Engineering Polymer-Based Porous Membrane for Sustainable Lithium-Ion Battery Separators
Abstract
:1. Introduction
2. Characteristics Outline of LIB Separators and Polymeric Membranes
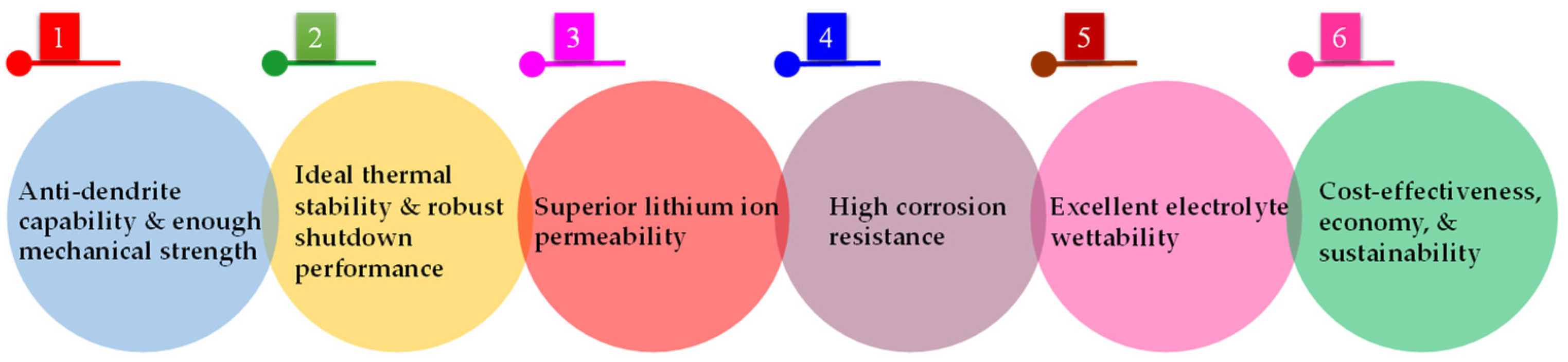
2.1. Requirements and Features of LIB Separators
2.1.1. Anti-Dendrite Capability and Enough Mechanical Strength
2.1.2. Ideal Thermal Stability and Robust Shutdown Performance
2.1.3. Superior Lithium Ion Permeability
2.1.4. High Corrosion Resistance
2.1.5. Excellent Electrolyte Wettability
2.1.6. Cost-Effectiveness, Economy, and Sustainability
2.2. Physical and Chemical Properties of Separator Membranes
2.2.1. Thickness
2.2.2. Tensile Strength
2.2.3. Puncture Resistance
2.2.4. Porosity and Pore Size
2.2.5. Electrolyte Absorption and Retention Rate
2.2.6. Dimensional Stability
2.2.7. Air Permeability
2.2.8. Electrochemical Performance
3. Manufacturing: Dry and Wet Methods Based on Uniaxial/Biaxial Stretching
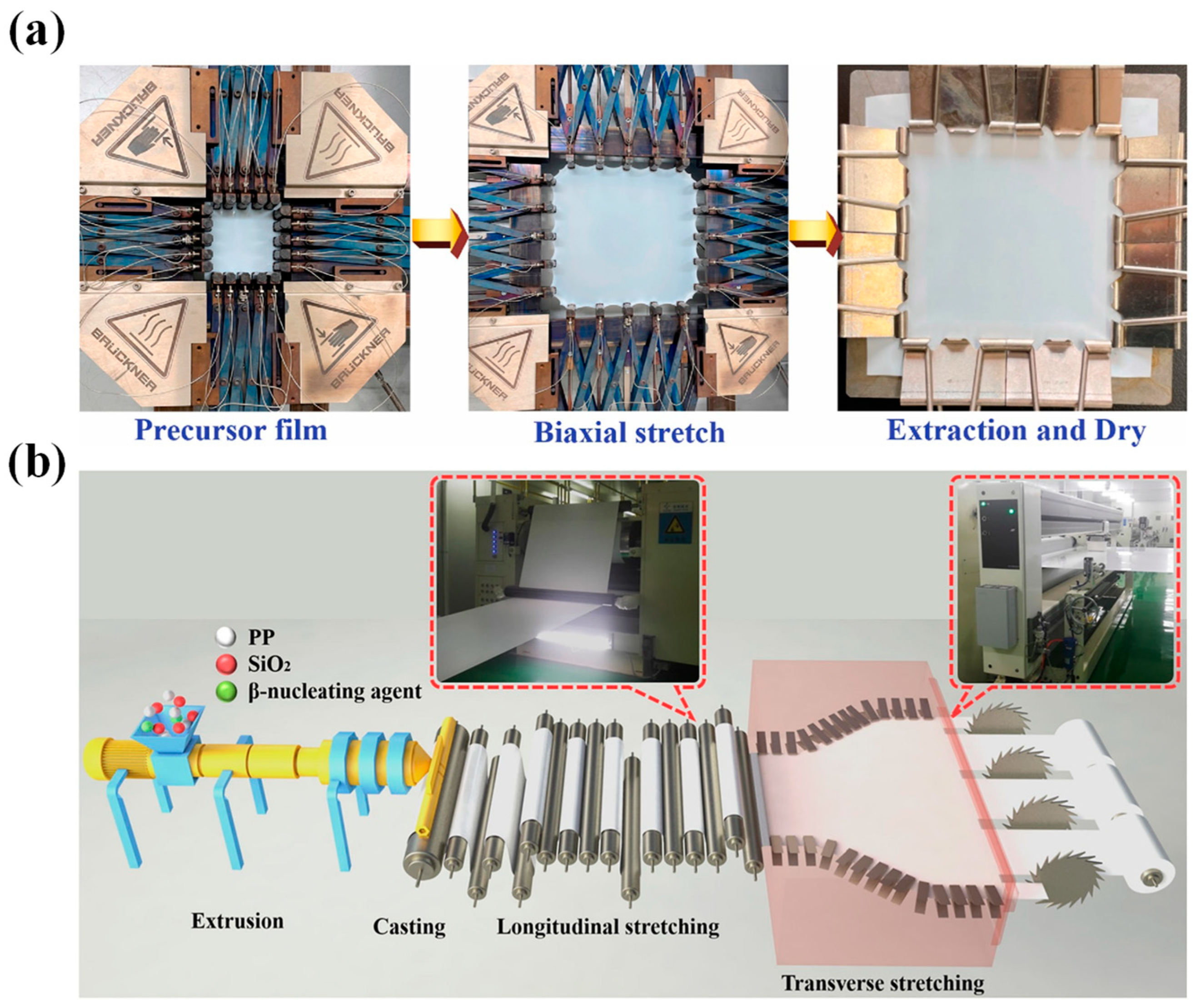
3.1. Uniaxial/Biaxial Stretching of Separator Membrane
3.2. Dry Method
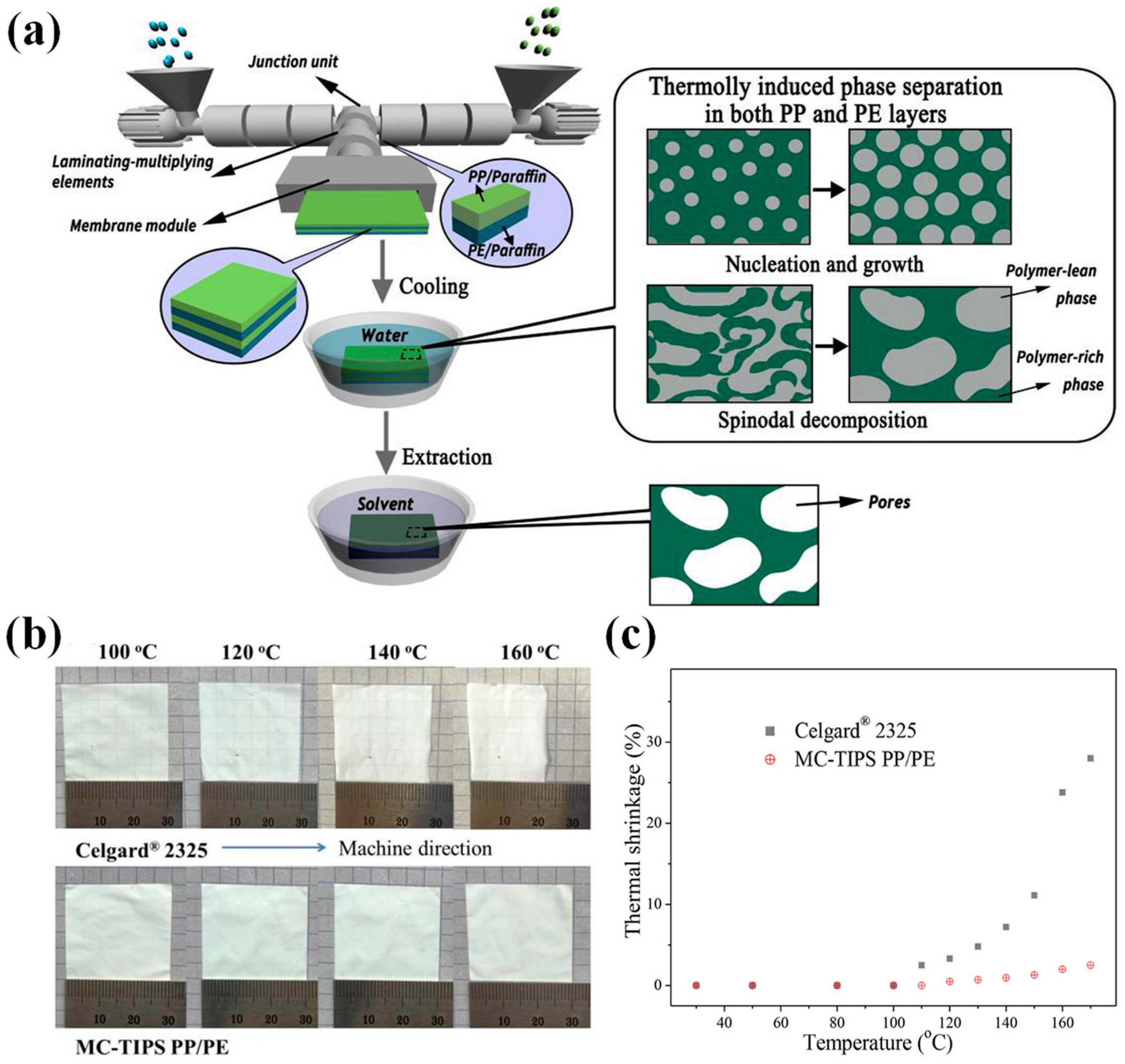
3.3. Wet Method
4. Recent Progress in Polymer-Based Porous Separator Membranes
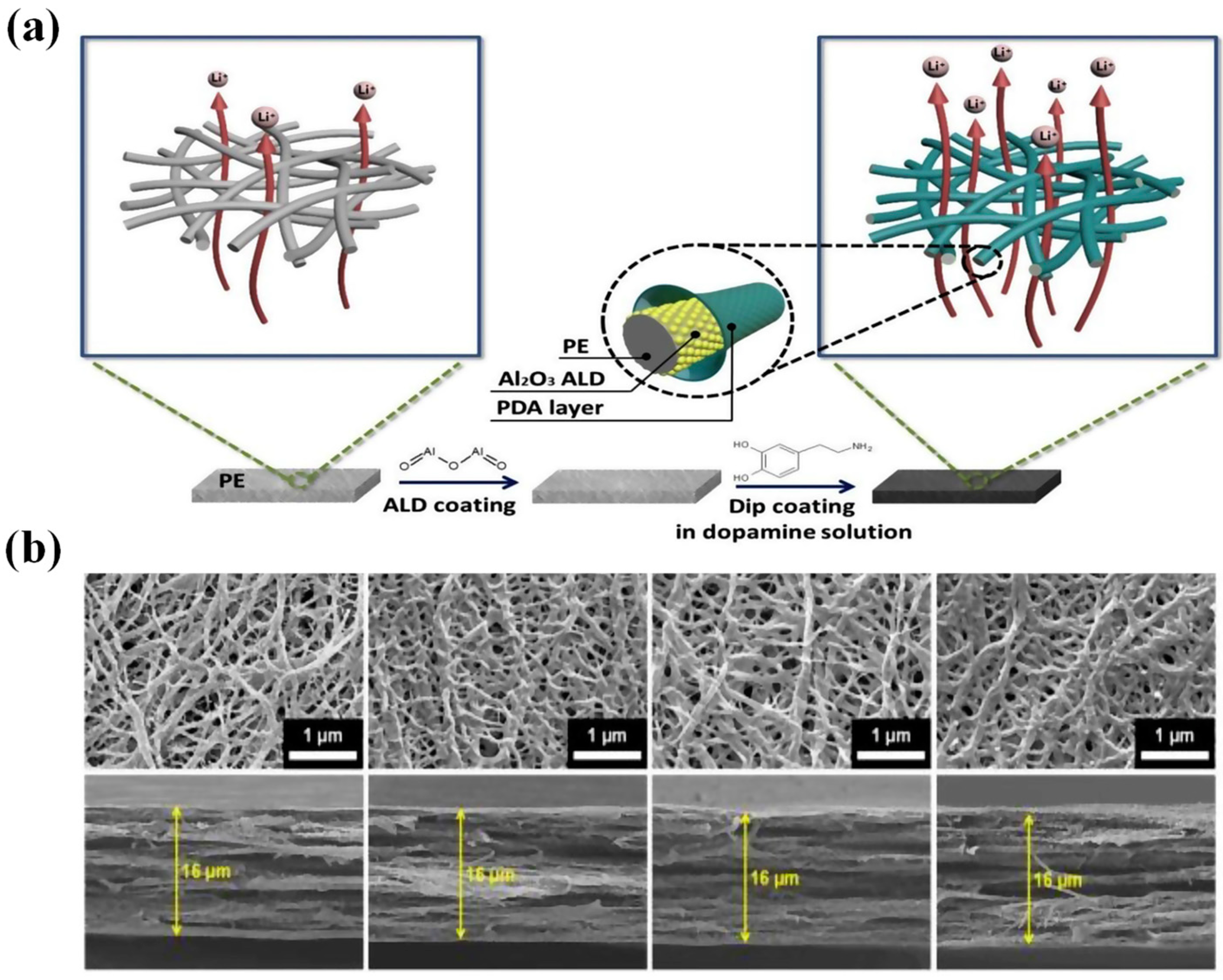
4.1. PE-Based Separator Membranes
4.2. PP-Based Separator Membranes
4.3. PVDF-Based Separator Membranes
4.4. Other Polymer-Based Separator Membranes
| Substrate Material | Additives | Thickness (μm) | Porosity (%) | Thermal Shrinkage (%) | Electrolyte Uptake (%) | Ionic Conductivity (mS cm−1) | Battery Performance | Ref. |
|---|---|---|---|---|---|---|---|---|
| PE | Al2O3 | 26 | 55.20 | 0% at 130 °C for 0.5 h | 144 | 0.75 | 68.4% (89.3 mAh g−1) after 200 cycles at 1 C | [182] |
| PE | γ-AlOOH | 26 | 55.63 | 0% at 170 °C for 0.5 h | 187 | 1.00 | 75.1% (95.1 mAh g−1) after 200 cycles at 1 C | [182] |
| PE | BPO | 85 | 0.89 | 98.1% after 70 cycles | [183] | |||
| PE | MA | 16 | 0.306 | >135 cycles at 1.5 mA cm−2 (1 C) | [184] | |||
| PE | SiO2–PZS | 20.4 ± 1.2 | 155.2 ± 14.3 | 1.04 | 115 mAh g−1 at 8 C; 81.69% after 100 cycles at 0.5 C | [185] | ||
| PE | HDPE wax/γ-AlOOH | 10 | 44.0 (85 °C); 10.3 (130 °C) | <5% at 130 °C for 1 h | 136.8 | 0.32 | 190.1 mAh g−1 (85 °C) at 0.1 C; 181.8 mAh g−1 (85 °C) at 0.1 C; 81% over 200 cycles | [186] |
| PE | PAI | 14.5–15.0 | 35–55 | 1.7% (MD) and 1.1% (TD) at 130 °C for 0.5 h | 98.8–99.5% after 10 cycles at 0 °C | [188] | ||
| PE | CA–PEO–LPSQ | 26 ± 1.5 | 9% at 120 °C for 1 h | 185 | 5.79 | 82% after 300 cycles at 0.5 C | [189] | |
| UHMWPE | SiO2 | 500 | 1.7% (MD) and 1% (TD) at 120 °C for 1 h | 472 ± 11.5 | 3.38 ± 0.62 | 165 mAh g−1 at 0.1 C; 123 mAh g−1 at 5 C; 99.93% after 300 cycles at 0.5 C | [99] | |
| UHMWPE | PMP | 65.4 ± 1.1 | 0.7% (MD) and 1.6% (TD) at 120 °C for 1 h | 259.7% | 1.17 | 172.8 mAh g−1 at 0.1 C; 99.89% after 100 cycles at 1 C | [190] | |
| UHMWPE | 2 | 78.3 | [191] | |||||
| UHMWPE | liquid paraffin | 12/5 | 94/30 | [154] | ||||
| UHMWPE | nano-Al2O3 | 15.3 | 44.2 | 14.3% at 120 °C and 34.5% at 130 °C for 0.5 h | 129.8 | 0.94 | 139.4 mAh g−1 at 0.2 C; 89.4% after 200 cycles at 1 C | [192] |
| PP | PAAB–Li | 24 | 61.8 | 195.8 | 0.96 | 119.4 mAh g−1 at 1 C; 78.0% after 250 cycles at 1 C | [193] | |
| PP | SiO2/β-iPP | 18 | 42 | 113.7 | 0.82 | 67.2 mAh g−1 at 7 C; 87% after 200 cycles | [155] | |
| PP | SiO2–TEOS | 25.6 | 4.6% at 150 °C for 0.5 h | 0.16 | 140 mAh g−1 at 0.2 C; 96.14% after 65 cycles at 0.2 C | [194] | ||
| PP | PI | 24 | 54.78 | 0% at 150 °C for 0.5 h | 207.62 | 0.35 | 144.3 mAh g−1 at 5 C; 80.1% after 200 cycles at 1 C | [195] |
| PP | ZIF-67–H2O | 30 | 57.17 | 3.33% at 120 °C for 0.5 h | 147 | 0.62 | 80.0% after 100 cycles at 1 C under 25 °C; 37.8% after 100 cycles at 1 C under 55 °C | [196] |
| PP | ZIF-67–CH3OH | 30 | 52.53 | 3.33% at 120 °C for 0.5 h | 142 | 0.78 | 86.9% after 100 cycles at 1 C under 25 °C; 61.5% after 100 cycles at 1 C under 55 °C | [196] |
| PP | BTCEAD | 38.2 ± 1.30 (8% GD); 32.7 ± 2.24 (18% GD); 24.3 ± 1.49 (31% GD) | 144 ± 5.9 (8% GD); 212 ± 4.5 (18% GD); 292 ± 9.4 (31% GD) | 0.56 (8% GD); 0.51 (18% GD) 0.37 (31% GD) | 84.59% after 175 cycles at 0.5 C (8% GD); 97.97% after 175 cycles at 0.5 C (18% GD) | [198] | ||
| PP/PE | 25 | 54.6 | 0% at 160 °C for 0.5 h | 157 | 1.46 | 98% (coulombic efficiency) after 30 cycles at 0.2 C | [199] | |
| PP/PAN/cotton | 63 | <4% at 160 °C for 1 h | 269 | 1.99 | 166.7 mAh g−1 at 1 C; 93.8% after 100 cycles at 1 C | [200] | ||
| PVDF | PMMA | 70–80 | 2.18 | 133.3 mAh g−1 at 4 C; 130.7 mAh g−1 after 200 cycles at 1 C | [204] | |||
| PVDF | SiO2/PMMA | 30 | 77 | 406 | 4.0 | 158 mAh g−1 after 50 cycles at 0.2 C | [205] | |
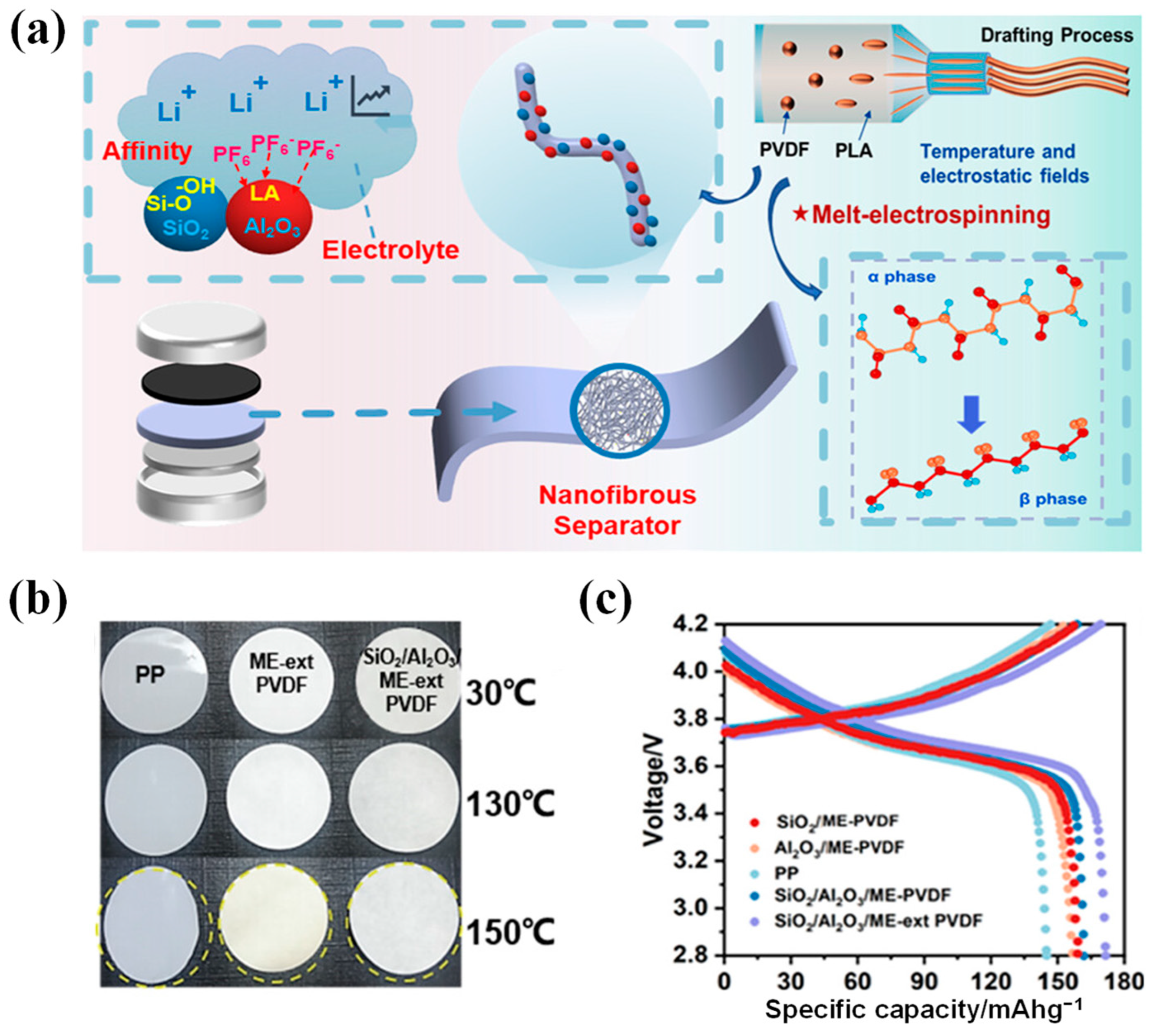
| PVDF | PLA/SiO2/Al2O3 | 25 | 468.46 | 2.24 | 86.42% after 100 cycles at 0.2 C | [75] | ||
| PVDF | HFP | 100–180 | >300 | 3.8 | 38 mAh g−1 at 2 C | [207] | ||
| PVDF–HFP | TiN | 25 | 30% at 175 °C for 1 h | 192 | 1.02 | 156 mAh g−1 at 1 C; 98% after 1000 cycles | [208] | |
| PU | graphene/LiAc | 61 | 0% at 170 °C for 1 h | 224.1 | 2.47 | 164.59 mAh g−1 at 0.2 C; 151.95 mAh g−1 at 0.5 C; 132.96 mAh g−1 at 1 C; 95% after 50 cycles at 0.5 C | [210] | |
| PU | Al2O3 | 29.1 | 63.7 | 0% at 211 °C | 371 | 0.65 | 139.5 mAh g−1 at 5 C; 82.7% after 200 cycles at 1 C | [211] |
| PAN/PMMA | PDA | 65 | 56.1 | 421 | 3.61 | 172 mAh g−1 at 0.2 C; 162.3 mAh g−1 after 200 cycles at 0.2 C | [213] | |
| PI | DBP/Gly | 10.5 | 80 (LiTFSI); 76 (LiPF6) | 0% at 180 °C for 0.5 h | 200 (LiTFSI); 220 (LiPF6) | 0.54 (LiTFSI); 0.55 (LiPF6) | 75.2 mAh g−1 at 2 C; 87.2% after 100 cycles at 0.5 C | [215] |
| CCN | 12 | 0.4 (CCN-0M); 1.6 (CCN-1M); 5.4 (CCN-3M); 4.6 (CCN-5M) | 1.16 (CCN-0M); 0.59 (CCN-1M); 0.57 (CCN-3M); 0.58 (CCN-5M) at 170 °C for 0.5 h | 0.45 | 100% (140 mAh g−1) after 49 cycles at 120 °C (CCN-3M) | [216] | ||
