Chitosan-Based Composites: Development and Perspective in Food Preservation and Biomedical Applications
Abstract
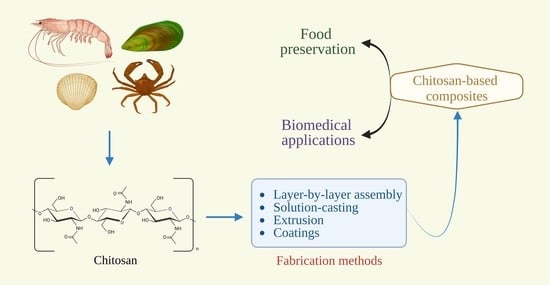
:1. Introduction
2. Fabrication Methods for Composite Films/Coatings
2.1. Solution Casting
2.2. Layer-by-Layer Assembly
2.3. Extrusion
2.4. Coatings (Spraying, Dipping, or Spreading)
- Preparation of raw materials through mixing the right proportions of chitosan and fillers.
- Preparation of coating samples through various methods such as irradiating, heating, mixing, and steam flash pasteurizing.
- Sanitizing of the food samples through sodium hypochlorite.
- Application of the chitosan-based composite solutions to food through the sterile spreader.
- Drying under specific circumstances.
- Packaging and storage in suitable conditions [41].
2.5. Crosslinking
2.6. Electrospinning
- (1)
- Formation of the cone-shaped jet from a pendant droplet by charging it in an electric field.
- (2)
- Lengthening of the charged jet.
- (3)
- Stretching and thinning of the charged jet under the electric field causes bending instability.
- (4)
- Solidification and collection of the jet as solid fibers on a grounded collector [54].
3. Improvisation of Chitosan-Based Composites
4. Application of Chitosan-Based Composite in the Food Industry
4.1. Utilization of Chitosan-Based Composite in the Preservation of Seafood
4.2. Utilization of Chitosan-Based Composite in the Preservation of Meat and Meat Products
4.3. Utilization of Chitosan-Based Composite in the Preservation of Postharvest Foods
4.4. Utilization of Chitosan-Based Composite in the Monitoring of Freshness/Spoilage
5. Application of Chitosan-Based Composite in the Biomedical Industry
5.1. Utilization of Chitosan-Based Products in Drug Delivery
5.2. Utilization of Chitosan-Based Products in Tissue Engineering
5.3. Utilization of Chitosan-Based Products in Wound Healing
6. Miscellaneous
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent Advances in Chitosan-Based Applications—A Review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M.; Harding, D.; Sashiwa, H. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Local Sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Sagheer, F.A.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Marine Sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Mohite, P.; Shah, S.R.; Singh, S.; Rajput, T.; Munde, S.; Ade, N.; Prajapati, B.G.; Paliwal, H.; Mori, D.D.; Dudhrejiya, A.V. Chitosan and chito-oligosaccharide: A versatile biopolymer with endless grafting possibilities for multifarious applications. Front. Bioeng. Biotechnol. 2023, 11, 1190879. [Google Scholar] [CrossRef]
- Fatullayeva, S.; Tagiyev, D.; Zeynalov, N.; Mammadova, S.; Aliyeva, E. Recent Advances of Chitosan-Based Polymers in Biomedical Applications and Environmental Protection. J. Polym. Res. 2022, 29, 259. [Google Scholar] [CrossRef]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and Sulfated Chitosan Derivatives for Biomedical Applications: A Review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Sajwan, M.; Alsuwayt, B.; Asif, M. Synthesis, Characterization and Anticoagulant Activity of Chitosan Derivatives. Saudi Pharm. J. 2020, 28, 25–32. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. In Vitro Antioxidant Activity of Chitosan Aqueous Solution: Effect of Salt Form. Trop. J. Pharm. Res. 2012, 11, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Tzeng, H.P.; Liu, S.H.; Chiang, M.T. Antidiabetic Properties of Chitosan and Its Derivatives. Mar. Drugs 2022, 20, 784. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, D.N.; Prashanth, K.V.H.; Tharanathan, R.N. A Review on Potential Anti-Diabetic Mechanisms of Chitosan and Its Derivatives. Carbohydr. Polym. Technol. Appl. 2022, 3, 100188. [Google Scholar] [CrossRef]
- Shakil, M.S.; Mahmud, K.M.; Sayem, M.; Niloy, M.S.; Halder, S.K.; Hossen, M.S.; Uddin, M.F.; Hasan, M.A. Using Chitosan or Chitosan Derivatives in Cancer Therapy. Polysaccharides 2021, 2, 795–816. [Google Scholar] [CrossRef]
- Ding, J.; Guo, Y. Recent Advances in Chitosan and Its Derivatives in Cancer Treatment. Front. Pharmacol. 2022, 13, 888740. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Thi Dao, U.T.; Thi Bui, Q.P.; Bach, G.L.; Ha Thuc, C.N.; Ha Thuc, H. Enhanced Antimicrobial Activities and Physiochemical Properties of Edible Film Based on Chitosan Incorporated with Sonneratia caseolaris (L.) Engl. Leaf Extract. Prog. Org. Coat. 2020, 140, 105487. [Google Scholar] [CrossRef]
- Kumar, A.; Hasan, M.; Mangaraj, S.; Pravitha, M.; Verma, D.K.; Srivastav, P.P. Trends in Edible Packaging Films and Its Prospective Future in Food: A Review. Appl. Food Res. 2022, 2, 100118. [Google Scholar]
- Tokatlı, K.; Demirdöven, A. Effects of Chitosan Edible Film Coatings on the Physicochemical and Microbiological Qualities of Sweet Cherry (Prunus avium L.). Sci. Hortic. 2020, 259, 108656. [Google Scholar] [CrossRef]
- Hu, W.; Sarengaowa; Feng, K. Effect of Edible Coating on the Quality and Antioxidant Enzymatic Activity of Postharvest Sweet Cherry (Prunus avium L.) during Storage. Coatings 2022, 12, 581. [Google Scholar] [CrossRef]
- Bigi, F.; Haghighi, H.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Characterization of Chitosan-Hydroxypropyl Methylcellulose Blend Films Enriched with Nettle or Sage Leaf Extract for Active Food Packaging Applications. Food Hydrocoll. 2021, 120, 106979. [Google Scholar] [CrossRef]
- Prajapati, B. Chitosan A Marine Medical Polymer and Its Lipid Lowering Capacity. Internet J. Health 2008, 9, 1–6. [Google Scholar]
- Sun, J.; Li, Y.; Cao, X.; Yao, F.; Shi, L.; Liu, Y. A Film of Chitosan Blended with Ginseng Residue Polysaccharides as an Antioxidant Packaging for Prolonging the Shelf Life of Fresh-Cut Melon. Coatings 2022, 12, 468. [Google Scholar] [CrossRef]
