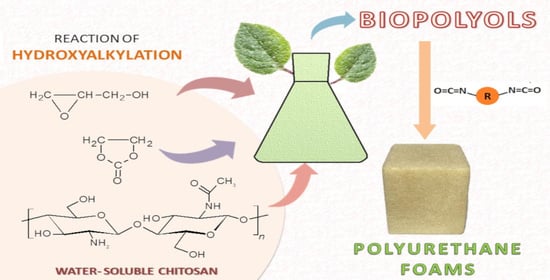
Polyols and Polyurethane Foams Based on Water-Soluble Chitosan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
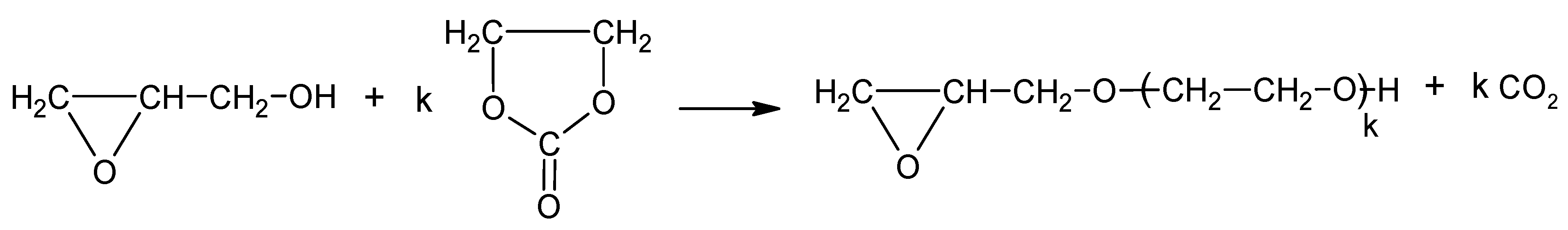
2.2. Synthesis of Polyols
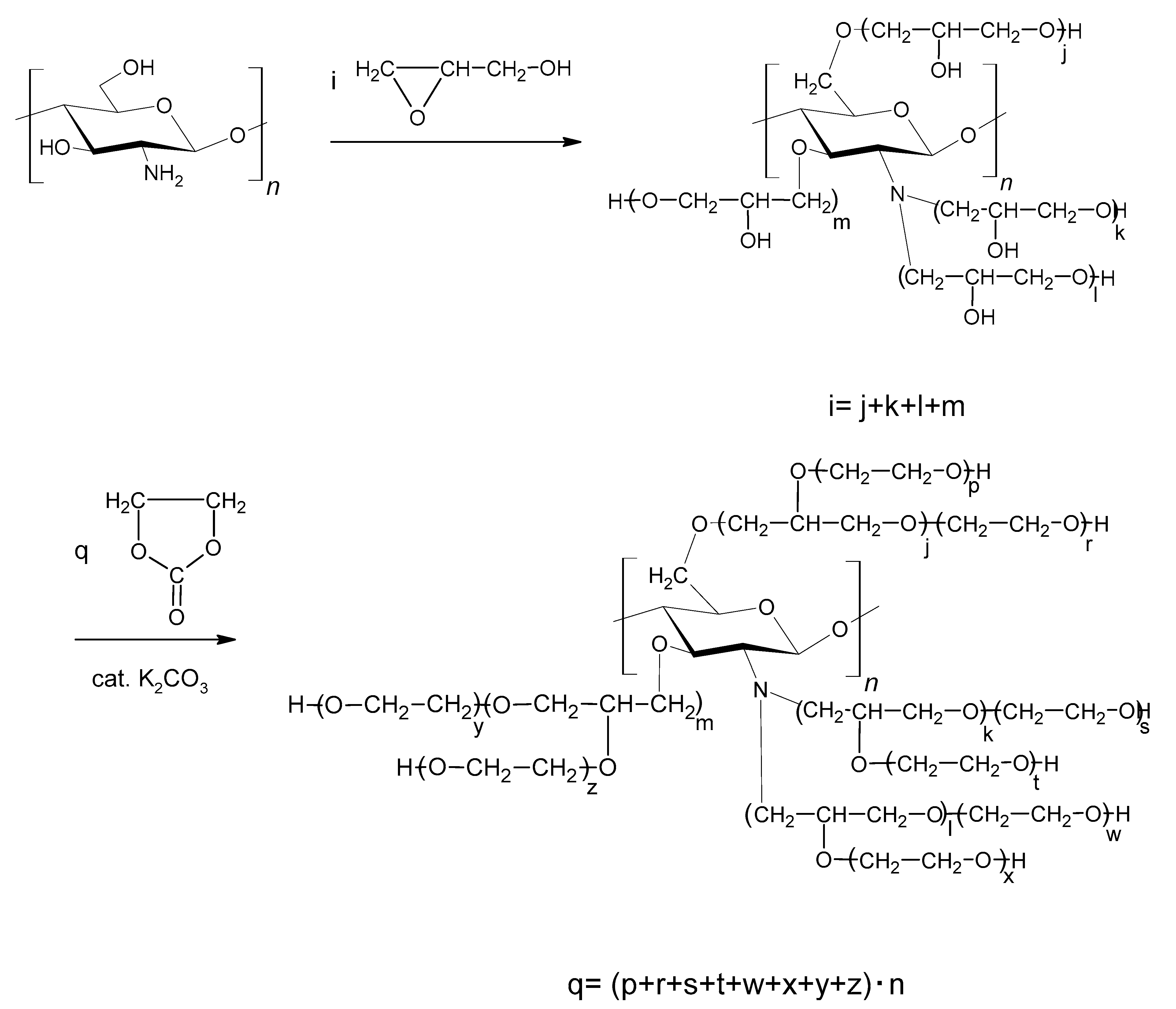
2.2.1. Synthesis 1: Polyol (CS + H2O + GL) + EC