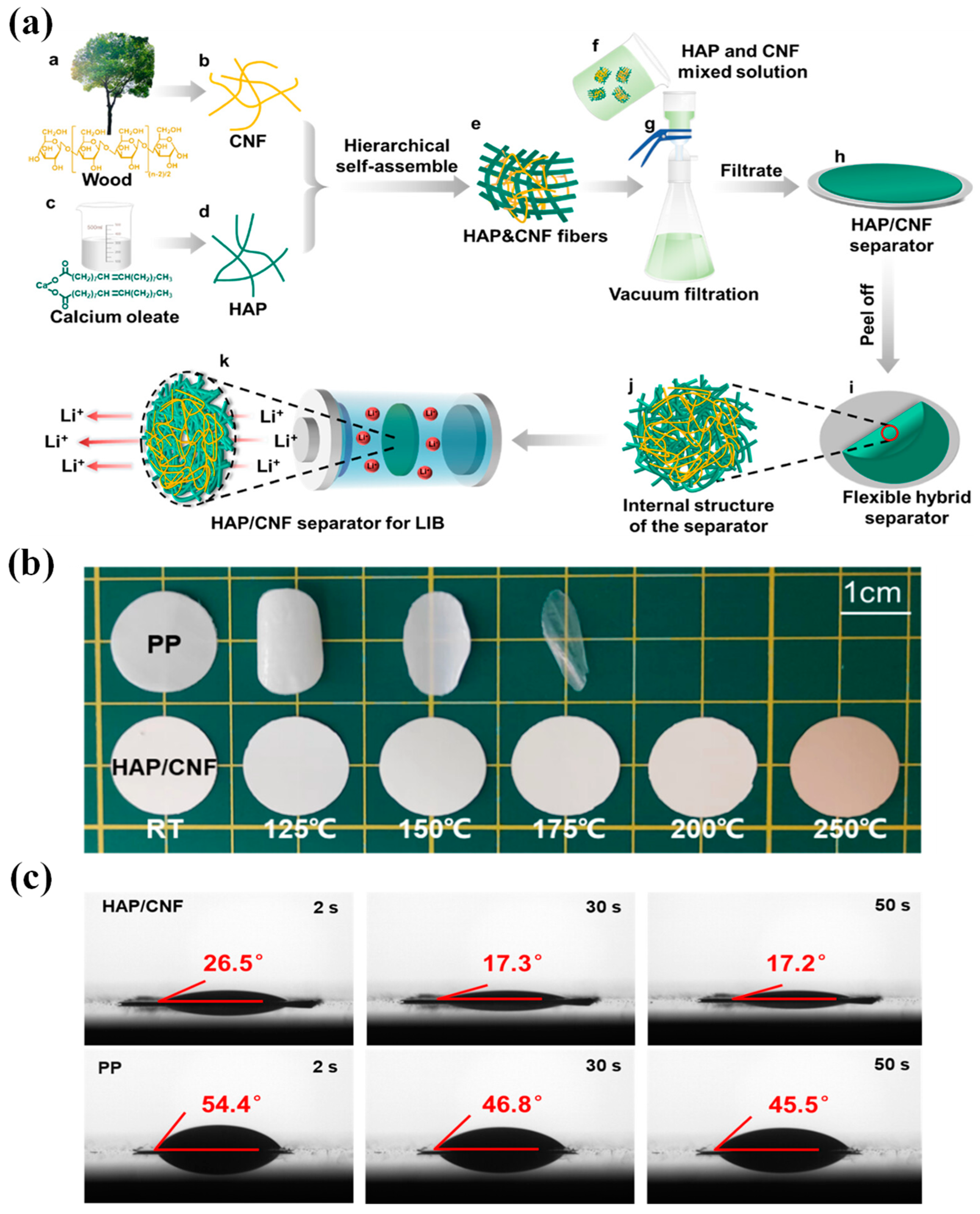
| HAP/CNF | 28 | 76 | 0% at 250 °C for 0.5 h | 162 | 0.81 | [217] |
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Di Lecce, D.; Verrelli, R.; Hassoun, J. Lithium-ion batteries for sustainable energy storage: Recent advances towards new cell configurations. Green Chem. 2017, 19, 3442–3467. [Google Scholar] [CrossRef]
- Chae, B.-G.; Park, S.Y.; Song, J.H.; Lee, E.; Jeon, W.S. Evolution and expansion of Li concentration gradient during charge–discharge cycling. Nat. Commun. 2021, 12, 3814. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief history of early lithium-battery development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef]
- Nishi, Y. The development of lithium ion secondary batteries. Chem. Rec. 2001, 1, 406–413. [Google Scholar] [CrossRef]
- Lin, L.; Ning, H.; Song, S.; Xu, C.; Hu, N. Flexible electrochemical energy storage: The role of composite materials. Compos. Sci. Technol. 2020, 192, 108102. [Google Scholar] [CrossRef]
- Babiker, D.M.D.; Usha, Z.R.; Wan, C.; Hassaan, M.M.E.; Chen, X.; Li, L. Recent progress of composite polyethylene separators for lithium/sodium batteries. J. Power Sources 2023, 564, 232853. [Google Scholar] [CrossRef]
- McCrossan, C.; Shankaravelu, K. A review of the second life electric vehicle battery landscape from a business and technology perspective. In Proceedings of the 2021 IEEE Green Technologies Conference (GreenTech), Denver, CO, USA, 7–9 April 2021; pp. 416–423. [Google Scholar]
- Manjakkal, L.; Jain, A.; Nandy, S.; Goswami, S.; Tiago Carvalho, J.; Pereira, L.; See, C.H.; Pillai, S.C.; Hogg, R.A. Sustainable electrochemical energy storage devices using natural bast fibres. Chem. Eng. J. 2023, 465, 142845. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef] [PubMed]
- Weidenkaff, A.; Wagner-Wenz, R.; Veziridis, A. A world without electronic waste. Nat. Rev. Mater. 2021, 6, 462–463. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, H. Advanced thin film cathodes for lithium ion batteries. Research 2020, 2020, 2969510. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Lv, T.; Dong, K.; Liu, Y.; Qi, Y.; Cao, S.; Chen, T. Ultrafast self-assembly of supramolecular hydrogels toward novel flame-retardant separator for safe lithium ion battery. J. Colloid Interface Sci. 2023, 649, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chuan, X. Recent developments in natural mineral-based separators for lithium-ion batteries. RSC Adv. 2021, 11, 16633–16644. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, H.; Kong, X.; Huang, W.; Tsao, Y.; Mackanic, D.G.; Wang, K.; Wang, X.; Huang, W.; Choudhury, S.; et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 2020, 5, 526–533. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Weng, S.; Li, R.; Lu, D.; Deng, T.; Zhang, S.; Lv, L.; Qi, J.; Xiao, X.; et al. Multifunctional solvent molecule design enables high-voltage Li-ion batteries. Nat. Commun. 2023, 14, 2211. [Google Scholar] [CrossRef]
- Ryu, M.; Hong, Y.-K.; Lee, S.-Y.; Park, J.H. Ultrahigh loading dry-process for solvent-free lithium-ion battery electrode fabrication. Nat. Commun. 2023, 14, 1316. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, K.; Wu, F.; Bai, Y.; Wu, C. Ionic liquid-based electrolytes for aluminum/magnesium/sodium-ion batteries. Energy Mater. Adv. 2021, 2021, 9204217. [Google Scholar] [CrossRef]
- Dong, W.; Lin, T.; Huang, J.; Wang, Y.; Zhang, Z.; Wang, X.; Yuan, X.; Lin, J.; Chen, I.-W.; Huang, F. Electrodes with electrodeposited water-excluding polymer coating enable high-voltage aqueous supercapacitors. Research 2020, 2020, 4178179. [Google Scholar] [CrossRef] [PubMed]
- Langner, T.; Sieber, T.; Rietig, A.; Merk, V.; Pfeifer, L.; Acker, J. A phenomenological and quantitative view on the degradation of positive electrodes from spent lithium-ion batteries in humid atmosphere. Sci. Rep. 2023, 13, 5671. [Google Scholar] [CrossRef] [PubMed]
- Tajik, M.; Makui, A.; Tosarkani, B.M. Sustainable cathode material selection in lithium-ion batteries using a novel hybrid multi-criteria decision-making. J. Energy Storage 2023, 66, 107089. [Google Scholar] [CrossRef]
- Hao, H.; Hutter, T.; Boyce, B.L.; Watt, J.; Liu, P.; Mitlin, D. Review of multifunctional separators: Stabilizing the cathode and the anode for alkali (Li, Na, and K) metal–sulfur and selenium batteries. Chem. Rev. 2022, 122, 8053–8125. [Google Scholar] [CrossRef]
- Gu, Q.-Q.; Xue, H.-J.; Li, Z.-W.; Song, J.-C.; Sun, Z.-Y. High-performance polyethylene separators for lithium-ion batteries modified by phenolic resin. J. Power Sources 2021, 483, 229155. [Google Scholar] [CrossRef]
- Li, Y.; Yu, L.; Hu, W.; Hu, X. Thermotolerant separators for safe lithium-ion batteries under extreme conditions. J. Mater. Chem. A 2020, 8, 20294–20317. [Google Scholar] [CrossRef]
- Lin, W.; Wang, F.; Wang, H.; Li, H.; Fan, Y.; Chan, D.; Chen, S.; Tang, Y.; Zhang, Y. Thermal-stable separators: Design principles and strategies towards safe lithium-ion battery operations. ChemSusChem 2022, 15, e202201464. [Google Scholar] [CrossRef]
- Cheng, N.; Ren, L.; Xu, X.; Du, Y.; Dou, S.X. Application of organic-inorganic hybrids in lithium batteries. Mater. Today Phys. 2020, 15, 100289. [Google Scholar] [CrossRef]
- Wu, Y.; Lei, D.; Wang, C. The formation of LiAl5O8 nanowires from bulk Li-Al alloy enables dendrite-free Li metal batteries. Mater. Today Phys. 2021, 18, 100395. [Google Scholar] [CrossRef]
- Luo, W.; Cheng, S.; Wu, M.; Zhang, X.; Yang, D.; Rui, X. A review of advanced separators for rechargeable batteries. J. Power Sources 2021, 509, 230372. [Google Scholar] [CrossRef]