- El-Hefian, E.A.; Nasef, M.M.; Yahaya, A.H. Preparation and Characterization of Chitosan/Polyvinyl Alcohol Blends—A Rheological Study. J. Chem. 2010, 7, S349–S357. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Becerra, J.; Sudre, G.; Royaud, I.; Montserret, R.; Verrier, B.; Rochas, C.; Delair, T.; David, L. Tuning the Hydrophilic/Hydrophobic Balance to Control the Structure of Chitosan Films and Their Protein Release Behavior. AAPS PharmSciTech 2017, 18, 1070–1083. [Google Scholar] [CrossRef]
- Basumatary, K.; Daimary, P.; Das, S.K.; Thapa, M.; Singh, M.; Mukherjee, A.; Kumar, S. Lagerstroemia Speciosa Fruit-Mediated Synthesis of Silver Nanoparticles and Its Application as Filler in Agar Based Nanocomposite Films for Antimicrobial Food Packaging. Food Packag. Shelf Life 2018, 17, 99–106. [Google Scholar] [CrossRef]
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-Biofilm Activity and Food Packaging Application of Room Temperature Solution Process Based Polyethylene Glycol Capped Ag-ZnO-Graphene Nanocomposite. Mater. Sci. Eng. C 2018, 91, 743–753. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, X.; Li, W.; Song, R.; Li, J.; Li, Y.; Li, B.; Liu, S. Green and biodegradable composite films with novel antimicrobial performance based on cellulose. Food Chem. 2016, 197, 250–256. [Google Scholar] [CrossRef]
- Tu, H.; Wu, G.; Yi, Y.; Huang, M.; Liu, R.; Shi, X.; Deng, H. Layer-by-Layer Immobilization of Amphoteric Carboxymethyl Chitosan onto Biocompatible Silk Fibroin Nanofibrous Mats. Carbohydr. Polym. 2019, 210, 9–16. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of Chitosan-TiO2 Composite Film with Efficient Antimicrobial Activities under Visible Light for Food Packaging Applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Guzmán, E.; Mateos-Maroto, A.; Ruano, M.; Ortega, F.; Rubio, R.G. Layer-by-Layer Polyelectrolyte Assemblies for Encapsulation and Release of Active Compounds. Adv. Colloid Interface Sci. 2017, 249, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Rouster, P.; Dondelinger, M.; Galleni, M.; Nysten, B.; Jonas, A.M.; Glinel, K. Layer-by-Layer Assembly of Enzyme-Loaded Halloysite Nanotubes for the Fabrication of Highly Active Coatings. Colloids Surf. B 2019, 178, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, P.; Stevens, B.; Devlaming, I.; Grunlan, J.C. Polymer–Graphene Oxide Quadlayer Thin-Film Assemblies with Improved Gas Barrier. Langmuir 2015, 31, 5919–5927. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Areco, S.; Guz, L.; Famá, L.; Candal, R.; Goyanes, S. Bioactive Starch Nanocomposite Films with Antioxidant Activity and Enhanced Mechanical Properties Obtained by Extrusion Followed by Thermo-Compression. Food Hydrocoll. 2019, 96, 518–528. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Jia, R.; Dai, Y.; Dong, H.; Hou, H.; Guo, Q. High Performance Extrusion Blown Starch/Polyvinyl Alcohol/Clay Nanocomposite Films. Food Hydrocoll. 2018, 79, 534–543. [Google Scholar] [CrossRef]
- Alsadi, J.; Obaidat, M.T.; Ismail, R.; Trrad, I.; Aljamal, M.; Dahim, M.; Abuaddous, M.; Khodier, M.; Hatamleh, R.; Al-Mattarneh, H. Box–Behnken Design for Polycarbonate-Pigment Blending: Applications and Characterization Techniques. Polymers 2022, 14, 4860. [Google Scholar] [CrossRef] [PubMed]
- Matet, M.; Heuzey, M.C.; Ajji, A.; Sarazin, P. Plasticized Chitosan/Polyolefin Films Produced by Extrusion. Carbohydr. Polym. 2015, 117, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bie, P.; Liu, P.; Yu, L.; Li, X.; Chen, L.; Xie, F. The properties of antimicrobial films derived from poly(lactic acid)/starch/chitosan blended matrix. Carbohydr. Polym. 2013, 98, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Vargas, M.; Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Edible Chitosan Coatings for Fresh and Minimally Processed Foods. In Emerging Food Packaging Technologies, 1st ed.; Yam, K.L., Lee, D.S., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 66–95. [Google Scholar]
- Yu, D.; Jiang, Q.; Xu, Y.; Xia, W. The Shelf Life Extension of Refrigerated Grass Carp (Ctenopharyngodon idellus) Fillets by Chitosan Coating Combined with Glycerol Monolaurate. Int. J. Biol. Macromol. 2017, 101, 448–454. [Google Scholar] [CrossRef]
- Pushkala, R.; Raghuram, P.K.; Srividya, N. Chitosan Based Powder Coating Technique to Enhance Phytochemicals and Shelf Life Quality of Radish Shreds. Postharvest Biol. Technol. 2013, 86, 402–408. [Google Scholar] [CrossRef]
- Kashiri, M.; Cerisuelo, J.P.; Domínguez, I.; López-Carballo, G.; Muriel-Gallet, V.; Gavara, R.; Hernández-Muñoz, P. Zein Films and Coatings as Carriers and Release Systems of Zataria Multiflora Boiss. Essential Oil for Antimicrobial Food Packaging. Food Hydrocoll. 2017, 70, 260–268. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of Fungal Chitosan Incorporated with Pomegranate Peel Extract as Edible Coating for Microbiological, Chemical and Sensorial Quality Enhancement of Nile Tilapia Fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.H.; Qin, G.Z.; Tian, S.P. Influences of Preharvest Spraying Cryptococcus laurentii Combined with Postharvest Chitosan Coating on Postharvest Diseases and Quality of Table Grapes in Storage. LWT-Food Sci. Technol. 2010, 43, 596–601. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial effects of modified chitosan-based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157:H7 and Salmonella Typhimurium on green beans. Food Control 2015, 50, 215–222. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Shan, C.L.; Zhou, Q.; Fang, Y.; Wang, Y.L.; Xu, F.; Han, L.R.; Ibrahim, M.; Guo, L.B.; Xie, G.L.; et al. Synthesis, characterization, and antibacterial activity of crosslinked chitosan-glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef] [Green Version]