2.2.2. Synthesis 2: Polyol (CS + GLYC + GL) + EC
2.2.3. Synthesis 3: Polyol (CS + GL) + EC
2.2.4. Synthesis 4: Polyol (CS + GL + EC)
2.3. Analytical Methods
2.4. Physical Properties of Polyol
2.5. Polyurethane Foams
2.6. Properties of Foams
2.7. Biodegradation of Polyol and Foam
3. Results and Discussion
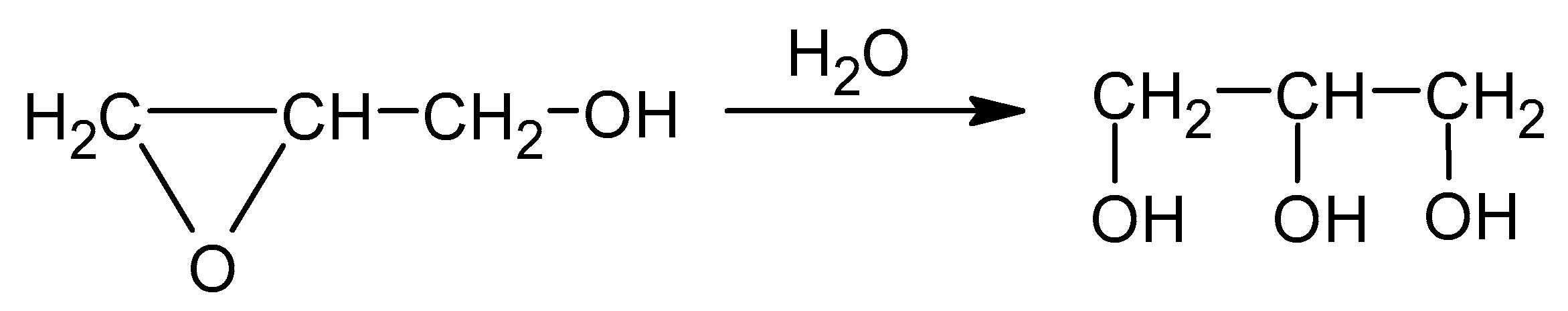
3.1. Obtaining of Polyols
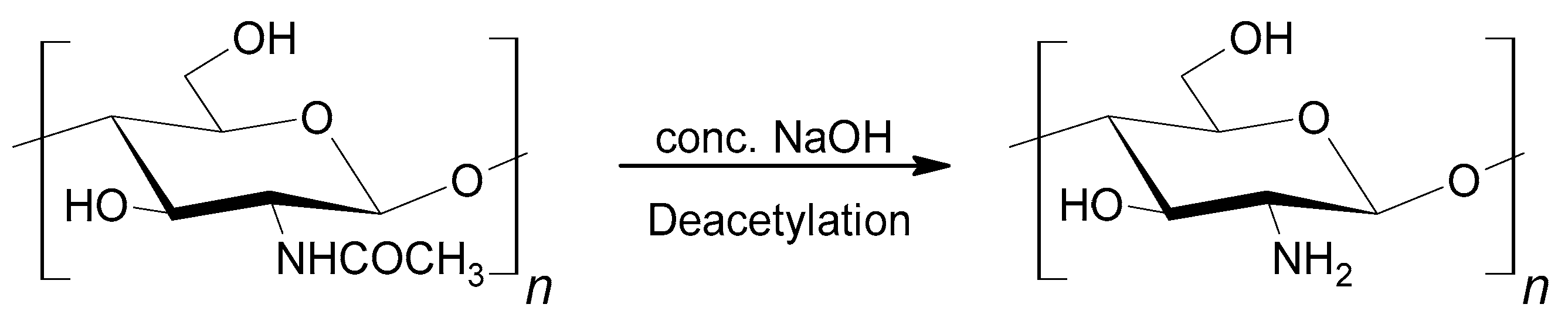
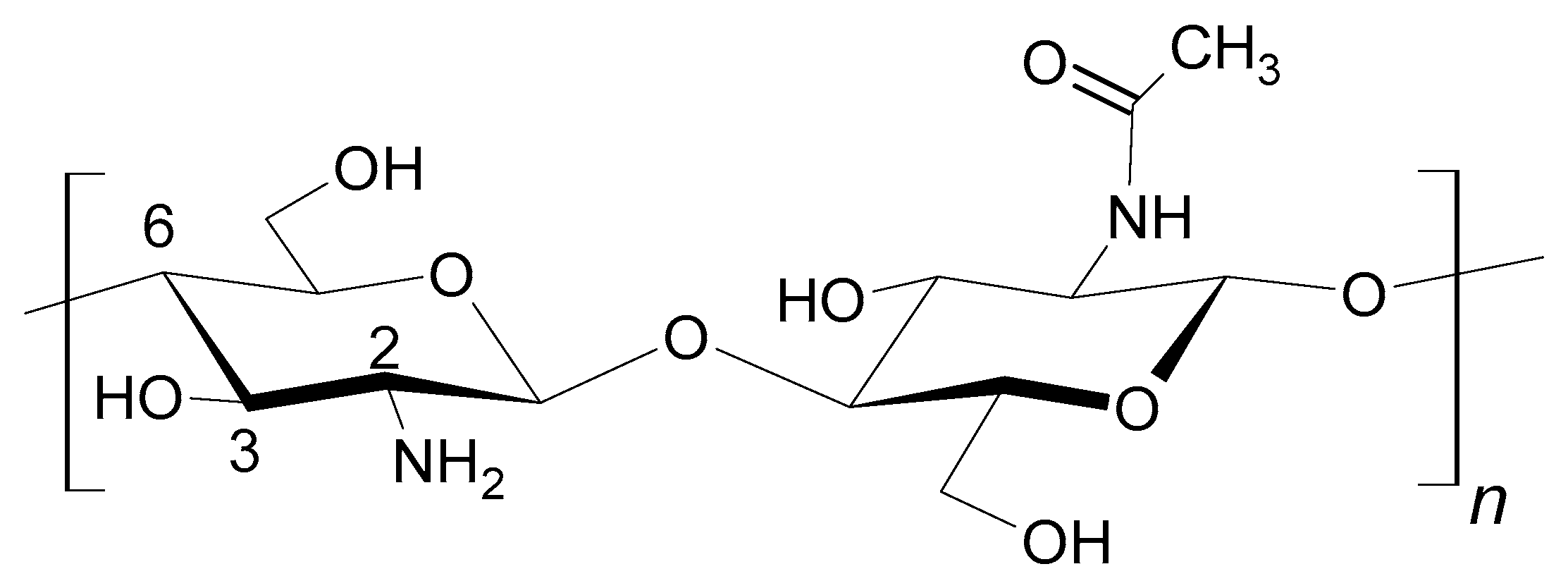
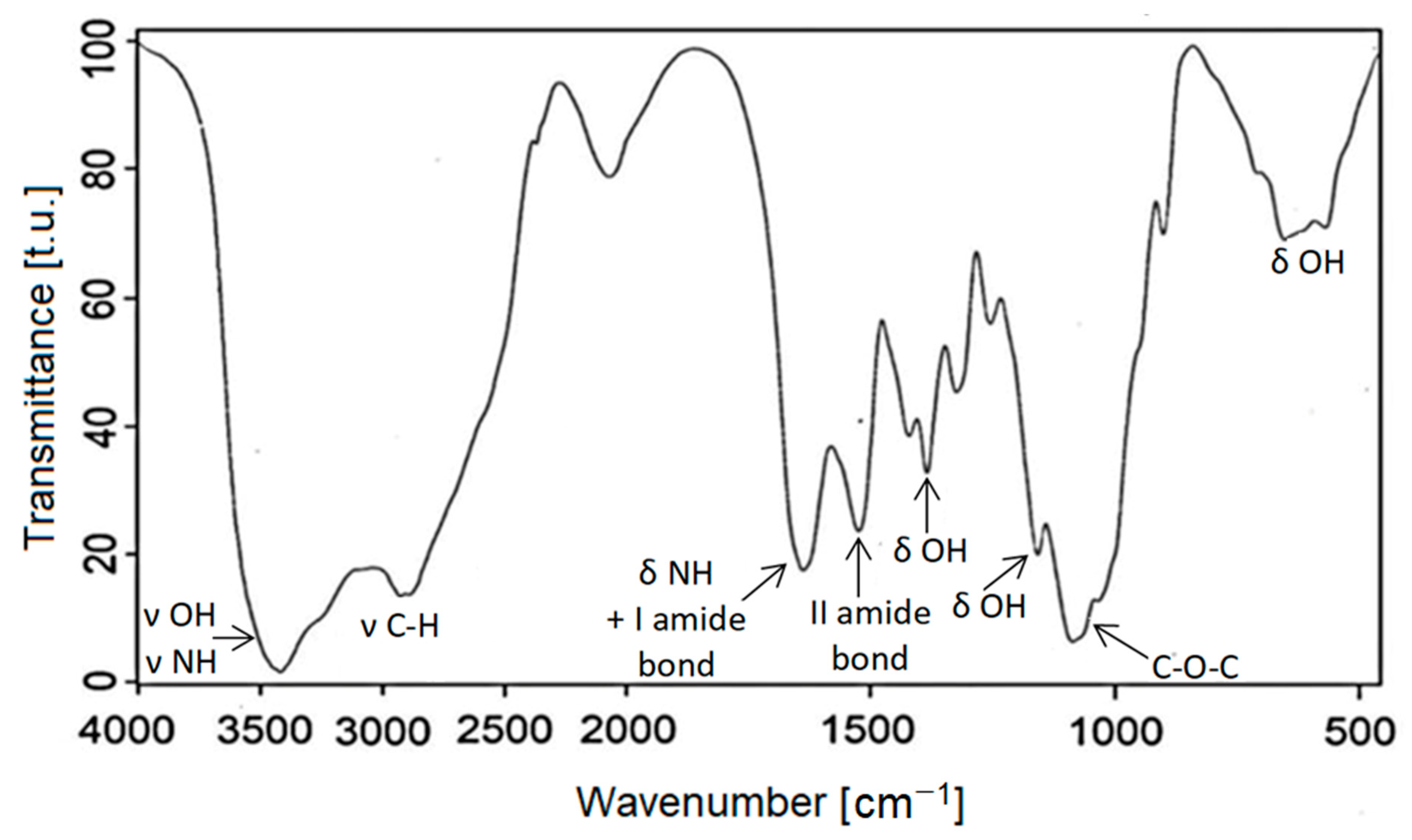
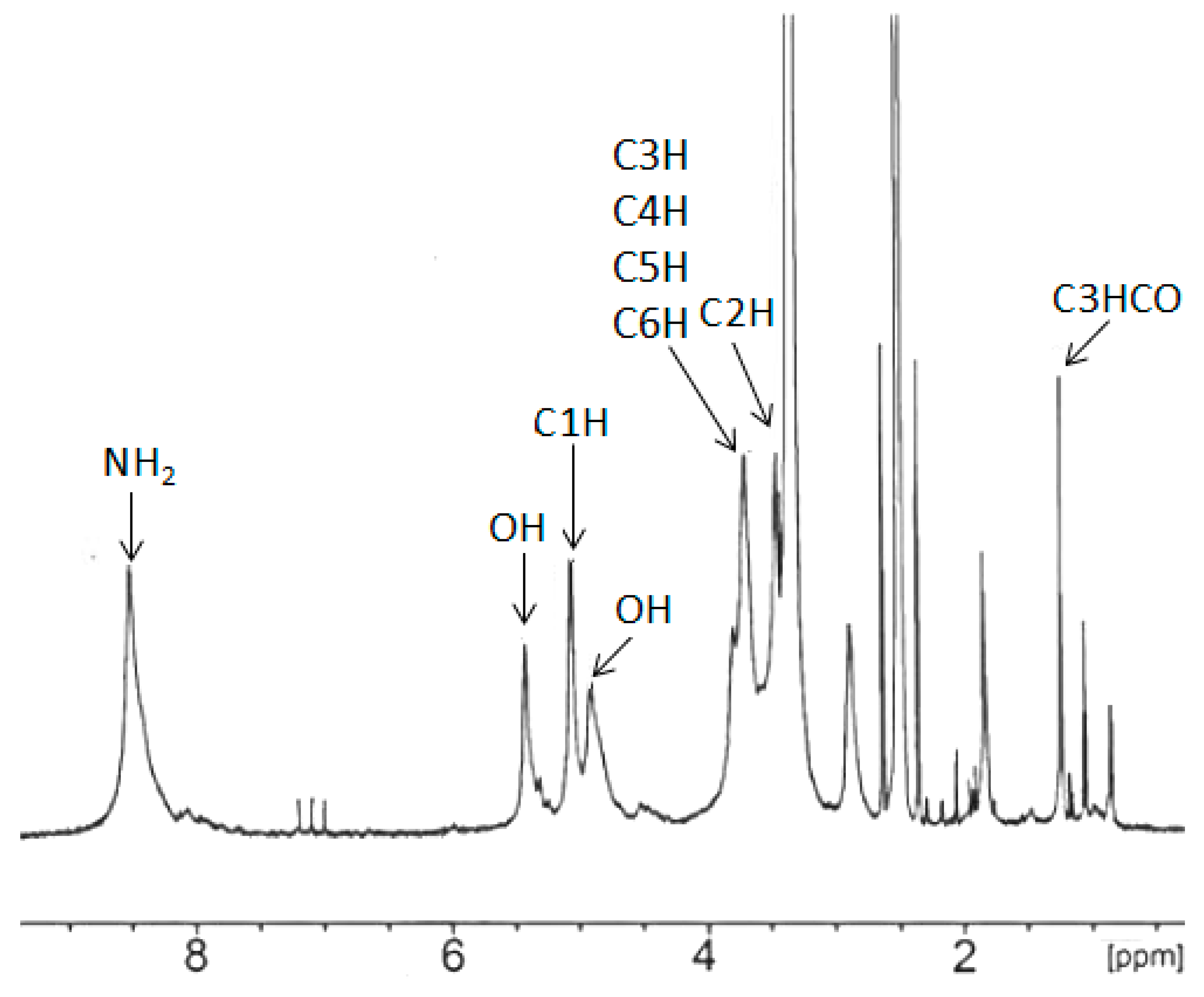
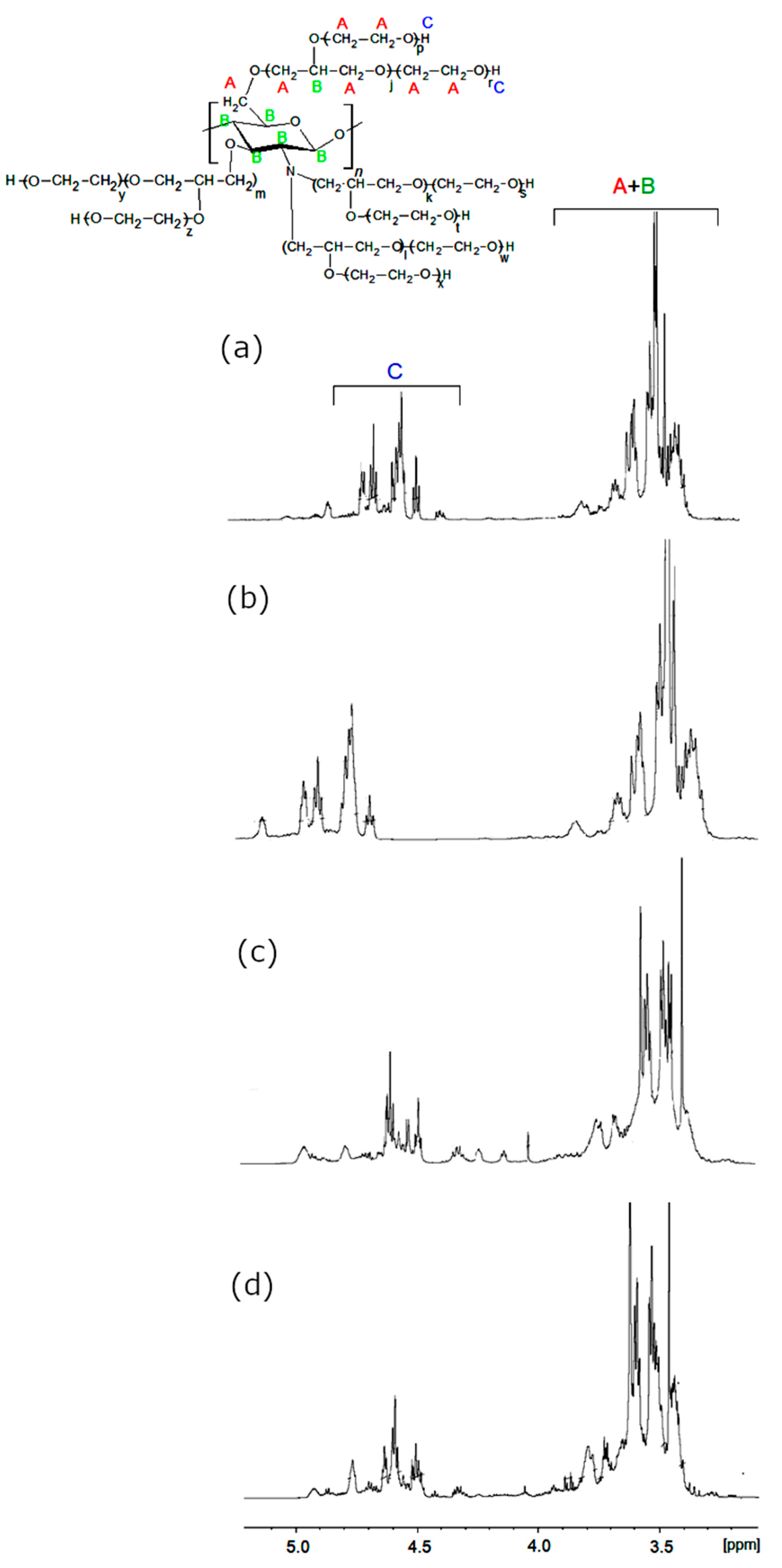
3.2. Composition and Structure of Polyols
3.3. Preparation of Polyurethane Foams
3.4. Properties of Polyurethane Foams
4. Summary and Conclusions
- Four new methods of the synthesis of chitosan-based polyols were elaborated using water-soluble chitosan, glycidol, and ethylene carbonate, with variable environments.