- Waqas, M.; Ali, S.; Feng, C.; Chen, D.; Han, J.; He, W. Recent development in separators for high-temperature lithium-ion batteries. Small 2019, 15, 1901689. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Liu, R.; Yuan, B.; Zhong, S.; Liu, J.; Dong, L.; Ji, Y.; Dong, Y.; Yang, C.; He, W. Poly(vinylidene fluoride) separators for next-generation lithium based batteries. Nano Select 2021, 2, 2308–2345. [Google Scholar] [CrossRef]
- Zhai, P.; Liu, K.; Wang, Z.; Shi, L.; Yuan, S. Multifunctional separators for high-performance lithium ion batteries. J. Power Sources 2021, 499, 229973. [Google Scholar] [CrossRef]
- Yoneda, H.; Nishimura, Y.; Doi, Y.; Fukuda, M.; Kohno, M. Development of microporous PE films to improve lithium ion batteries. Polym. J. 2010, 42, 425–437. [Google Scholar] [CrossRef]
- Mun, S.C.; Won, J.H. Manufacturing processes of microporous polyolefin separators for lithium-ion batteries and correlations between mechanical and physical properties. Crystals 2021, 11, 1013. [Google Scholar] [CrossRef]
- Deplancke, T.; Lame, O.; Rousset, F.; Seguela, R.; Vigier, G. Mechanisms of chain reentanglement during the sintering of UHMWPE nascent powder: Effect of molecular weight. Macromolecules 2015, 48, 5328–5338. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Y.; Yao, Y.; Wang, H.; Lin, T.; Gao, Y.; Wang, X.; Xue, G. Chain dynamics of partially disentangled UHMWPE around melting point characterized by 1H low-field solid-state NMR. Polymers 2023, 15, 1910. [Google Scholar] [CrossRef]
- Shen, H.; He, L.; Fan, C.; Xie, B.; Yang, W.; Yang, M. Improving the integration of HDPE/UHMWPE blends by high temperature melting and subsequent shear. Mater. Lett. 2015, 138, 247–250. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.; Ma, L.; Xu, X.; Zhang, P.; Yu, H. Explorations of complex thermally induced phase separation (C-TIPS) method for manufacturing novel diphenyl ether polysulfate flat microporous membranes. J. Membr. Sci. 2022, 659, 120739. [Google Scholar] [CrossRef]
- Cheng, Q.; Cui, Z.; Li, J.; Qin, S.; Yan, F.; Li, J. Preparation and performance of polymer electrolyte based on poly(vinylidene fluoride)/polysulfone blend membrane via thermally induced phase separation process for lithium ion battery. J. Power Sources 2014, 266, 401–413. [Google Scholar] [CrossRef]
- Kim, J.F.; Kim, J.H.; Lee, Y.M.; Drioli, E. Thermally induced phase separation and electrospinning methods for emerging membrane applications: A review. AIChE J. 2016, 62, 461–490. [Google Scholar] [CrossRef]
- Lee, J.T.; Jo, C.; De Volder, M. Bicontinuous phase separation of lithium-ion battery electrodes for ultrahigh areal loading. Proc. Natl. Acad. Sci. USA 2020, 117, 21155–21161. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, J.I.; Moon, J.; Jeong, J.; Park, J.H. Electron beam induced strong organic/inorganic grafting for thermally stable lithium-ion battery separators. Appl. Surf. Sci. 2018, 444, 339–344. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, R.; Xiang, S.; Hu, J.; Zheng, X. Preparation and lithium-ion separation property of ZIF-8 membrane with excellent flexibility. Membranes 2023, 13, 500. [Google Scholar] [CrossRef] [PubMed]
- Shkrob, I.A.; Luo, M.; Rodrigues, M.-T.F.; Trask, S.E.; Abraham, D.P. Fast charging of Li-ion cells: Effect of separator membranes and mapping of “safe lines” to avoid Li plating. J. Power Sources 2022, 549, 232086. [Google Scholar] [CrossRef]
- Heidari, A.A.; Mahdavi, H. Recent development of polyolefin-based microporous separators for Li−ion batteries: A review. Chem. Rec. 2020, 20, 570–595. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.P.; Fidalgo-Marijuan, A.; Martins, P.M.; Queirós, J.M.; Gonçalves, R.; Gutiérrez-Pardo, A.; Aguesse, F.; Costa, C.M.; Lanceros-Mendez, S. Porous composite bifunctional membranes for lithium-ion battery separator and photocatalytic degradation applications: Toward multifunctionality for circular economy. Adv. Energy Sustain. Res. 2021, 2, 2100046. [Google Scholar] [CrossRef]
- Suresh Kumar, R.; Jithin, K.V.; Rajesh, P.K. Lithium-ion ferrous phosphate prismatic cell aging analysis and assessment for the development of battery management systems. J. Energy Storage 2023, 70, 108093. [Google Scholar] [CrossRef]
- Kanchan, B.K.; Randive, P. Investigation on capacity extension through non-uniform anode microstructure in lithium-ion battery. Int. J. Heat Mass Transfer 2023, 214, 124413. [Google Scholar] [CrossRef]
- Akhmetova, K.; Tatykayev, B.; Kalybekkyzy, S.; Sultanov, F.; Bakenov, Z.; Mentbayeva, A. One-step fabrication of all-in-one flexible nanofibrous lithium-ion battery. J. Energy Storage 2023, 65, 107237. [Google Scholar] [CrossRef]
- Ding, L.; Li, D.; Liu, L.; Zhang, P.; Du, F.; Wang, C.; Zhang, D.; Zhang, S.; Zhang, S.; Yang, F. Dependence of lithium metal battery performances on inherent separator porous structure regulation. J. Energy Chem. 2023, 84, 436–447. [Google Scholar] [CrossRef]
- Serra, J.P.; Uranga, J.; Gonçalves, R.; Costa, C.M.; de la Caba, K.; Guerrero, P.; Lanceros-Mendez, S. Sustainable lithium-ion battery separators based on cellulose and soy protein membranes. Electrochim. Acta 2023, 462, 142746. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, G.; Hu, Y.; Sun, J.; Xu, J.; Wang, L.; Wang, H.; Cheng, C. Ionic conductivity and cycling stability-enhanced composite separator using hollow halloysite nanotubes constructed on PP nonwoven through polydopamine-induced water-based coating method. Colloids Surf. A Physicochem. Eng. Asp. 2023, 667, 131403. [Google Scholar] [CrossRef]