- Salehi, R.; Arami, M.; Mahmoodi, N.M.; Bahrami, H.; Khorramfar, S. Novel biocompatible composite (chitosan–zinc oxide nanoparticle): Preparation, characterization and dye adsorption properties. Colloids Surf. B Biointerfaces 2010, 80, 86–93. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Camargo, T.P.; Gonçalves, N.S.; Neves, A.; Laranjeira, M.C.; Fávere, V.T. Chitosan crosslinked with a metal complexing agent: Synthesis, characterization and copper (II) ions adsorption. React. Funct. Polym. 2008, 68, 572–579. [Google Scholar] [CrossRef]
- Tsai, W.B.; Chen, Y.R.; Liu, H.L.; Lai, J.Y. Fabrication of UV-crosslinked chitosan scaffolds with conjugation of RGD peptides for bone tissue engineering. Carbohydr. Polym. 2011, 85, 129–137. [Google Scholar] [CrossRef]
- Yeh, J.T.; Chen, C.L.; Huang, K.S. Synthesis and properties of chitosan/SiO2 hybrid materials. Mater. Lett. 2007, 61, 1292–1295. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Genipin-Crosslinked Gelatin/Chitosan-Based Functional Films Incorporated with Rosemary Essential Oil and Quercetin. Materials 2022, 15, 3769. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Wilson, L.D. An Overview of the Design of Chitosan-Based Fiber Composite Materials. J. Compos. Sci. 2021, 5, 160. [Google Scholar] [CrossRef]
- Loscertales, I.G.; Barrero, A.; Guerrero, I.; Cortijo, R.; Marquez, M.; Ganan-Calvo, A.M. Micro/nano encapsulation via electrified coaxial liquid jets. Science 2002, 295, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Kimura, E.; Sato, T.; Fujita, S.; Shiratori, S. Fabrication of blend biodegradable nanofibrous nonwoven mats via multi-jet electrospinning. Polymer 2004, 45, 1895–1902. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Sapkota, S.; Chou, S.F. Electrospun Chitosan-based Fibers for Wound Healing Applications. J. Biomater. 2020, 4, 51–57. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the Functionality of Chitosan- and Alginate-Based Active Edible Coatings/Films for the Preservation of Fruits and Vegetables: A Review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Jiang, A.; Patel, R.; Padhan, B.; Palimkar, S.; Galgali, P.; Adhikari, A.; Varga, I.; Patel, M. Chitosan Based Biodegradable Composite for Antibacterial Food Packaging Application. Polymers 2023, 15, 2235. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of Chitosan Concentration on Mechanical and Barrier Properties of Corn Starch/Chitosan Films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef]
- Escamilla-García, M.; Reyes-Basurto, A.; García-Almendárez, B.E.; Hernández-Hernández, E.; Calderón-Domínguez, G.; Rossi-Márquez, G.; Regalado-González, C. Modified Starch-Chitosan Edible Films: Physicochemical and Mechanical Characterization. Coatings 2017, 7, 224. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M.; Akyuz, L.; Sargin, I.; Mujtaba, M.; Salaberria, A.M.; Labidi, J.; Cakmak, Y.S.; Koc, B.; Baran, T.; Ceter, T. Incorporation of Sporopollenin Enhances Acid–Base Durability, Hydrophobicity, and Mechanical, Antifungal and Antioxidant Properties of Chitosan Films. J. Ind. Eng. Chem. 2017, 47, 236–245. [Google Scholar] [CrossRef]
- Younis, H.G.R.; Zhao, G. Physicochemical Properties of the Edible Films from the Blends of High Methoxyl Apple Pectin and Chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Luo, H.; Dai, Z.; Nie, M.; Hashim, M.M.; Liu, C.; Song, J.; Li, D. Chitosan-Based Biodegradable Active Food Packaging Film Containing Chinese Chive (Allium tuberosum) Root Extract for Food Application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and Characterization of Chitosan-Based Antimicrobial Active Food Packaging Film Incorporated with Apple Peel Polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of Chitosan-Based Biodegradable Active Films Using Bio-Waste Enriched with Polyphenol Propolis Extract Envisaging Food Packaging Applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef]
- Wu, C.; Tian, J.; Li, S.; Wu, T.; Hu, Y.; Chen, S.; Sugawara, T.; Ye, X. Structural Properties of Films and Rheology of Film-Forming Solutions of Chitosan Gallate for Food Packaging. Carbohydr. Polym. 2016, 146, 10–19. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Zhang, J.; Dai, W.; Gao, X.; Zhou, Y.; Wan, X.; Puligundla, P. Preparation and Characterization of Antioxidant Edible Chitosan Films Incorporated with Epigallocatechin Gallate Nanocapsules. Carbohydr. Polym. 2017, 171, 300–306. [Google Scholar] [CrossRef]
- Vilela, C.; Pinto, R.J.B.; Coelho, J.; Domingues, M.R.M.; Daina, S.; Sadocco, P.; Santos, S.A.O.; Freire, C.S.R. Bioactive Chitosan/Ellagic Acid Films with UV-Light Protection for Active Food Packaging. Food Hydrocoll. 2017, 73, 120–128. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of Protocatechuic Acid Incorporation on the Physical, Mechanical, Structural and Antioxidant Properties of Chitosan Film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Bai, R.; Liu, Y.; Liu, J.; Liu, J. Preparation and Characterization of Antioxidant and Antimicrobial Packaging Films Based on Chitosan and Proanthocyanidins. Int. J. Biol. Macromol. 2019, 134, 11–19. [Google Scholar] [CrossRef]
- Yang, K.; Dang, H.; Liu, L.; Hu, X.; Li, X.; Ma, Z.; Wang, X.; Ren, T. Effect of Syringic Acid Incorporation on the Physical, Mechanical, Structural and Antibacterial Properties of Chitosan Film for Quail Eggs Preservation. Int. J. Biol. Macromol. 2019, 141, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J.; Le, X. Molecular Interactions, Characterization and Antimicrobial Activity of Curcumin–Chitosan Blend Films. Food Hydrocoll. 2016, 52, 564–572. [Google Scholar] [CrossRef]