- The chitosan-derived polyols can be obtained in water in the presence of glycerol or no-solvent conditions. These polyols are suitable to obtain polyurethane foams.
- The polyurethane foams obtained from these polyols have properties analogous for typical rigid PUFs, except for increased thermal resistance in comparison with classic ones. They can withstand long-term thermal exposure at 175 °C. Additionally, with thermal exposure of the obtained PUFs at 150 °C, the compressive strength of the annealed PUF considerably increased.
- The chitosan-based polyols and polyurethane foams obtained from them were biodegradable: the polyol was completely biodegraded, while the PUF obtained thereof was 52% biodegradable within 28 days in the soil biodegradation oxygen demand test.
- Polyurethane foams obtained from polyols based on chitosan converted by the reaction with glycidol and ethylene carbonate in water or in glycerol have useful thermal conductivity, dimensional stability, compressive strength, and low water absorption. Their high thermal resistance renders them as promising candidates to use as thermal insulating materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swinarew, B. Polyurethanes—Modern versatile materials. Part 2, Polyurethane foam. Przetwórstwo Tworzyw 2015, 21, 428–434. [Google Scholar]
- Datta, J.; Włoch, M. Elastomer Engineering; Gdańsk University of Technology: Gdańsk, Poland, 2017. [Google Scholar]
- Pan, X.; Saddler, J. Effect or replacing polyol by organosolv and kraft lignin on the property and structure of rigid polyurethane foam. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prociak, A.; Rokicki, G.; Ryszkowska, J. Polyurethane Materials; PWN: Warsaw, Poland, 2014. [Google Scholar]
- Noreen, A.; Zia, K.N.; Zuber, M.; Tabasum, S.; Zahoor, A.S. Bio-based polyurethane: An efficient and environment friendly coating systems: A review. Prog. Org. Coat. 2016, 91, 25–32. [Google Scholar] [CrossRef]
- Mosiewicki, M.; Rojek, P.; Michałowski, S.; Aranguren, M.; Prociak, A. Rapeseed Oil—Based Polyurethane Foams Modified with Glycerol and Cellulose Micro/Nanocrystals. J. Appl. Polym. Sci. 2015, 132, 41602. [Google Scholar] [CrossRef]
- Zlatanić, A.; Lava, C.; Zhang, W.; Petrović, Z. Effect of structure on properties of polyols and polyurethanes based on different vegetable oils. J. Appl. Polym. Sci. 2004, 42, 809–819. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czupryński, B. Biodegradable, Flame-retardant and Bio-Based rigid Polyurethane/Polyisocyanurate Foams for Thermal Insulation Application. Polymers 2019, 11, 1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatakeyama, H.; Hatakeyama, T. Environmentally Compatible Hybrid-Type Polyurethane Foams Containing Saccharide and Lignin Components. Macromol. Symp. 2005, 24, 219–226. [Google Scholar] [CrossRef]
- Lubczak, R.; Szczęch, D.; Broda, D.; Wojnarowska-Nowak, R.; Kus-Liśkiewicz, M.; Dębska, B.; Lubczak, J. Polyetherols and polyurethane foams from starch. Polym. Test. 2021, 93, 106884. [Google Scholar] [CrossRef]
- Szpiłyk, M.; Lubczak, R.; Lubczak, J. The biodegradable cellulose-derived polyol and polyurethane foam. Polym. Test. 2021, 100, 107250. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug. Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Pakdel, P.M.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for waste water treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. 1-Fundamentals of Chitosan for Biomedical Applications, Chitosan Based Biomaterials; Woodhead Publishing: Sawston, UK, 2017; Volume 1. [Google Scholar]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef] [Green Version]
- Crini, G. Chitin and chitosan: Production, properties, and applications. In Chitin and Chitosan. Discoveries and Applications for Sustainability; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Singha, N.R.; Deb, M.; Chattopadhyay, P.K. Chitin and chitosan-based blends and composites. In Biodegradable Polymers, Blends and Composites; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rhman, A. Review of chitosan composite as a heavy metal adsorbent: Material preparation and properties. Carbohydr. Polym. 2021, 259, 117613. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, F.; Atyabi, S.M.; Norouzian, D.; Zandi, M.; Bakhshi, H.; Irani, S. Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int. J. Biol. Macromol. 2018, 115, 243–248. [Google Scholar] [CrossRef]
- Borsagli, F.G.L.M.; Carvalho, I.C.; Mansur, H.S. Amino acid-grafted and N-acylated chitosan thiomers: Construction of 3D bio-scaffolds for potential cartilage repair applications. Int. J. Biol. Macromol. 2018, 114, 270–282. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Desbriéres, J.; Guibal, E. Chitosan for waste water treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Aboelfetoh, E.F.; Aboubaraka, A.E.; Ebeid, E.M. Binary coagulation system (graphene oxide/chitosan) for polluted surface water treatment. J. Environ. Manag. 2021, 288, 112481. [Google Scholar] [CrossRef] [PubMed]