- Zhu, X.; Roy, J.C.; Li, X.; Li, J.; Zhang, L. Toward improved sustainability in lithium ion batteries using bio-based materials. Trends Chem. 2023, 5, 393–403. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Jeon, H.; Yeon, D.; Kim, B.-H.; Cho, K.Y.; Ryou, M.-H.; Lee, Y.M. In-depth correlation of separator pore structure and electrochemical performance in lithium-ion batteries. J. Power Sources 2016, 325, 732–738. [Google Scholar] [CrossRef]
- Rajagopalan Kannan, D.R.; Terala, P.K.; Moss, P.L.; Weatherspoon, M.H. Analysis of the separator thickness and porosity on the performance of lithium-ion batteries. Int. J. Electrochem. 2018, 2018, 1925708. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-safety separators for lithium-ion batteries and sodium-ion batteries: Advances and perspective. Energy Storage Mater. 2021, 41, 522–545. [Google Scholar] [CrossRef]
- Zhong, S.; Yuan, B.; Guang, Z.; Chen, D.; Li, Q.; Dong, L.; Ji, Y.; Dong, Y.; Han, J.; He, W. Recent progress in thin separators for upgraded lithium ion batteries. Energy Storage Mater. 2021, 41, 805–841. [Google Scholar] [CrossRef]
- Chen, D.L.; Zhang, Z.Y.; Zhang, Y.M.; Luo, K.; Yi, H.L. Numerical investigation of electroconvection transport of polymer electrolyte solutions on a perfectly selective membrane. Colloid Surf. A Physicochem. Eng. Asp. 2023, 673, 131813. [Google Scholar] [CrossRef]
- Enayati-Gerdroodbar, A.; Eliseeva, S.N.; Salami-Kalajahi, M. A review on the effect of nanoparticles/matrix interactions on the battery performance of composite polymer electrolytes. J. Energy Storage 2023, 68, 107836. [Google Scholar] [CrossRef]
- Lv, H.; Wei, Z.; Han, C.; Yang, X.; Tang, Z.; Zhang, Y.; Zhi, C.; Li, H. Cross-linked polyaniline for production of long lifespan aqueous iron||organic batteries with electrochromic properties. Nat. Commun. 2023, 14, 3117. [Google Scholar] [CrossRef]
- Wu, J.; Ye, H.; Zhang, T. Applications of polymer materials in lithium-ion batteries: Polymeric electrolytes and separators. In Proceedings of the 2021 3rd International Academic Exchange Conference on Science and Technology Innovation (IAECST), Guangzhou, China, 10–12 December 2021; pp. 1156–1162. [Google Scholar]
- Zhang, J.; Yan, J.; Zhao, Y.; Zhou, Q.; Ma, Y.; Zi, Y.; Zhou, A.; Lin, S.; Liao, L.; Hu, X.; et al. High-strength and machinable load-bearing integrated electrochemical capacitors based on polymeric solid electrolyte. Nat. Commun. 2023, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Xiao, M.; Wang, S.; Han, D.; Li, Y.; Meng, Y. Polymer-based solid electrolytes: Material selection, design, and application. Adv. Funct. Mater. 2021, 31, 2007598. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer electrolytes for lithium-based batteries: Advances and prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Foran, G.; Verdier, N.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. Thermal and electrochemical properties of solid polymer electrolytes prepared via lithium salt-catalyzed epoxide ring opening polymerization. Appl. Sci. 2021, 11, 1561. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on polymer-based composite electrolytes for lithium batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef]
- Cannarella, J.; Liu, X.; Leng, C.Z.; Sinko, P.D.; Gor, G.Y.; Arnold, C.B. Mechanical properties of a battery separator under compression and tension. J. Electrochem. Soc. 2014, 161, F3117–F3122. [Google Scholar] [CrossRef]
- Plaimer, M.; Breitfuß, C.; Sinz, W.; Heindl, S.F.; Ellersdorfer, C.; Steffan, H.; Wilkening, M.; Hennige, V.; Tatschl, R.; Geier, A.; et al. Evaluating the trade-off between mechanical and electrochemical performance of separators for lithium-ion batteries: Methodology and application. J. Power Sources 2016, 306, 702–710. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, L.; Zhang, W.; Li, Z.; Xie, X.; Huang, Y. Reducing the thickness of solid-state electrolyte membranes for high-energy lithium batteries. Energy Environ. Sci. 2021, 14, 12–36. [Google Scholar] [CrossRef]
- Costa, C.M.; Rodrigues, H.M.; Gören, A.; Machado, A.V.; Silva, M.M.; Lanceros-Méndez, S. Preparation of poly(vinylidene fluoride) lithium-ion battery separators and their compatibilization with ionic liquid-a green solvent approach. ChemistrySelect 2017, 2, 5394–5402. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Y.-C.; Jenkins, D.M.; Chernova, N.A.; Chung, Y.; Radhakrishnan, B.; Chu, I.-H.; Fang, J.; Wang, Q.; Omenya, F.; et al. Thermal stability and reactivity of cathode materials for Li-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 7013–7021. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Huang, A.-C.; Tang, Y.; Liu, Y.-C.; Wu, Z.-H.; Zhou, H.-L.; Li, Z.-P.; Shu, C.-M.; Jiang, J.-C.; Xing, Z.-X. Thermal stability analysis of lithium-ion battery electrolytes based on lithium bis(trifluoromethanesulfonyl)imide-lithium difluoro(oxalato)borate dual-salt. Polymers 2021, 13, 707. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yao, X.; Zhang, F.; Ang, E.H.; Rong, S.; Zhao, K.; He, K.; Xiang, H. Nanofiber membrane coated with lithiophilic polydopamine for lithium metal batteries. J. Membr. Sci. 2023, 685, 121951. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, S.; Ji, C.; Tang, F.; Zhang, L.; Huang, F. Enhancing the β phase of poly(vinylidene fluoride) nanofibrous membranes for thermostable separators in lithium-ion batteries. ACS Appl. Nano Mat. 2023, 6, 10340–10350. [Google Scholar] [CrossRef]
- Jin, Y.; Ai, Z.; Song, Y.; Zhang, X.; Shi, J.; Ma, C. Enhanced lithium storage performance of Si/C composite nanofiber membrane with carbon coating as binder-free and self-supporting anode for lithium-ion battery. Mater. Res. Bull. 2023, 167, 112429. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, J.; Zhang, L.; Li, Y.; Wang, H.; Wang, L. Na3V2(PO4)3-decorated separator as an improved catalysis ceramic layer for high-performance lithium sulfur batteries. Ionics 2023, 29, 2271–2285. [Google Scholar] [CrossRef]