- Musella, E.; Ouazzani, I.C.E.; Mendes, A.R.; Rovera, C.; Farris, S.; Mena, C.; Teixeira, P.; Poças, F. Preparation and Characterization of Bioactive Chitosan-Based Films Incorporated with Olive Leaves Extract for Food Packaging Applications. Coatings 2021, 11, 1339. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J.; Qin, Y.; Bai, R.; Zhang, X.; Liu, J. Antioxidant and PH-Sensitive Films Developed by Incorporating Purple and Black Rice Extracts into Chitosan Matrix. Int. J. Biol. Macromol. 2019, 137, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical Properties, Antioxidant and Antimicrobial Activity of Chitosan Films Containing Carvacrol and Pomegranate Peel Extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant Edible Films Based on Chitosan and Starch Containing Polyphenols from Thyme Extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; Erim, F.B. Antimicrobial and Physical Properties of Chitosan Films Incorporated with Turmeric Extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango Leaf Extract Incorporated Chitosan Antioxidant Film for Active Food Packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of Antioxidant and Intelligent PH-Sensing Packaging Films by Incorporating Purple-Fleshed Sweet Potato Extract into Chitosan Matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Z.H.; Qin, Y.Y.; Zhao, T.R.; Cheng, C.S. Characterization of Antioxidant Chitosan Film Incorporated with Pomegranate Peel Extract. Adv. Mater. Res. 2013, 706–708, 24–27. [Google Scholar] [CrossRef]
- Tan, Y.M.; Lim, S.H.; Tay, B.Y.; Lee, M.W.; Thian, E.S. Functional Chitosan-Based Grapefruit Seed Extract Composite Films for Applications in Food Packaging Technology. Mater. Res. Bull. 2015, 69, 142–146. [Google Scholar] [CrossRef]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and Characterization of Chitosan Based Edible Films from Berberis Crataegina’s Fruit Extract and Seed Oil. Innov. Food Sci. Emerg. Technol. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Kadam, D.; Shah, N.; Palamthodi, S.; Lele, S.S. An Investigation on the Effect of Polyphenolic Extracts of Nigella Sativa Seedcake on Physicochemical Properties of Chitosan-Based Films. Carbohydr. Polym. 2018, 192, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Vitchayakitti, W. Improving Functional Properties of Chitosan Films as Active Food Packaging by Incorporating with Propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, F.; Feng, Z.; Fan, X.; Pan, Z.; Zhou, J. Antioxidant Activity and Physicochemical Properties of Chitosan Films Incorporated with Lycium Barbarum Fruit Extract for Active Food Packaging. Int. J. Food Sci. Technol. 2015, 50, 458–464. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Wang, J.; Jiang, G.; Du, F.; Liu, X. Effects of Herba lophatheri Extract on the Physicochemical Properties and Biological Activities of the Chitosan Film. Int. J. Biol. Macromol. 2019, 133, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yong, H.; Yao, X.; Chen, D.; Kan, J.; Liu, J. Effect of Starch Aldehyde-Catechin Conjugates on the Structural, Physical and Antioxidant Properties of Quaternary Ammonium Chitosan/Polyvinyl Alcohol Films. Food Hydrocoll. 2022, 124, 107279. [Google Scholar] [CrossRef]
- Lan, W.; Wang, S.; Chen, M.; Sameen, D.E.; Lee, K.J.; Liu, Y. Developing Poly(Vinyl Alcohol)/Chitosan Films Incorporate with d-Limonene: Study of Structural, Antibacterial, and Fruit Preservation Properties. Int. J. Biol. Macromol. 2020, 145, 722–732. [Google Scholar] [CrossRef]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in Situ Enhanced PVA/Chitosan Biodegradable Films for Food Packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef]
- Cao, W.; Yan, J.; Liu, C.; Zhang, J.; Wang, H.; Gao, X.; Yan, H.; Niu, B.; Li, W. Preparation and Characterization of Catechol-Grafted Chitosan/Gelatin/Modified Chitosan-AgNP Blend Films. Carbohydr. Polym. 2020, 247, 116643. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Rodrigues, P.F.; Lopes, A.; Silva, R.J.; Caldeira, J.; Duarte, M.P.; Fernandes, F.B.; Coelhoso, I.M.; et al. Physical and Morphological Characterization of Chitosan/Montmorillonite Films Incorporated with Ginger Essential Oil. Coatings 2019, 9, 700. [Google Scholar] [CrossRef] [Green Version]
- Grande, C.D.; Mangadlao, J.; Fan, J.; De Leon, A.; Delgado-Ospina, J.; Rojas, J.G.; Rodrigues, D.F.; Advincula, R. Chitosan Crosslinked Graphene Oxide Nanocomposite Films with Antimicrobial Activity for Application in Food Industry. Macromol. Symp. 2017, 374, 1600114. [Google Scholar] [CrossRef]
- Inanli, A.G.; Tümerkan, E.T.A.; Abed, N.E.; Regenstein, J.M.; Özogul, F. The Impact of Chitosan on Seafood Quality and Human Health: A Review. Trends Food Sci. Technol. 2020, 97, 404–416. [Google Scholar] [CrossRef]
- Yu, D.; Li, P.; Xu, Y.; Jiang, Q.; Xia, W. Physicochemical, Microbiological, and Sensory Attributes of Chitosan-Coated Grass Carp (Ctenopharyngodon idellus) Fillets Stored at 4 °C. Int. J. Food Prop. 2016, 20, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Ge, Y.; Li, Y.; Xiang, Y.; Jiang, Y.; Hu, Y. Quality Enhancement of Large Yellow Croaker Treated with Edible Coatings Based on Chitosan and Lysozyme. Int. J. Biol. Macromol. 2018, 120, 1072–1079. [Google Scholar] [CrossRef]
- Hussain, M.A.; Sumon, T.A.; Mazumder, S.K.; Ali, M.M.; Jang, W.J.; Abualreesh, M.H.; Sharifuzzaman, S.M.; Brown, C.L.; Lee, H.T.; Lee, E.W.; et al. Essential Oils and Chitosan as Alternatives to Chemical Preservatives for Fish and Fisheries Products: A Review. Food Control 2021, 129, 108244. [Google Scholar] [CrossRef]
- Xin, S.; Xiao, L.; Dong, X.; Li, X.; Wang, Y.; Hu, X.; Sameen, D.E.; Qin, W.; Zhu, B. Preparation of Chitosan/Curcumin Nanoparticles Based Zein and Potato Starch Composite Films for Schizothorax prenati Fillet Preservation. Int. J. Biol. Macromol. 2020, 164, 211–221. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Zhao, L.; Chen, L.; He, Y.; Yang, H. Vacuum Impregnation of Fish Gelatin Combined with Grape Seed Extract Inhibits Protein Oxidation and Degradation of Chilled Tilapia Fillets. Food Chem. 2019, 294, 316–325. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Y.; Cao, A. The Physiochemical and Preservation Properties of Fish Sarcoplasmic Protein/Chitosan Composite Films Containing Ginger Essential Oil Emulsions. J. Food Process Eng. 2020, 43, e13495. [Google Scholar] [CrossRef]