- Szpiłyk, M.; Lubczak, R.; Walczak, M.; Lubczak, J. Polyol and polyurethane foam from cellulose hydrolysate. J. Chem. Technol. Biotechnol. 2021, 96, 881–889. [Google Scholar] [CrossRef]
- Lubczak, R.; Szczęch, D.; Lubczak, J. From starch to oligoetherols and polyurethane foams. Polym. Bull. 2020, 77, 5725–5751. [Google Scholar] [CrossRef]
- Fernandes, S.; Freire, C.; Neto, C.P.; Gandini, A. The bulk oxypropylation of chitin and chitosan and the characterization of the ensuing polyol. Green Chem. 2008, 10, 93–97. [Google Scholar] [CrossRef]
- Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Sanjay, K.J. A New Horizon in Modifications of Chitosan: Syntheses and Applications. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 91–181. [Google Scholar] [CrossRef]
- Zhu, C.; Zou, S.; Rao, Z.; Min, L.; Liu, M.; Liu, L.; Fan, L. Preparation and characterization of hydroxypropyl chitosan modified with nisin. Int. J. Biol. Macromol. 2017, 105, 1017–1024. [Google Scholar] [CrossRef]
- Fan, L.; Zou, S.; Ge, H.; Xiao, Y.; Wen, H.; Feng, H.; Liu, M.; Nie, M. Preparation and characterization of hydroxypropyl chitosan modified with collagen peptide. Int. J. Biol. Macromol. 2016, 93, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Pedro, R.; Pereira, S.; Goycoolea, F.M.; Schmitt, C.C.; Neumann, M.G. Self-aggregated nanoparticles of N-dodecyl, N′-glycidyl(chitosan) as pH-responsive drug delivery systems for quercetin. J. Appl. Polym. Sci. 2018, 135, 45678. [Google Scholar] [CrossRef]
- Park, J.H.; Kwon, S.; Lee, M.; Chung, H.; Kim, J.H.; Kim, Y.S.; Park, R.W.; Kim, I.S.; Seo, S.B.; Kwon, I.C.; et al. Self-assembled nanoparticles based on glycol chitosan bearing hydrophobic moieties as carriers for doxorubicin: In vivo biodistribution and anti-tumor activity. Biomaterials 2006, 27, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Park, K.-H.; Park, I.S.; Na, K. Glycol chitosan as a stabilizer for protein en-capsulated into poly(lactide-co-glycolide) microparticle. Int. J. Pharm. 2007, 338, 310–316. [Google Scholar] [CrossRef]
- Uscátegui, Y.L.; Arévalo-Alquichire, S.J.; Gómez-Tejedor, J.A.; Vallés-Lluch, A.; Diaz, L.E.; Valero, M.F. Polyurethane-based bio-adhesive synthesized from polyols derived from castor oil (Ricinuscommunis) and low concentration of chitosan. J. Mater. Res. 2017, 32, 3699–3711. [Google Scholar] [CrossRef]
- Zuo, D.-Y.; Tao, Y.-Z.; Chen, Y.-B.; Xu, W.-L. Preparation and characterization of blend membranes of polyurethane and superfine chitosan powder. Polym. Bull. 2009, 62, 713–725. [Google Scholar] [CrossRef]
- Schio, R.; da Rosa, B.C.; Goncalves, J.O.; Pinto, L.A.A.; Mallmann, E.S.; Dotto, G.L. Synthesis of a bio–based polyurethane/chitosan composite foam using ricin oleic acid for the adsorption of Food Red 17 dye. Int. J. Biol. Macromol. 2019, 121, 373–380. [Google Scholar] [CrossRef]
- Silva, S.S.; Menezes, S.M.C.; Garcia, R.B. Synthesis and characterization of polyurethane-g-chitosan. Eur. Polym. J. 2003, 39, 1515–1519. [Google Scholar] [CrossRef]
- Zia, K.M.; Anjum, S.; Zuber, M.; Mujahid, M.; Jamil, T. Synthesis and molecular characterization of chitosan based polyurethane elastomers using aromatic diisocyanate. Int. J. Biol. Macromol. 2014, 66, 26–32. [Google Scholar] [CrossRef]
- Qin, H.; Wang, K. Study on preparation and performance of PEG-based polyurethane foams modified by the chitosan with different molecular weight. Int. J. Biol. Macromol. 2019, 140, 877–885. [Google Scholar] [CrossRef]
- Lee, H.C.; Jeong, Y.G.; Min, B.G.; Lyoo, W.S.; Lee, S.C. Preparation and acid dye adsorption behavior of polyurethane/chitosan composite foams. Fibers Polym. 2009, 10, 636–642. [Google Scholar] [CrossRef]
- Sasidharan, A.P.; Meera, V.; Raphael, V.P. Investigations on characteristics of polyurethane foam impregnated with nanochitosan and nanosilver/silver oxide and its effective nessin phosphate removal. Environ. Sci. Pollut. Res. 2021, 28, 12980–12992. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R.; Arul, J.; Charlet, G. Intrinsic Viscosity-Molecular Weight relationship for Chitosan. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2591–2598. [Google Scholar] [CrossRef]
- Wang, W.; Bo, S.; Li, S.; Qiin, W. Determination of the Mark-Houwink equation for chitosans with different degrees of deacetylation. Int. J. Biol. Macromol. 1991, 13, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Brojer, Z.; Hertz, Z.; Penczek, P. Epoxy Resins; WNT: Warsaw, Poland, 1972. [Google Scholar]
- Kijowska, D.; Wołowiec, S.; Lubczak, J. Kinetics and mechanism of initial steps of synthesis of polyetherols from melamine and ethylene carbonate. J. Appl. Polym. Sci. 2004, 93, 294–300. [Google Scholar] [CrossRef]
- Standards PN-93/C-89052.03; Polyethers for Polyurethanes. Test Methods. Determination of the Hydroxyl Number. Polish Committee for Standardization: Warsaw, Poland, 1993.