- Murali, D.R.L.; Banihashemi, F.; Lin, J.Y.S. Zeolite membrane separators for fire-safe Li-ion batteries–effects of crystal shape and membrane pore structure. J. Membr. Sci. 2023, 680, 121743. [Google Scholar] [CrossRef]
- Wu, D.; Dong, N.; Wang, R.; Qi, S.; Liu, B.; Wu, D. In situ construction of High-safety and Non-flammable polyimide “Ceramic” Lithium-ion battery separator via SiO2 Nano-Encapsulation. Chem. Eng. J. 2021, 420, 129992. [Google Scholar] [CrossRef]
- Liu, K.; Yang, C.; Li, X.; Dong, N.; Liu, B.; Tian, G.; Qi, S.; Wu, D. Controllable coaxial coating of boehmite on the surface of polyimide nanofiber membrane and its application as a separator for lithium-ion batteries. Energy Technol. 2022, 10, 2100982. [Google Scholar]
- Choi, H.; Lee, B.-S. Pilot scale hybrid organic/inorganic coatings on a polyolefin separator to enhance dimensional stability for thermally stable long-life rechargeable batteries. Polymers 2022, 14, 4474. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Y.; Hu, Q.; Guo, S.; Yu, L.; Li, Q.; Liu, Q.; Hu, X. Safer lithium-ion batteries from the separator aspect: Development and future perspectives. Energy Environ. Mater. 2021, 4, 336–362. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Xie, X.; Kretschmer, K.; Huang, X.; Sun, B.; Wang, G. Porous poly(vinylidene fluoride-co-hexafluoropropylene) polymer membrane with sandwich-like architecture for highly safe lithium ion batteries. J. Membr. Sci. 2014, 472, 133–140. [Google Scholar] [CrossRef]
- Moon, J.; Jeong, J.Y.; Kim, J.I.; Kim, S.; Park, J.H. An ultrathin inorganic-organic hybrid layer on commercial polymer separators for advanced lithium-ion batteries. J. Power Sources 2019, 416, 89–94. [Google Scholar] [CrossRef]
- Wen, R.; Gao, Z.; Luo, L.; Cui, X.; Tang, J.; Zheng, Z.; Zhang, J. Sandwich-structured electrospun all-fluoropolymer membranes with thermal shut-down function and enhanced electrochemical performance. Nanocomposites 2022, 8, 64–73. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Zhu, J.; Gao, Y. A critical review of thermal runaway prediction and early-warning methods for lithium-ion batteries. Energy Mater. Adv. 2023, 4, 0008. [Google Scholar] [CrossRef]
- Abraham, K.M. Directions in secondary lithium battery research and development. Electrochim. Acta 1993, 38, 1233–1248. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Doeff, M.M.; Xin, H.L. Chemical and structural stability of lithium-ion battery electrode materials under electron beam. Sci. Rep. 2014, 4, 5694. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Bree, G.; Hao, H.; Stoeva, Z.; John Low, C.T. Monitoring state of charge and volume expansion in lithium-ion batteries: An approach using surface mounted thin-film graphene sensors. RSC Adv. 2023, 13, 7045–7054. [Google Scholar] [CrossRef]
- Francis, C.F.J.; Kyratzis, I.L.; Best, A.S. Lithium-ion battery separators for ionic-liquid electrolytes: A review. Adv. Mater. 2020, 32, 1904205. [Google Scholar] [CrossRef]
- Yao, Z.; Seong, H.J.; Jang, Y.-S. Environmental toxicity and decomposition of polyethylene. Ecotoxicol. Environ. Saf. 2022, 242, 113933. [Google Scholar] [CrossRef]
- Zaribaf, F.P.; Gill, H.S.; Pegg, E.C. Chemical stability of oil-infused polyethylene. J. Biomater. Appl. 2020, 35, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cen, J.; Li, W.; Pan, W.; Zhang, Y.; Yin, H.; Hu, J.; Liu, S. A general strategy toward synthesis of well-defined polypeptides with complex chain topologies. CCS Chem. 2022, 4, 3864–3877. [Google Scholar] [CrossRef]
- Li, L.; Cen, J.; Pan, W.; Zhang, Y.; Leng, X.; Tan, Z.; Yin, H.; Liu, S. Synthesis of polypeptides with high-fidelity terminal functionalities under NCA monomer-starved conditions. Research 2021, 2021, 9826046. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.W.S.; Teo, J.Y.Q.; Loh, X.J.; Lim, J.Y.C. Polyolefins and polystyrene as chemical resources for a sustainable future: Challenges, advances, and prospects. ACS Mater. Lett. 2021, 3, 1660–1676. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, H.; Lee, Y.; Esken, D.; Dehe, D.; Song, H.; Kim, D. Nanostructured reactive alumina particles coated with water-soluble binder on the polyethylene separator for highly safe lithium-ion batteries. J. Power Sources 2021, 506, 230119. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Zhu, J.; Lu, Y.; Ge, Y.; Zhang, X. High-strength, thermally stable nylon 6,6 composite nanofiber separators for lithium-ion batteries. J. Mater. Sci. 2017, 52, 5232–5241. [Google Scholar] [CrossRef]
- Babiker, D.M.D.; Wan, C.; Mansoor, B.; Usha, Z.R.; Yu, R.; Habumugisha, J.C.; Chen, W.; Chen, X.; Li, L. Superior lithium battery separator with extraordinary electrochemical performance and thermal stability based on hybrid UHMWPE/SiO2 nanocomposites via the scalable biaxial stretching process. Compos. Part B Eng. 2021, 211, 108658. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Roy, J. The synthesis and applications of TiO2 nanoparticles derived from phytochemical sources. J. Ind. Eng. Chem. 2022, 106, 1–19. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Ai, X.; Yang, H.; Cao, Y. TiO2 ceramic-grafted polyethylene separators for enhanced thermostability and electrochemical performance of lithium-ion batteries. J. Membr. Sci. 2016, 504, 97–103. [Google Scholar] [CrossRef]
- Croce, F.; Settimi, L.; Scrosati, B. Superacid ZrO2-added, composite polymer electrolytes with improved transport properties. Electrochem. Commun. 2006, 8, 364–368. [Google Scholar] [CrossRef]
