993; ISBN 978-0-521-40972-8. [Google Scholar]
- Luo, Y.-R. Handbook of Bond Dissociation Energies in Organic Compounds; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-8493-1589-3. [Google Scholar]
- Thermal Decomposition of Aryl—Alicyclic Polyimides Studied by Thermogravimetry/Mass Spectrometry and Pyrolysis—Gas Chromatography/Mass Spectrometry. J. Anal. Appl. Pyrolysis 1992, 23, 229–243. [CrossRef]

| Sample | DGEBA (g) | EPU (g) | ITPU (g) | Epoxide Value (mol (100 g−1) | D230 (g) |
|---|---|---|---|---|---|
| EP | 100 | -- | -- | 0.51 | 29.33 |
| EPU10 | 90 | 10 | -- | 0.47 | 27.03 |
| EPU20 | 80 | 20 | -- | 0.42 | 24.15 |
| EPU30 | 70 | 30 | -- | 0.38 | 21.85 |
| EPU50 | 50 | 50 | -- | 0.29 | 16.68 |
| ITPU10 | 90 | -- | 10 | 0.45 | 25.88 |
| Sample | Tg (°C) | E′ (MPa) | ν (10−3 mol·cm−3) | HPW (°C) | Peak Height of Tan Delta |
|---|---|---|---|---|---|
| EP | 81.32 | 9.61 | 1.09 | 11.94 | 1.38 |
| EPU10 | 78.73 | 9.73 | 1.02 | 13.57 | 1.16 |
| EPU20 | 78.70 | 8.79 | 0.92 | 14.79 | 0.99 |
| EPU30 | 74.50 | 7.54 | 0.80 | 18.63 | 0.78 |
| EPU50 | 66.81 | 5.42 | 0.59 | 28.16 | 0.52 |
| ITPU10 | 82.21 | 9.61 | 1.08 | 13.02 | 1.18 |
| Sample | T5% (°C) | Tm (°C) | DTR (°C) | Dm (%/min) | Char Residue at 800 °C (%) |
|---|---|---|---|---|---|
| EP | 367.07 | 394.2 | 132.5 | 45.29 | 8.55 |
| EPU10 | 363.7 | 395.2 | 137.5 | 45.69 | 5.82 |
| EPU20 | 360.6 | 393.9 | 145 | 38.58 | 5.58 |
| EPU30 | 358.7 | 395.7 | 152.5 | 35.52 | 5.73 |
| EPU50 | 339.2 | 400.0 | 170 | 31.09 | 5.11 |
| ITPU10 | 368.6 | 405.1 | 133 | 43.73 | 14.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Huang, J.; Wang, Y.; Li, W.; Nie, X. Eco-Friendly Epoxy-Terminated Polyurethane-Modified Epoxy Resin with Efficient Enhancement in Toughness. Polymers 2023, 15, 2803. https://doi.org/10.3390/polym15132803
Zhang K, Huang J, Wang Y, Li W, Nie X. Eco-Friendly Epoxy-Terminated Polyurethane-Modified Epoxy Resin with Efficient Enhancement in Toughness. Polymers. 2023; 15(13):2803. https://doi.org/10.3390/polym15132803
Chicago/Turabian StyleZhang, Kun, Jinrui Huang, Yigang Wang, Wenbin Li, and Xiaoan Nie. 2023. "Eco-Friendly Epoxy-Terminated Polyurethane-Modified Epoxy Resin with Efficient Enhancement in Toughness" Polymers 15, no. 13: 2803. https://doi.org/10.3390/polym15132803