- Chen, L.; Jiao, D.; Zhou, B.; Zhu, C.; Liu, J.; Zhang, D.; Liu, H. Shrimp (Penaeus monodon) Preservation by Using Chitosan and Tea Polyphenol Coating Combined with High-Pressure Processing. Food Sci. Nutr. 2022, 10, 3395–3404. [Google Scholar] [CrossRef]
- Rezaeifar, M.; Mehdizadeh, T.; Mojaddar Langroodi, A.; Rezaei, F. Effect of Chitosan Edible Coating Enriched with Lemon Verbena Extract and Essential Oil on the Shelf Life of Vacuum Rainbow Trout (Oncorhynchus mykiss). J. Food Saf. 2020, 40, e12781. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Afshari, A.; Aminzare, M.; Raeisi, M.; Zeinali, T. Effect of Different Types of Active Biodegradable Films Containing Lactoperoxidase System or Sage Essential Oil on the Shelf Life of Fish Burger during Refrigerated Storage. LWT-Food Sci. Technol. 2020, 117, 108633. [Google Scholar] [CrossRef]
- Demircan, B.; Özdestan-Ocak, Ö. Effects of Lemon Essential Oil and Ethyl Lauroyl Arginate on the Physico-Chemical and Mechanical Properties of Chitosan Films for Mackerel Fillet Coating Application. J. Food Meas. Charact. 2021, 15, 1499–1508. [Google Scholar] [CrossRef]
- Zamani, F.; Khoshkhoo, Z.; Hosseini, S.E.; Basti, A.A.Z.; Azizi, M.H. Chitosan Nano-Coating Incorporated with Green Cumin (Cuminum cyminum) Extracts: An Active Packaging for Rainbow Trout (Oncorhynchus mykiss) Preservation. J. Food Meas. Charact. 2022, 16, 1228–1240. [Google Scholar] [CrossRef]
- Huang, L. Growth Kinetics of Escherichia coli O157:H7 in Mechanically-Tenderized Beef. Int. J. Food Microbiol. 2010, 140, 40–48. [Google Scholar] [CrossRef]
- Laine, E.S.; Scheftel, J.M.; Boxrud, D.J.; Vought, K.J.; Danila, R.N.; Elfering, K.M.; Smith, K.E. Outbreak of Escherichia coli O157:H7 Infections Associated with Nonintact Blade-Tenderized Frozen Steaks Sold by Door-to-Door Vendors. J. Food Prot. 2005, 68, 1198–1202. [Google Scholar] [CrossRef]
- Serrano-León, J.S.; Bergamaschi, K.B.; Yoshida, C.M.P.; Saldaña, E.; Selani, M.M.; Rios-Mera, J.D.; Alencar, S.M.; Contreras-Castillo, C.J. Chitosan Active Films Containing Agro-Industrial Residue Extracts for Shelf Life Extension of Chicken Restructured Product. Food Res. Int. 2018, 108, 93–100. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Noipha, S. Active Film from Chitosan Incorporating Green Tea Extract for Shelf Life Extension of Pork Sausages. Food Hydrocoll. 2012, 27, 102–108. [Google Scholar] [CrossRef]
- Liu, F.; Chang, W.; Chen, M.; Xu, F.; Ma, J.; Zhong, F. Tailoring Physicochemical Properties of Chitosan Films and Their Protective Effects on Meat by Varying Drying Temperature. Carbohydr. Polym. 2019, 212, 150–159. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-Starch Film Containing Pomegranate Peel Extract and Thymus Kotschyanus Essential Oil Can Prolong the Shelf Life of Beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Effect of Chitosan-Gelatin Coating Containing Nano-Encapsulated Tarragon Essential Oil on the Preservation of Pork Slices. Meat Sci. 2020, 166, 108137. [Google Scholar] [CrossRef]
- Gaba, A.B.M.; Hassan, M.A.; Abd El-Tawab, A.A.; Abdelmonem, M.A.; Morsy, M.K. Protective Impact of Chitosan Film Loaded Oregano and Thyme Essential Oil on the Microbial Profile and Quality Attributes of Beef Meat. Antibiotics 2022, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hwang, K.T. Changes in Physicochemical Properties of Mulberry Fruits (Morus alba L.) during Ripening. Sci. Hortic. 2017, 217, 189–196. [Google Scholar] [CrossRef]
- Ali, S.; Nawaz, A.; Ejaz, S.; Haider, S.T.A.; Alam, M.W.; Javed, H.U. Effects of Hydrogen Sulfide on Postharvest Physiology of Fruits and Vegetables: An Overview. Sci. Hortic. 2019, 243, 290–299. [Google Scholar] [CrossRef]
- Martínez, K.; Ortiz, M.; Albis, A.; Castañeda, C.G.G.; Valencia, M.E.; Tovar, C.D.G. The Effect of Edible Chitosan Coatings Incorporated with Thymus Capitatus Essential Oil on the Shelf-Life of Strawberry (Fragaria × ananassa) during Cold Storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Li, W.; Wang, Q.; Li, X.; Xu, Q.; Guo, X.; Bi, X.; Liu, X.; Shui, Y.; Lin, H.; et al. Antimicrobial Nanoparticles Incorporated in Edible Coatings and Films for the Preservation of Fruits and Vegetables. Molecules 2019, 24, 1695. [Google Scholar] [CrossRef] [Green Version]
- Adiletta, G.; Zampella, L.; Coletta, C.; Petriccione, M. Chitosan Coating to Preserve the Qualitative Traits and Improve Antioxidant System in Fresh Figs (Ficus carica L.). Agriculture 2019, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Wang, H.; Wang, L. Physical Properties and Antibacterial Activity of Quaternized Chitosan/Carboxymethyl Cellulose Blend Films. LWT-Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M.M. Application of Chitosan-Based Apple Peel Polyphenols Edible Coating on the Preservation of Strawberry (Fragaria ananassa cv Hongyan) Fruit. J. Food Process. Preserv. 2021, 45, e15018. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Koushesh Saba, M.; Emamifar, A. Aloe Vera and Ascorbic Acid Coatings Maintain Postharvest Quality and Reduce Microbial Load of Strawberry Fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, H.; Guo, X.; Bi, X.; Liu, X.; Xu, Q.; Wang, Q.; Li, W.; Li, X.; Shui, Y.; et al. Effect of Chitosan/Nano-TiO2 Composite Coatings on the Postharvest Quality and Physicochemical Characteristics of Mango Fruits. Sci. Hortic. 2020, 263, 109135. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhou, S.; Sun, Q. Preservation Effect of Degradable Chitosan Starch Antibacterial Composite Membrane on Red Grape. J. Food Saf. Qual. 2017, 8, 1579–1584. [Google Scholar]
- Yang, G.; Yue, J.; Gong, X.; Qian, B.; Wang, H.; Deng, Y.; Zhao, Y. Blueberry Leaf Extracts Incorporated Chitosan Coatings for Preserving Postharvest Quality of Fresh Blueberries. Postharvest Biol. Technol. 2014, 92, 46–53. [Google Scholar] [CrossRef]