- Suharto, Y.; Lee, Y.; Yu, J.-S.; Choi, W.; Kim, K.J. Microporous ceramic coated separators with superior wettability for enhancing the electrochemical performance of sodium-ion batteries. J. Power Sources 2018, 376, 184–190. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Jin, C.; Wood Iii, D.L.; Singler, T.J.; Li, J. On electrolyte wetting through lithium-ion battery separators. Extreme Mech. Lett. 2020, 40, 100960. [Google Scholar] [CrossRef]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Jeon, D.H. Enhancing electrode wettability in lithium-ion battery via particle-size ratio control. Appl. Mater. Today 2021, 22, 100976. [Google Scholar] [CrossRef]
- Li, D.; Shi, D.; Xia, Y.; Qiao, L.; Li, X.; Zhang, H. Superior thermally stable and nonflammable porous polybenzimidazole membrane with high wettability for high-power lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 8742–8750. [Google Scholar] [CrossRef]
- Jeon, D.H. Wettability in electrodes and its impact on the performance of lithium-ion batteries. Energy Storage Mater. 2019, 18, 139–147. [Google Scholar] [CrossRef]
- Zhu, B.; Zheng, T.; Xiong, J.; Shi, X.; Cheng, Y.-J.; Xia, Y. A lithium-ion battery cathode with enhanced wettability toward an electrolyte fabricated by a fast light curing of photoactive slurry. Energy Fuels 2022, 36, 3313–3318. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, C.; Li, X. Enhanced electrolyte retention capability of separator for lithium-ion battery constructed by decorating ZIF-67 on bacterial cellulose nanofiber. Cellulose 2021, 28, 3097–3112. [Google Scholar] [CrossRef]
- Xie, Y.; Zou, H.; Xiang, H.; Xia, R.; Liang, D.; Shi, P.; Dai, S.; Wang, H. Enhancement on the wettability of lithium battery separator toward nonaqueous electrolytes. J. Membr. Sci. 2016, 503, 25–30. [Google Scholar] [CrossRef]
- Wood, D.L.; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Pérez, J.M.; Colledani, M. Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, A.K.; Xu, Z.; Li, G.; Wang, X. Current trends in sourcing, recycling, and regeneration of spent lithium-ion batteries—A review. Renewables 2023, 1, 294–315. [Google Scholar] [CrossRef]
- Yang, M.; Chen, L.; Li, H.; Wu, F. Air/water stability problems and solutions for lithium batteries. Energy Mater. Adv. 2022, 2022, 9842651. [Google Scholar] [CrossRef]
- Yang, T.; Zheng, Y.; Liu, Y.; Luo, D.; Yu, A.; Chen, Z. Reviving low-temperature performance of lithium batteries by emerging electrolyte systems. Renewables 2023, 1, 2–20. [Google Scholar] [CrossRef]
- Bicy, K.; Gueye, A.B.; Rouxel, D.; Kalarikkal, N.; Thomas, S. Lithium-ion battery separators based on electrospun PVDF: A review. Surf. Interfaces 2022, 31, 101977. [Google Scholar] [CrossRef]
- Deng, K.; Qin, J.; Wang, S.; Ren, S.; Han, D.; Xiao, M.; Meng, Y. Effective suppression of lithium dendrite growth using a flexible single-ion conducting polymer electrolyte. Small 2018, 14, 1801420. [Google Scholar] [CrossRef]
- ASTM D882-10; Standard Test Methods for Tensile Properties of Thin Plastic Sheeting, Annual Book of ASTM. American Society for Testing and Materials: Philadelphia, PA, USA, 2010.
- Zhang, X.; Sahraei, E.; Wang, K. Li-ion battery separators, mechanical integrity and failure mechanisms leading to soft and hard internal shorts. Sci. Rep. 2016, 6, 32578. [Google Scholar] [CrossRef]
- ASTM D3763; Standard Test Method for High Speed Puncture Properties of Plastics Using Load and Displacement Sensors. ASTM International: West Conshohocken, PA, USA, 2012.
- Wang, Y.; Yin, C.; Song, Z.; Wang, Q.; Lan, Y.; Luo, J.; Bo, L.; Yue, Z.; Sun, F.; Li, X. Application of PVDF organic particles coating on polyethylene separator for lithium ion batteries. Materials 2019, 12, 3125. [Google Scholar] [CrossRef]
- Reizabal, A.; Gonçalves, R.; Fidalgo-Marijuan, A.; Costa, C.M.; Pérez, L.; Vilas, J.-L.; Lanceros-Mendez, S. Tailoring silk fibroin separator membranes pore size for improving performance of lithium ion batteries. J. Membr. Sci. 2020, 598, 117678. [Google Scholar] [CrossRef]
- Costa, C.M.; Lee, Y.-H.; Kim, J.-H.; Lee, S.-Y.; Lanceros-Méndez, S. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Sheng, L.; Xu, R.; Zhang, H.; Bai, Y.; Song, S.; Liu, G.; Wang, T.; Huang, X.; He, J. The morphology of polyethylene (PE) separator for lithium-ion battery tuned by the extracting process. J. Electroanal. Chem. 2020, 873, 114391. [Google Scholar] [CrossRef]
- Lee, H.; Alcoutlabi, M.; Toprakci, O.; Xu, G.; Watson, J.V.; Zhang, X. Preparation and characterization of electrospun nanofiber-coated membrane separators for lithium-ion batteries. J. Solid State Electrochem. 2014, 18, 2451–2458. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Guan, M.; Shang, Z.; Sun, Y.; Lu, Z.; Li, H.; An, X.; Liu, H. Nanofibrillated cellulose (NFC) as a pore size mediator in the preparation of thermally resistant separators for lithium ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 4838–4844. [Google Scholar] [CrossRef]
- Gigova, A. Investigation of the porous structure of battery separators using various porometric methods. J. Power Sources 2006, 158, 1054–1061. [Google Scholar] [CrossRef]
- Wu, T.; Wang, K.; Xiang, M.; Fu, Q. Progresses in manufacturing techniques of lithium-ion battery separators in china. Chin. J. Chem. 2019, 37, 1207–1215. [Google Scholar] [CrossRef]
- Yang, M.; Hou, J. Membranes in lithium ion batteries. Membranes 2012, 2, 367–383. [Google Scholar] [CrossRef]
- ASTM D726-94; Standard Test Method for Resistance of Nonporous Paper to Passage of Air. ASTM International: West Conshohocken, PA, USA, 1994.