- Nizioł, J.; Zieliński, Z.; Rode, W.; Ruman, T. Matrix-free laser desorption-ionization with silver nanoparticle enhanced steel targets. Int. J. Mass Spectrom. 2013, 335, 22–32. [Google Scholar] [CrossRef]
- Polish (European) Standards PN-EN ISO 845-2000; Cellular Plastics and Rubbers. Determination of Apparent (Bulk) Density. Polish Committee for Standardization: Warsaw, Poland, 2001.
- Polish (European) Standards PN-EN ISO 2896-1986; Cellular Plastics, Rigid. Determination of Water Absorption. Polish Committee for Standardization: Warsaw, Poland, 2001.
- Polish (European) Standards PN-EN ISO 2796-1986; Cellular Plastics, Rigid. Test of Dimensional Stability. Polish Committee for Standardization: Warsaw, Poland, 1986.
- Polish (European) Standards PN- EN ISO 844-1978; Cellular Plastics, Compression Test for Rigid Materials. Polish Committee for Standardization: Warsaw, Poland, 2010.
- Standard ISO17556-2019; Plastics—Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved. ISO/TC 61 Plastics, Subcommittee SC14 Environmental Aspect. Technical Committee: Warsaw, Poland, 2019.
- Standard ISO11274:2019; Soil Quality—Determination of the Water-Retention Characteristic—Laboratory Methods. Chemical and Physical Characterization. Hydrological Properties of Soils Technical Committee ISO/TC 190/SC 3: Warsaw, Poland, 2020.
- Standard ISO 10390-2005; Soil Quality—Determination of pH. ISO/TC 190/SC 3Chemical Characteristics of Soils. Technical Committee: Geneva, Switzerland, 2005.
- Czupryński, B. Questions of Chemistry and Technology of Polyurethanes; The Publishing House of the Academy of Bydgoszcz: Bydgoszcz, Poland, 2004. [Google Scholar]
- Wirpsza, Z. Polyurethanes: Chemistry, Technology, Application; WNT: Warsaw, Poland, 1991. [Google Scholar]
- Diab, M.A.; El-Sonbati, A.Z.; Bader, D.M.D. Thermal stability and degradation of chitosan modified by benzophenone. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 1057–1062. [Google Scholar] [CrossRef]








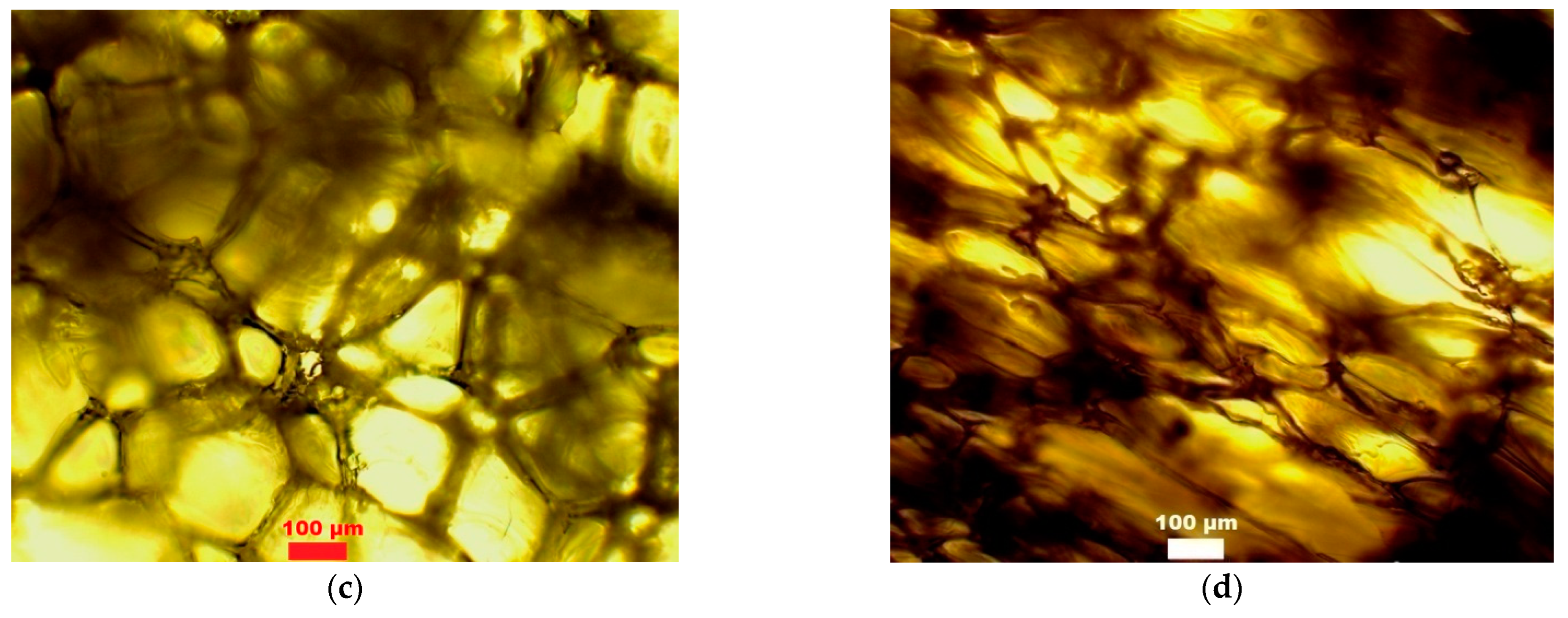
| Entry | Signal Position, m/z | Relative Intensity of Signal (%) | Molecular Ion Structure | Calc. Molecular Weight (g/mol) |
|---|---|---|---|---|
| 1 | 96.929 | 43.84 | GL + Na+ | 97.027 |
| 2 | 112.895 | 100.00 | GL + K+ | 113.000 |
| 3 | 114.898 | 44.54 | GL + H2O + Na+ | 115.037 |
| 4 | 119.934 | 11.95 | GL + OE + H+ | 119.071 |
| 5 | 196.334 | 30.58 | GL + 2OE + CH3OH | 194.115 |
| 6 | 210.700 | 4.12 | 2GL + H2O + OE | 210.110 |
| 7 | 230.217 | 6.74 | 2GL + OE + K+ | 231.063 |
| 8 | 257.024 | 9.33 | 2GL + 2OE + Na+ | 259.116 |
| 9 | 274.287 | 7.92 | 2GL + 2OE + K+ | 275.090 |
| 10 | 287.078 | 8.44 | 3GL + OE-H2O + K+ | 287.090 |