- Sun, M.; Liu, N.; Ni, S.; Bian, H.; Fu, Y.; Chen, X. Poplar Hot Water Extract Enhances Barrier and Antioxidant Properties of Chitosan/Bentonite Composite Film for Packaging Applications. Polymers 2019, 11, 1614. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, X.; Jiang, W. Development of Antioxidant Chitosan Film with Banana Peels Extract and Its Application as Coating in Maintaining the Storage Quality of Apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An Overview of Natural Antimicrobials Role in Food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Ponce, A.; Roura, S.I.; Moreira, M.R. Casein and Chitosan Polymers: Use in Antimicrobial Packaging. In Antimicrobial Food Packaging, 1st ed.; Barros-Velázquez, J., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 455–466. [Google Scholar]
- Ye, Y.; Guo, H.; Sun, X. Recent Progress on Cell-Based Biosensors for Analysis of Food Safety and Quality Control. Biosens. Bioelectron. 2019, 126, 389–404. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/Corn Starch Blend Films with Extract from Brassica oleraceae (Red Cabbage) as a Visual Indicator of Fish Deterioration. LWT-Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi Tirtashi, F.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Cellulose/Chitosan PH-Responsive Indicator Incorporated with Carrot Anthocyanins for Intelligent Food Packaging. Int. J. Biol. Macromol. 2019, 136, 920–926. [Google Scholar] [CrossRef]
- Snyman, D.; Hamman, J.H.; Kotze, J.S.; Rollings, J.E.; Kotzé, A.F. The Relationship between the Absolute Molecular Weight and the Degree of Quaternisation of N-Trimethyl Chitosan Chloride. Carbohydr. Polym. 2002, 50, 145–150. [Google Scholar] [CrossRef]
- Wang, T.; Farajollahi, M.; Choi, Y.S.; Lin, I.T.; Marshall, J.E.; Thompson, N.M.; Kar-Narayan, S.; Madden, J.D.W.; Smoukov, S.K. Electroactive Polymers for Sensing. Interface Focus 2016, 6, 20160026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Li, P.; Guo, Z. Cationic Chitosan Derivatives as Potential Antifungals: A Review of Structural Optimization and Applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Goycoolea, F.M.; Sosnik, A.; das Neves, J. Chitosan and Chitosan Derivatives for Biological Applications: Chemistry and Functionalization. Int. J. Carbohydr. Chem. 2011, 2011, 802693. [Google Scholar] [CrossRef] [Green Version]
- Dilamian, M.; Montazer, M.; Masoumi, J. Antimicrobial Electrospun Membranes of Chitosan/Poly(Ethylene Oxide) Incorporating Poly(Hexamethylene Biguanide) Hydrochloride. Carbohydr. Polym. 2013, 94, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan Biomaterials for Current and Potential Dental Applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, S.; Ishii, K.; Nadai, T.; Miyazaki, S. The Use of Chitin and Chitosan as Drug Carriers. Chem. Pharm. Bull. 1981, 29, 3067–3069. [Google Scholar] [CrossRef] [Green Version]
- Ailincai, D.; Porzio, W.; Marin, L. Hydrogels Based on Imino-Chitosan Amphiphiles as a Matrix for Drug Delivery Systems. Polymers 2020, 12, 2687. [Google Scholar] [CrossRef]
- Zuo, R.; Shi, J.; Jiang, S.; Chu, M.; Wang, Q.; Kong, L.; Kang, Q.; Guo, Y.P.; Guan, J. Promotion of the Genipin Crosslinked Chitosan-Fiber Hydrogel Loaded with Sustained Release of Clemastine Fumarate in Diabetic Wound Repair. Int. J. Biol. Macromol. 2023, 226, 900–914. [Google Scholar] [CrossRef]
- Dahan, W.M.; Mohammad, F.; Ezzat, A.R.O.; Atta, A.M.; Al-Tilasi, H.H.; Al-Lohedan, H.A. Enhanced Delivery of Insulin through Acrylamide-Modified Chitosan Containing Smart Carrier System. Gels 2022, 8, 701. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Mathiyalagan, R.; Nguyen, M.T.; Tran, T.T.; Murugesan, M.; Ho, T.N.; Huong, H.; Yang, D.C.; Li, Y.; Thambi, T. Ionically Crosslinked Alginate-Chitosan Core-Shell Hydrogel Beads for Oral Delivery of Insulin. Int. J. Biol. Macromol. 2022, 222, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Yari, K.; Gharati, G.; Akbari, I. Evaluating Effect of Salt Leaching Method on Release and Swelling Rate of Metformin Nanoparticles Loaded-Chitosan/Polyvinyl Alcohol Porous Composite. Int. J. Biol. Macromol. 2023, 227, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Farhadnejad, H.; Mortazavi, S.A.; Jamshidfar, S.; Rakhshani, A.; Motasadizadeh, H.; Fatahi, Y.; Mahdieh, A.; Darbasizadeh, B. Montmorillonite-Famotidine/Chitosan Bio-Nanocomposite Hydrogels as a Mucoadhesive/Gastroretentive Drug Delivery System. Iran. J. Pharm. Res. 2022, 21, e127035. [Google Scholar] [CrossRef]
- Rezaei, A.; Hooman Vahidi, S.; Nasrabadi, M.; Ali Beyramabadi, S.; Morsali, A. Quantum Chemical Study of 2-Hydroxypropyl-β-Cyclodextrin and Genipin-Crosslinked Chitosan Nanocarriers Functionalized with Cytarabine Anticancer Drug. J. Mol. Liq. 2022, 367, 120427. [Google Scholar] [CrossRef]
- Rui, Q.; Gao, J.; Yin, Z.Z.; Li, J.; Cai, W.; Wu, D.; Kong, Y. A Biodegradable PH and Glutathione Dual-Triggered Drug Delivery System Based on Mesoporous Silica, Carboxymethyl Chitosan and Oxidized Pullulan. Int. J. Biol. Macromol. 2023, 224, 1294–1302. [Google Scholar] [CrossRef]
- Kamaci, M.; Kaya, I. Chitosan Based Hybrid Hydrogels for Drug Delivery: Preparation, Biodegradation, Thermal, and Mechanical Properties. Polym. Adv. Technol. 2023, 34, 779–788. [Google Scholar] [CrossRef]
- Pujana, M.A.; Pérez-Álvarez, L.; Iturbe, L.C.C.; Katime, I. Water Soluble Folate-Chitosan Nanogels Crosslinked by Genipin. Carbohydr. Polym. 2014, 101, 113–120. [Google Scholar] [CrossRef]
- Barkhordari, S.; Alizadeh, A.; Yadollahi, M.; Namazi, H. One-Pot Synthesis of Magnetic Chitosan/Iron Oxide Bio-Nanocomposite Hydrogel Beads as Drug Delivery Systems. Soft Mater. 2020, 19, 373–381. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Abdolahinia, E.D.; Nakhlband, A.; Aslzad, S.; Fathi, M.; Barar, J.; Omidi, Y. Smart Chitosan–Folate Hybrid Magnetic Nanoparticles for Targeted Delivery of Doxorubicin to Osteosarcoma Cells. Colloids Surf. B Biointerfaces 2022, 220, 112911. [Google Scholar] [CrossRef]