- Mun, S.C.; Won, J.H. Estimating the permeability of the ceramic coating on lithium-ion battery separators via the ideal laminate theory. Front. Energy Res. 2022, 10, 928179. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, W.; Cao, S.; Chen, X. A figure of merit for fast-charging Li-ion battery materials. ACS Nano 2022, 16, 8525–8530. [Google Scholar] [CrossRef]
- Zahn, R.; Lagadec, M.F.; Hess, M.; Wood, V. Improving ionic conductivity and lithium-ion transference number in lithium-ion battery separators. ACS Appl. Mater. Interfaces 2016, 8, 32637–32642. [Google Scholar] [CrossRef]
- Wang, J.; Huang, W.; Pei, A.; Li, Y.; Shi, F.; Yu, X.; Cui, Y. Improving cyclability of Li metal batteries at elevated temperatures and its origin revealed by cryo-electron microscopy. Nat. Energy 2019, 4, 664–670. [Google Scholar] [CrossRef]
- Banerjee, A.; Ziv, B.; Shilina, Y.; Luski, S.; Aurbach, D.; Halalay, I.C. Acid-scavenging separators: A novel route for improving Li-ion batteries’ durability. ACS Energy Lett. 2017, 2, 2388–2393. [Google Scholar] [CrossRef]
- Cronau, M.; Szabo, M.; König, C.; Wassermann, T.B.; Roling, B. How to measure a reliable ionic conductivity? the stack pressure dilemma of microcrystalline sulfide-based solid electrolytes. ACS Energy Lett. 2021, 6, 3072–3077. [Google Scholar] [CrossRef]
- Yang, H.; Wu, N. Ionic conductivity and ion transport mechanisms of solid-state lithium-ion battery electrolytes: A review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar] [CrossRef]
- Lu, D.; Shao, Y.; Lozano, T.; Bennett, W.D.; Graff, G.L.; Polzin, B.; Zhang, J.; Engelhard, M.H.; Saenz, N.T.; Henderson, W.A.; et al. Failure mechanism for fast-charged lithium metal batteries with liquid electrolytes. Adv. Energy Mater. 2015, 5, 1400993. [Google Scholar] [CrossRef]
- Stroe, D.I.; Swierczynski, M.; Stan, A.I.; Knap, V.; Teodorescu, R.; Andreasen, S.J. Diagnosis of lithium-ion batteries state-of-health based on electrochemical impedance spectroscopy technique. In Proceedings of the 2014 IEEE Energy Conversion Congress and Exposition (ECCE), Pittsburgh, PA, USA, 14–18 September 2014; pp. 4576–4582. [Google Scholar]
- Huang, X.; Hitt, J. Lithium ion battery separators: Development and performance characterization of a composite membrane. J. Membr. Sci. 2013, 425–426, 163–168. [Google Scholar] [CrossRef]
- Oh, J.; Jin, D.; Kim, K.; Song, D.; Lee, Y.M.; Ryou, M.-H. Improving the cycling performance of lithium-ion battery Si/graphite anodes using a soluble polyimide binder. ACS Omega 2017, 2, 8438–8444. [Google Scholar] [CrossRef]
- Eddahech, A.; Briat, O.; Vinassa, J.-M. Lithium-ion battery performance improvement based on capacity recovery exploitation. Electrochim. Acta 2013, 114, 750–757. [Google Scholar] [CrossRef]
- Sohn, J.-Y.; Im, J.S.; Gwon, S.-J.; Choi, J.-H.; Shin, J.; Nho, Y.-C. Preparation and characterization of a PVDF-HFP/PEGDMA-coated PE separator for lithium-ion polymer battery by electron beam irradiation. Radiat. Phys. Chem. 2009, 78, 505–508. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Shi, J.; Li, Y.; You, J.; Bian, F. Crystallization-templated high-performance PVDF separator used in lithium-ion batteries. J. Membr. Sci. 2023, 670, 121359. [Google Scholar] [CrossRef]
- Folayan, T.-O.; Zhan, R.; Huang, K.; Pan, L. Improved separation between recycled anode and cathode materials from Li-ion batteries using coarse flake particle flotation. ACS Sustain. Chem. Eng. 2023, 11, 2917–2926. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, L.-Z.; Kang, Y.-Q.; Wang, L.; Zhou, Y.-N.; Liu, X.-Y.; Li, T.; Li, Y.-X.; Liang, Z.; Zhang, Z.-X.; et al. A novel three-step approach to separate cathode components for lithium-ion battery recycling. Rare Metals 2021, 40, 1431–1436. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Kundu, M.; Gören, A.; Silva, M.M.; Costa, C.M.; Liu, L.; Lanceros-Méndez, S. Optimization of filler type within poly(vinylidene fluoride-co-trifluoroethylene) composite separator membranes for improved lithium-ion battery performance. Compos. Part B Eng. 2016, 96, 94–102. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, X.; Fu, C.; Chen, C.; Ran, X. Effect of electron beam irradiation on structural and thermal properties of gamma poly (vinylidene fluoride) (γ-PVDF) films. Radiat. Phys. Chem. 2018, 144, 48–55. [Google Scholar] [CrossRef]
- Valentino, L.; Matsumoto, M.; Dichtel, W.R.; Mariñas, B.J. Development and performance characterization of a polyimine covalent organic framework thin-film composite nanofiltration membrane. Environ. Sci. Technol. 2017, 51, 14352–14359. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, G.; MacInnis, K.; Olah, A.; Baer, E. Structure-property relationships of microporous membranes produced by biaxial orientation of compatibilized PP/Nylon 6 blends. Polymer 2018, 145, 148–156. [Google Scholar] [CrossRef]
- Lizundia, E.; Costa, C.M.; Alves, R.; Lanceros-Méndez, S. Cellulose and its derivatives for lithium ion battery separators: A review on the processing methods and properties. Carbohydr. Polym. Technol. Appl. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, F.; Cao, Y.; Xiang, M.; Kang, J.; Wu, T.; Fu, Q. Investigation on cavitation behavior of ultrahigh molecular weight polyethylene during stretching in wet process and dry process. Polymer 2021, 230, 124081. [Google Scholar] [CrossRef]
- Ding, L.; Yan, N.; Zhang, S.; Xu, R.; Wu, T.; Yang, F.; Cao, Y.; Xiang, M. Facile manufacture technique for lithium-ion batteries composite separator via online construction of fumed SiO2 coating. Mater. Des. 2022, 215, 110476. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for lithium-ion batteries: A review on the production processes and recent developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Ding, Q.; Jiang, J.; Li, G.; Mai, K. β-Crystallization of isotactic polypropylene in the presence of β-nucleating agent and different crystallinity poly(ethylene terephthalate). Thermochim. Acta 2013, 559, 17–22. [Google Scholar] [CrossRef]
- Zhang, D.-X.; Ding, L.; Yang, F.; Lan, F.; Cao, Y.; Xiang, M. Comparison of the structural evolution of β polypropylene during the sequential and simultaneous biaxial stretching process. Chin. J. Polym. Sci. 2021, 39, 620–631. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Chrissafis, K.; Bikiaris, D.N. β-Nucleated polypropylene: Processing, properties and nanocomposites. Polym. Rev. 2015, 55, 596–629. [Google Scholar] [CrossRef]
- Carmeli, E.; Ottonello, S.; Wang, B.; Menyhárd, A.; Müller, A.J.; Cavallo, D. Competing crystallization of α- and β-phase induced by β-nucleating agents in microdroplets of isotactic polypropylene. CrystEngComm 2022, 24, 1966–1978. [Google Scholar] [CrossRef]
- Deng, J.; Xie, J.; Zhang, G.; Yang, X. Research progress of cross-linked fiber membranes for lithium-ion battery separators. Chem. Eng. Sci. 2023, 280, 118970. [Google Scholar] [CrossRef]
- Din, M.M.U.; Murugan, R. Metal coated polypropylene separator with enhanced surface wettability for high capacity lithium metal batteries. Sci. Rep. 2019, 9, 16795. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Xu, H.; Song, Y.; He, X. Polyimides as promising materials for lithium-ion batteries: A review. Nano-Micro Lett. 2023, 15, 135. [Google Scholar] [CrossRef]
- Wu, S.L.; Qiao, J.; Guan, J.; Chen, H.M.; Wang, T.; Wang, C.; Wang, Y. Nascent disentangled UHMWPE: Origin, synthesis, processing, performances and applications. Eur. Polym. J. 2023, 184, 111799. [Google Scholar] [CrossRef]
- Rezaeian, A.; Hanifpour, A.; Teimoury, H.R.; Nekoomanesh-Haghighi, M.; Ahmadi, M.; Bahri-Laleh, N. Synthesis of highly spherical Ziegler–Natta catalyst by employing span 80 as an emulsifier suitable for UHMWPE production. Polym. Bull. 2023, 80, 1625–1639. [Google Scholar] [CrossRef]
- Gote, R.P.; Romano, D.; van der Eem, J.; Zhao, J.; Zhou, F.; Rastogi, S. Unprecedented mechanical properties in linear UHMWPE using a heterogeneous catalytic system. Macromolecules 2023, 56, 361–378. [Google Scholar] [CrossRef]
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-high-molecular-weight-polyethylene (UHMWPE) as a promising polymer material for biomedical applications: A concise review. Polymers 2020, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Yu, J.; Wang, Y.; Hu, Z. Oriented shish-kebab like ultra-high molecular weight polyethylene membrane for direct contact membrane distillation. Sep. Purif. Technol. 2022, 290, 120847. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Zheng, G.; Zhang, D. Enhanced thermal performance and impact strength of UHMWPE/recycled-PA6 blends synthesized via a melting extrusion route. Adv. Mater. Sci. Eng. 2016, 2016, 8089525. [Google Scholar] [CrossRef]
- Ahmad, M.; Wahit, M.U.; Kadir, M.R.A.; Dahlan, K.Z.M.; Jawaid, M. Thermal and mechanical properties of ultrahigh molecular weight polyethylene/high-density polyethylene/polyethylene glycol blends. J. Polym. Eng. 2013, 33, 599–614. [Google Scholar] [CrossRef]
- Cao, M.; Chen, L.; Xu, R.; Fang, Q. Effect of the temperature on ballistic performance of UHMWPE laminate with limited thickness. Compos. Struct. 2021, 277, 114638. [Google Scholar] [CrossRef]
- Sobieraj, M.C.; Rimnac, C.M. Ultra high molecular weight polyethylene: Mechanics, morphology, and clinical behavior. J. Mech. Behav. Biomed. Mater. 2009, 2, 433–443. [Google Scholar] [CrossRef]
- Xu, J.; Li, N.; Zhu, Y. Preparation of fiber core support UHMWPE/SiO2 composite hollow fiber membrane toward enhancing structure stability and antifouling. Polym. Eng. Sci. 2022, 62, 472–485. [Google Scholar] [CrossRef]
- Basko, A.; Pochivalov, K. Current state-of-the-art in membrane formation from ultra-high molecular weight polyethylene. Membranes 2022, 12, 1137. [Google Scholar] [CrossRef]
- Pochivalov, K.; Basko, A.; Lebedeva, T.; Yurov, M.; Yushkin, A.; Volkov, A.; Bronnikov, S. Controlled swelling of monolithic films as a facile approach to the synthesis of UHMWPE membranes. Membranes 2023, 13, 422. [Google Scholar] [CrossRef]
- Yang, K.; Xu, Z.; Li, R.; Liu, Y.; Sun, W.; Tang, Y.; Liu, X.; Fu, Q. Thickness effects of surface direct fluorination and plasma modification on ultra-high molecular weight polyethylene ultrathin membranes. Macromol. Mater. Eng. 2023, 308, 2200557. [Google Scholar] [CrossRef]
- Chhetri, S.; Bougherara, H. A comprehensive review on surface modification of UHMWPE fiber and interfacial properties. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106146. [Google Scholar] [CrossRef]
- Ahn, Y.-k.; Kwon, Y.K.; Kim, K.J. Surface-modified polyethylene separator with hydrophilic property for enhancing the electrochemical performance of lithium-ion battery. Int. J. Energy Res. 2020, 44, 6651–6659. [Google Scholar] [CrossRef]