- Moura, M.J.; Gil, M.H.; Figueiredo, M.M. Cisplatin Delivery Systems Based on Different Drug Encapsulation Techniques. Eur. Polym. J. 2019, 113, 357–364. [Google Scholar] [CrossRef]
- Li, N.; Lin, J.; Liu, C.; Zhang, Q.; Li, R.; Wang, C.; Zhao, C.; Lu, L.; Zhou, C.; Tian, J.; et al. Temperature- and PH-Responsive Injectable Chitosan Hydrogels Loaded with Doxorubicin and Curcumin as Long-Lasting Release Platforms for the Treatment of Solid Tumors. Front. Bioeng. Biotechnol. 2022, 10, 1043939. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and Its Derivatives for Tissue Engineering Applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Tsai, S.P.; Wang, D.M.; Chang, Y.N.; Hsieh, H.J. Preparation of γ-PGA/Chitosan Composite Tissue Engineering Matrices. Biomaterials 2005, 26, 5617–5623. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In Vitro Characterization of Chitosan–Gelatin Scaffolds for Tissue Engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef] [PubMed]
- Sarasam, A.; Madihally, S.V. Characterization of Chitosan–Polycaprolactone Blends for Tissue Engineering Applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar] [CrossRef]
- Ressler, A. Chitosan-Based Biomaterials for Bone Tissue Engineering Applications: A Short Review. Polymers 2022, 14, 3430. [Google Scholar] [CrossRef] [PubMed]
- Vukajlovic, D.; Parker, J.; Bretcanu, O.; Novakovic, K. Chitosan Based Polymer/Bioglass Composites for Tissue Engineering Applications. Mater. Sci. Eng. C 2019, 96, 955–967. [Google Scholar] [CrossRef]
- Dasgupta, S.; Maji, K.; Nandi, S.K. Investigating the Mechanical, Physiochemical and Osteogenic Properties in Gelatin-Chitosan-Bioactive Nanoceramic Composite Scaffolds for Bone Tissue Regeneration: In Vitro and in Vivo. Mater. Sci. Eng. C 2019, 94, 713–728. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B.; Li, M.; Li, J.; Wan, Y. Enhanced Dual Network Hydrogels Consisting of Thiolated Chitosan and Silk Fibroin for Cartilage Tissue Engineering. Carbohydr. Polym. 2020, 227, 115335. [Google Scholar] [CrossRef]
- Patel, B.; Manne, R.; Patel, D.B.; Gorityala, S.; Palaniappan, A.; Kurakula, M. Chitosan as Functional Biomaterial for Designing Delivery Systems in Cardiac Therapies. Gels 2021, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, W.; Shi, G.; Zhu, F.; He, X.; Zhu, Z.; Chen, H. Human Cardiac Extracellular Matrix chitosan gelatin Composite Scaffold and Its Endothelialization. Exp. Ther. Med. 2020, 19, 1225–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, X.; Li, M.; Wang, X.; Liang, J.; Wang, X.; Wu, S.; Long, M.; Hu, C. An Injectable Chitosan/Dextran/β-Glycerophosphate Hydrogel as Cell Delivery Carrier for Therapy of Myocardial Infarction. Carbohydr. Polym. 2020, 229, 115516. [Google Scholar] [CrossRef] [PubMed]
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT Nanofibers as Electrically Conductive Scaffolds for Cardiovascular Tissue Engineering. Int. J. Biol. Macromol. 2019, 140, 278–287. [Google Scholar] [CrossRef]
- Tayebi, T.; Baradaran-Rafii, A.; Hajifathali, A.; Rahimpour, A.; Zali, H.; Shaabani, A.; Niknejad, H. Biofabrication of Chitosan/Chitosan Nanoparticles/Polycaprolactone Transparent Membrane for Corneal Endothelial Tissue Engineering. Sci. Rep. 2021, 11, 7060. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Li, P.; Zhu, C.; Lin, Z. The Wound Dressings and Their Applications in Wound Healing and Management. Health Sci. J. 2019, 13, 662. [Google Scholar]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Kojima, K.; Okamoto, Y.; Kojima, K.; Miyatake, K.; Fujise, H.; Shigemasa, Y.; Minami, S. Effects of Chitin and Chitosan on Collagen Synthesis in Wound Healing. J. Vet. Med. Sci. 2004, 66, 1595–1598. [Google Scholar] [CrossRef] [Green Version]
- Asghar, M.A.; Yousuf, R.I.; Shoaib, M.H.; Asghar, M.A. Antibacterial, Anticoagulant and Cytotoxic Evaluation of Biocompatible Nanocomposite of Chitosan Loaded Green Synthesized Bioinspired Silver Nanoparticles. Int. J. Biol. Macromol. 2020, 160, 934–943. [Google Scholar] [CrossRef]
- Deng, P.; Jin, W.; Liu, Z.; Gao, M.; Zhou, J. Novel Multifunctional Adenine-Modified Chitosan Dressings for Promoting Wound Healing. Carbohydr. Polym. 2021, 260, 117767. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, X.; Zheng, S.; Wang, Y.; Li, Z.; Zhang, H.; Nie, L.; Zhang, Y.; Zhao, Y.; Yang, X. Bio-Adhesive Catechol-Modified Chitosan Wound Healing Hydrogel Dressings through Glow Discharge Plasma Technique. Chem. Eng. J. 2022, 427, 130843. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Encapsulation of Food Bioactives and Nutraceuticals by Various Chitosan-Based Nanocarriers. Food Hydrocoll. 2020, 105, 105774. [Google Scholar] [CrossRef]
- Je, J.Y.; Kim, S.K. Chitosan as Potential Marine Nutraceutical. Adv. Food Nutr. Res. 2012, 65, 121–135. [Google Scholar] [PubMed]
- Hashemi Gahruie, H.; Niakousari, M. Antioxidant, Antimicrobial, Cell Viability and Enzymatic Inhibitory of Antioxidant Polymers as Biological Macromolecules. Int. J. Biol. Macromol. 2017, 104, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Harkin, C.; Mehlmer, N.; Woortman, D.V.; Brück, T.B.; Brück, W.M. Nutritional and Additive Uses of Chitin and Chitosan in the Food Industry. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2019; Volume 36, pp. 1–43. [Google Scholar]
- Kabanov, V.L.; Novinyuk, L.V. Chitosan application in food technology: A review of rescent advances. Food Syst. 2020, 3, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Taştan, Ö.; Baysal, T. Chitosan as a Novel Clarifying Agent on Clear Apple Juice Production: Optimization of Process Conditions and Changes on Quality Characteristics. Food Chem. 2017, 237, 818–824. [Google Scholar] [CrossRef]