- Baek, M.; Yoo, J.; Kim, Y.; Jang, Y.-J.; Seo, H.; Lee, S.-M.; Jo, H.; Woo, S.-G.; Kim, J.-H. Surface modification of polyethylene separator for Li-ion batteries via imine formation. Int. J. Energy Res. 2023, 2023, 4624762. [Google Scholar] [CrossRef]
- Hu, T.; Mu, Y.; Chen, Y.; Zhou, C.; Zhao, G.; Dong, G. Combined effects of nanoparticle and stretch-induced orientation on crystal structure and properties of UHMWPE/TiO2 composite microporous membranes. Polym. Compos. 2023, 44, 3580–3593. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, Z.; Tao, Y.; Xu, M.; Liao, J. Transforming hydrophobicity of high-density polyethylene surface to hydrophilicity and superoleophobicity by surface grafted with polyvinyl alcohols for oil contaminants cleanup. Colloid Surf. A Physicochem. Eng. Asp. 2022, 655, 130313. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Lan, Y.; Song, Z.; Luo, J.; Wei, X.; Sun, F.; Yue, Z.; Yin, C.; Zhou, L.; et al. Aqueous aluminide ceramic coating polyethylene separators for lithium-ion batteries. Solid State Ion. 2020, 345, 115188. [Google Scholar] [CrossRef]
- Kim, K.J.; Kwon, Y.K.; Yim, T.; Choi, W. Functional separator with lower resistance toward lithium ion transport for enhancing the electrochemical performance of lithium ion batteries. J. Ind. Eng. Chem. 2019, 71, 228–233. [Google Scholar] [CrossRef]
- Sheng, L.; Song, L.; Gong, H.; Pan, J.; Bai, Y.; Song, S.; Liu, G.; Wang, T.; Huang, X.; He, J. Polyethylene separator grafting with polar monomer for enhancing the lithium-ion transport property. J. Power Sources 2020, 479, 228812. [Google Scholar] [CrossRef]
- Fu, W.; Xu, R.; Zhang, X.; Tian, Z.; Huang, H.; Xie, J.; Lei, C. Enhanced wettability and electrochemical performance of separators for lithium-ion batteries by coating core-shell structured silica-poly(cyclotriphosphazene-co-4,4″-sulfonyldiphenol) particles. J. Power Sources 2019, 436, 226839. [Google Scholar] [CrossRef]
- Xiao, Y.; Fu, A.; Zou, Y.; Huang, L.; Wang, H.; Su, Y.; Zheng, J. High safety lithium-ion battery enabled by a thermal-induced shutdown separator. Chem. Eng. J. 2022, 438, 135550. [Google Scholar] [CrossRef]
- Boateng, B.; Zhang, X.; Zhen, C.; Chen, D.; Han, Y.; Feng, C.; Chen, N.; He, W. Recent advances in separator engineering for effective dendrite suppression of Li-metal anodes. Nano Select 2021, 2, 993–1010. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Ye, B.; Pang, P.; Ma, Z.; Chen, H.; Nan, J. A pore-controllable polyamine (PAI) layer-coated polyolefin (PE) separator for pouch lithium-ion batteries with enhanced safety. J. Solid State Electrochem. 2020, 24, 843–853. [Google Scholar] [CrossRef]
- Shin, S.C.; Kim, J.; Modigunta, J.K.R.; Murali, G.; Park, S.; Lee, S.; Lee, H.; Park, S.Y.; In, I. Bio-mimicking organic-inorganic hybrid ladder-like polysilsesquioxanes as a surface modifier for polyethylene separator in lithium-ion batteries. J. Membr. Sci. 2021, 620, 118886. [Google Scholar] [CrossRef]
- Habumugisha, J.C.; Usha, Z.R.; Yu, R.; Babiker, D.M.D.; Wan, C.X.; Chen, X.; Li, L.B. Thermally stable and high electrochemical performance ultra-high molecular weight polyethylene/poly(4-methyl-1-pentene) blend film used as Li-ion battery separator. Appl. Mater. Today 2021, 24, 101136. [Google Scholar] [CrossRef]
- Li, R.; Gao, P. Nanoporous UHMWPE membrane separators for safer and high-power-density rechargeable batteries. Glob. Chall. 2017, 1, 1700020. [Google Scholar] [CrossRef]
- Ding, L.; Li, D.; Du, F.; Zhang, D.; Zhang, S.; Wu, T. Novel preparation of lithium-ion battery wet-processed separator based on the synergistic effect of porous skeleton nano-Al2O3 in situ blending and synchro-draw. Polym. Int. 2023, 72, 61–70. [Google Scholar] [CrossRef]
- Yan, Y.; Kong, Q.R.; Sun, C.C.; Yuan, J.J.; Huang, Z.; Fang, L.F.; Zhu, B.K.; Song, Y.Z. Copolymer-assisted polypropylene separator for fast and uniform lithium ion transport in lithium-ion batteries. Chin. J. Polym. Sci. 2020, 38, 1313–1324. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, J.P.; Shang, Y.M.; Wang, L.; Fang, M.; Wang, J.L.; He, X.M. Surface modification of polyolefin separators for lithium ion batteries to reduce thermal shrinkage without thickness increase. J. Energy Chem. 2015, 24, 138–144. [Google Scholar] [CrossRef]
- Yu, J.; Dong, N.; Liu, B.; Tian, G.; Qi, S.; Wu, D. A newly-developed heat-resistance polyimide microsphere coating to enhance the thermal stability of commercial polyolefin separators for advanced lithium-ion battery. Chem. Eng. J. 2022, 442, 136314. [Google Scholar] [CrossRef]
- Chen, P.; Ren, H.; Yan, L.; Shen, J.; Wang, T.; Li, G.; Chen, S.; Cong, X.; Xie, J.; Li, W. Metal-organic frameworks enabled high-performance separators for safety-reinforced lithium ion battery. ACS Sustain. Chem. Eng. 2019, 7, 16612–16619. [Google Scholar] [CrossRef]
- Romano, D.; Marroquin-Garcia, R.; Gupta, V.; Rastogi, S. An unconventional route for synthesis and solid-state processing of low-entangled ultra-high molecular weight isotactic polypropylene. Macromol. Rapid Commun. 2023, 44, 2300039. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yue, F.S.; Zhao, Y.M.; Wang, S.S.; Li, Y.C.; Li, G.; Ge, X.C. Surface tailoring of polypropylene separators for lithium-ion batteries via N-hydroxyphthalimide catalysis. Eur. Polym. J. 2021, 152, 110487. [Google Scholar] [CrossRef]
- Li, Y.; Pu, H. Facile fabrication of multilayer separators for lithium-ion battery via multilayer coextrusion and thermal induced phase separation. J. Power Sources 2018, 384, 408–416. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Ma, C.; Huang, D.; Li, P.; Shi, Y.; Qu, C.; Shi, X. Preparation and properties of PP/PAN/cotton fibers composite membrane as lithium-ion battery separator with thermal shut-off function. Batteries 2023, 9, 113. [Google Scholar] [CrossRef]
- Shi, C.; Dai, J.; Shen, X.; Peng, L.; Li, C.; Wang, X.; Zhang, P.; Zhao, J. A high-temperature stable ceramic-coated separator prepared with polyimide binder/Al2O3 particles for lithium-ion batteries. J. Membr. Sci. 2016, 517, 91–99. [Google Scholar] [CrossRef]
- Ma, T.; Cui, Z.; Wu, Y.; Qin, S.; Wang, H.; Yan, F.; Han, N.; Li, J. Preparation of PVDF based blend microporous membranes for lithium ion batteries by thermally induced phase separation: I. Effect of PMMA on the membrane formation process and the properties. J. Membr. Sci. 2013, 444, 213–222. [Google Scholar] [CrossRef]
- Choi, N.-S.; Park, J.-K. New polymer electrolytes based on PVC/PMMA blend for plastic lithium-ion batteries. Electrochim. Acta 2001, 46, 1453–1459. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; He, C.; Li, J.; Li, Q.; Wang, C.; Xi, Y. Blending-based poly(vinylidene fluoride)/polymethyl methacrylate membrane for rechargeable lithium-ion batteries. Ionics 2019, 25, 5201–5211. [Google Scholar] [CrossRef]
- Fu, Q.; Lin, G.; Chen, X.; Yu, Z.; Yang, R.; Li, M.; Zeng, X.; Chen, J. Mechanically reinforced PVdF/PMMA/SiO2 composite membrane and its electrochemical properties as a separator in lithium-ion batteries. Energy Technol. 2018, 6, 144–152. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Lin, B.; Chu, F.; Ren, Y. PVDF-HFP-based composite electrolyte membranes having high conductivity and lithium-ion transference number for lithium metal batteries. ACS Appl. Energ. Mater. 2022, 5, 1031–1040. [Google Scholar] [CrossRef]
- Pinto, R.S.; Serra, J.P.; Barbosa, J.C.; Goncalves, R.; Silva, M.M.; Lanceros-Mendez, S.; Costa, C.M. Direct-ink-writing of electroactive polymers for sensing and energy storage applications. Macromol. Mater. Eng. 2021, 306, 2100372. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, X.; Li, S.; Zheng, Q.; Jiang, S.; Xu, Y.; He, B.; Ma, L.; Luo, Y.; Wang, Y.; et al. Boosting thermal and mechanical properties: Achieving high-safety separator chemically bonded with nano TiN particles for high performance lithium-ion batteries. Small 2023, 19, 2300378. [Google Scholar] [CrossRef] [PubMed]
- Zainab, G.; Wang, X.; Yu, J.; Zhai, Y.; Ahmed Babar, A.; Xiao, K.; Ding, B. Electrospun polyacrylonitrile/polyurethane composite nanofibrous separator with electrochemical performance for high power lithium ion batteries. Mater. Chem. Phys. 2016, 182, 308–314. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, C.; Liu, X.; Jiang, Y.; Ding, Y. Lithium acetate modified PU/graphene composites as separator for advanced Li-ion batteries. Micro Nano Lett. 2020, 15, 213–217. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, H.; Ouyang, C.; Hu, N.; Zha, G.; Hou, H. A high-temperature stable composite polyurethane separator coated Al2O3 particles for lithium ion battery. Compos. Commun. 2022, 33, 101217. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Zhang, X. Polymethylmethacrylate/polyacrylonitrile membranes via centrifugal spinning as separator in Li-ion batteries. Polymers 2015, 7, 629–643. [Google Scholar] [CrossRef]
- Zhang, T.; Qu, H.; Sun, K. Development of polydopamine coated electrospun PAN/PMMA nanofibrous membrane as composite separator for Lithium-ion batteries. Mater. Lett. 2019, 245, 10–13. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Yin, Y.; Guo, W.; Bai, Y.; Zhang, F.; Zhao, B.; Shen, F.; Han, X. Novel polyimide separator prepared with two porogens for safe lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 3610–3616. [Google Scholar] [CrossRef]
- Zhang, T.W.; Chen, J.L.; Tian, T.; Shen, B.; Peng, Y.D.; Song, Y.H.; Jiang, B.; Lu, L.L.; Yao, H.B.; Yu, S.H. Sustainable separators for high-performance lithium ion batteries enabled by chemical modifications. Adv. Funct. Mater. 2019, 29, 1902023. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Li, C.; Liang, Z.; Hu, X.; Liu, H.; Zhang, Z.; Cui, M.; Chen, G.; Wan, J.; et al. Highly thermally stable, highly electrolyte-wettable hydroxyapatite/cellulose nanofiber hybrid separators for lithium-ion batteries. ACS Appl. Energ. Mater. 2023, 6, 3862–3871. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Duan, Y. Engineering Polymer-Based Porous Membrane for Sustainable Lithium-Ion Battery Separators. Polymers 2023, 15, 3690. https://doi.org/10.3390/polym15183690
Li L, Duan Y. Engineering Polymer-Based Porous Membrane for Sustainable Lithium-Ion Battery Separators. Polymers. 2023; 15(18):3690. https://doi.org/10.3390/polym15183690
Chicago/Turabian StyleLi, Lei, and Yutian Duan. 2023. "Engineering Polymer-Based Porous Membrane for Sustainable Lithium-Ion Battery Separators" Polymers 15, no. 18: 3690. https://doi.org/10.3390/polym15183690