- Abdelmalek, B.E.; Sila, A.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-Chitin and Chitosan from Squid Gladius: Biological Activities of Chitosan and Its Application as Clarifying Agent for Apple Juice. Int. J. Biol. Macromol. 2017, 104, 953–962. [Google Scholar] [CrossRef]
- Gassara, F.; Antzak, C.; Ajila, C.M.; Sarma, S.J.; Brar, S.K.; Verma, M. Chitin and Chitosan as Natural Flocculants for Beer Clarification. J. Food Eng. 2015, 166, 80–85. [Google Scholar] [CrossRef]
- Abebe, L.S.; Chen, X.; Sobsey, M.D.; Gray, N.F.; Karanis, P. Chitosan Coagulation to Improve Microbial and Turbidity Removal by Ceramic Water Filtration for Household Drinking Water Treatment. Int. J. Environ. Res. Public Health 2016, 13, 269. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Mittal, A.; Benjakul, S. Chitosan Nanoparticles: Preparation, Food Applications and Health Benefits. Sci. Asia 2021, 47, 1–10. [Google Scholar] [CrossRef]
- Bokura, H.; Kobayashi, S. Chitosan Decreases Total Cholesterol in Women: A Randomized, Double-Blind, Placebo-Controlled Trial. Eur. J. Clin. Nutr. 2003, 57, 721–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Din, H.M.N.; El-Naggar, A.W.M. Characterization of Gamma Irradiated Concentrated Aqueous Solutions of Chitosan/Sodium Alginate Blends and Their Drug Uptake-Release Characters. J. Appl. Polym. Sci. 2011, 122, 2383–2390. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and Chitosan Coating Nanoparticles for the Treatment of Brain Disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Guidotti, M.; Soengas, R. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.M. Chitosan and Their Derivatives: Antibiofilm Drugs against Pathogenic Bacteria. Colloids Surf. B Biointerfaces 2020, 185, 110627. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of Chitosan in Food, Pharmaceuticals, Medicine, Cosmetics, Agriculture, Textiles, Pulp and Paper, Biotechnology, and Environmental Chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef] [Green Version]
- Lauritano, D.; Limongelli, L.; Moreo, G.; Favia, G.; Carinci, F. Nanomaterials for Periodontal Tissue Engineering: Chitosan-Based Scaffolds. A Systematic Review. Nanomaterials 2020, 10, 605. [Google Scholar] [CrossRef] [Green Version]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The Recent Advances in Scaffolds for Integrated Periodontal Regeneration. Bioact. Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef]
- Walczak, K.; Schierz, G.; Basche, S.; Petto, C.; Boening, K.; Wieckiewicz, M. Antifungal and Surface Properties of Chitosan-Salts Modified PMMA Denture Base Material. Molecules 2020, 25, 5899. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Wang, A. Preparation and Characterization of a Novel PH-Sensitive Chitosan-g-Poly (Acrylic Acid)/Attapulgite/Sodium Alginate Composite Hydrogel Bead for Controlled Release of Diclofenac Sodium. Carbohydr. Polym. 2009, 78, 731–737. [Google Scholar] [CrossRef]









| Sr. No. | Chitosan Composite | Application | Ref. |
|---|---|---|---|
| 1. | Chitosan and curcumin-based film | The composite improved the shelf life of Schizothoraxpranati fillets and prevented protein degradation, delayed lipid oxidation, and the production of volatile amines | [99] |
| 2. | Chitosan nanoparticle-based coating containing cinnamon-perilla essential oils and anthocyanidins | Protected the Red Sea Bream fillets from oxidation and amine development | [100] |
| 3. | Chitosan and tea polyphenols-based film | Delayed the lipid oxidation in shrimps during storage (28 days) | [102] |
| 4. | Chitosan, pomegranate peel extract, and thymus essential oil-based film | Increased the shelf life of fresh beef | [112] |
| 5. | Chitosan, oregano, and thyme essential oils-based film | Inhibited the proliferation of psychrophilic bacteria and increased the shelf life of meat | [114] |
| 6. | Chitosan/nano-titanium dioxide-based film | Decreased deterioration and water loss while delaying respiratory peaks in mangoes | [123] |
| 7. | Chitosan/starch-based film | The film exhibited antibacterial properties and prevented water loss from grapes | [124] |
| 8. | Chitosan/corn starch hydrogel film-embedded extract from Brassica oleraceae | Acted as a pH-based sensor and exhibited a distinct color as fish spoils due to its strong sensitivity to pH changes | [131] |
| 9. | Chitosan/PVA polymeric composite | Aided in the regulated release drugs | [144] |
| 10. | Thiolated chitosan and silk fibroin hydrogel | The platform supported chondrocyte proliferation and encouraged the deposition of chondrocyte-specific matrix | [162] |
| 11. | Human cardiac ECM, chitosan, and gelatin composite | Used as a 3D scaffold for a tissue-engineered heart patch | [164] |
| 12. | Chitosan, dextran, and β-glycerophosphate hydrogel loaded with mesenchymal stem cells | Used for the treatment of myocardial infarction | [165] |
| 13. | Chitosan-gallic acid hydrogel | Exhibited wound healing and hemostatic characteristic | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Yadav, S.; Pramanik, J.; Sivamaruthi, B.S.; Jayeoye, T.J.; Prajapati, B.G.; Chaiyasut, C. Chitosan-Based Composites: Development and Perspective in Food Preservation and Biomedical Applications. Polymers 2023, 15, 3150. https://doi.org/10.3390/polym15153150
Kumar A, Yadav S, Pramanik J, Sivamaruthi BS, Jayeoye TJ, Prajapati BG, Chaiyasut C. Chitosan-Based Composites: Development and Perspective in Food Preservation and Biomedical Applications. Polymers. 2023; 15(15):3150. https://doi.org/10.3390/polym15153150
Chicago/Turabian StyleKumar, Akash, Sangeeta Yadav, Jhilam Pramanik, Bhagavathi Sundaram Sivamaruthi, Titilope John Jayeoye, Bhupendra G. Prajapati, and Chaiyavat Chaiyasut. 2023. "Chitosan-Based Composites: Development and Perspective in Food Preservation and Biomedical Applications" Polymers 15, no. 15: 3150. https://doi.org/10.3390/polym15153150