| 11 | 305.108 | 15.14 | 3GL + OE + K+ | 305.100 |
| 12 | 333.149 | 11.86 | 3GL + 2OE + Na+ | 333.153 |
| 13 | 349.134 | 23.33 | H2O + 3GL + 2OE + Na+ | 351.163 |
| 14 | 363.142 | 13.82 | 4GL + OE + Na+ | 363.163 |
| 15 | 393.992 | 16.36 | 2GL + 6OE −H2O | 394.220 |
| 16 | 473.184 | 9.06 | 6GL + OE − H2O + H+ | 471.244 |
| 17 | 590.878 | 16.40 | 7GL + 2OE − H2O + H+ | 589.307 |
| 18 | 627.395 | 4.04 | 7GL + 2OE + Na+ | 629.300 |
| 19 | 671.165 | 3.40 | 7GL + 3OE + Na+ | 673.326 |
| 20 | 707.320 | 3.34 | 8GL + 3OE − H2O + H+ | 707.370 |
| Entry | Signal Position, m/z | Relative Intensity of Signal (%) | Molecular Ion Structure | Calc. Molecular Weight (g/mol) |
|---|---|---|---|---|
| 1 | 96.912 | 13.60 | GL + Na+ | 97.027 |
| 2 | 101.080 | 6.77 | GL + OE-H2O + H+ | 101.060 |
| 3 | 108.899 | 13.69 | GL + CH3OH + H+ | 107.071 |
| 4 | 112.900 | 26.55 | GL + K+ | 113.000 |
| 5 | 114.877 | 15.43 | GLYC + Na+ | 115.037 |
| 6 | 144.010 | 7.41 | GL + 2OE − H2O | 144.079 |
| 7 | 156.861 | 9.66 | GL + OE + K+ | 157.027 |
| 8 | 186.206 | 11.18 | 2GL + K+ | 187.037 |
| 9 | 196.971 | 35.24 | GLYC + GL + CH3OH | 198.110 |
| 10 | 205.068 | 29.28 | 3GL-H2O + H+ | 205.108 |
| 11 | 233.106 | 32.64 | GLYC + GL + OE + Na+ | 233.100 |
| 12 | 249.080 | 100.00 | 3GL + OE − H2O + H+ | 249.133 |
| 13 | 263.077 | 20.49 | GLYC + 2GL + Na+ | 263.111 |
| 14 | 279.067 | 25.71 | GLYC + 2GL + K+ | 279.0845 |
| 15 | 293.107 | 99.16 | GLYC + GL + 2OE + K+ | 293.100 |
| 16 | 307.140 | 25.74 | GLYC + 2GL + OE + Na+ | 307.137 |
| 17 | 323.114 | 40.40 | GLYC + 2GL + OE + K+ | 323.111 |
| 18 | 351.163 | 16.91 | GLYC + 2GL + 2OE + Na+ | 351.163 |
| 19 | 367.139 | 30.35 | 4GL + 2OE−H2O + H+ | 367.197 |
| 20 | 397.183 | 14.63 | GLYC + 3GL + OE + K+ | 397.148 |
| 21 | 411.169 | 21.71 | GLYC + 4GL + Na+ | 411.184 |
| 22 | 425.201 | 15.63 | GLYC + 3GL + 2OE + Na+ | 425.200 |
| 23 | 441.154 | 13.31 | GLYC + 3GL + 2OE + K+ | 441.174 |
| 24 | 455.203 | 12.60 | GLYC + 4GL + OE + Na+ | 455.210 |
| 25 | 471.164 | 9.66 | 6GL + OE−H2O + H+ | 471.244 |
| 26 | 485.200 | 11.57 | GLYC + 5GL + Na+ | 485.221 |
| 27 | 515.211 | 12.60 | GLYC + 4GL + 2OE + K+ | 515.211 |
| 28 | 521.314 | 11.08 | GLYC + 4GL + 3OE + H+ | 521.281 |
| 29 | 590.906 | 20.07 | GLYC + 5GL + 2OE + K+ | 589.247 |
| Entry | Signal Position m/z | Relative Intensity of Signal (%) | Molecular Ion Structure | Calc. Molecular Weight (g/mol) |
|---|---|---|---|---|
| 1 | 96.930 | 6.08 | GL + Na+ | 97.026 |
| 2 | 101.063 | 14.40 | GL + OE − H2O + H+ | 101.060 |
| 3 | 112.901 | 76.62 | GL + K+ | 113.000 |
| 4 | 114.902 | 24.69 | GLYC + Na+ | 115.037 |
| 5 | 120.936 | 15.39 | GL + OE + H+ | 119.071 |
| 6 | 140.902 | 14.34 | GL + OE + Na+ | 141.053 |
| 7 | 156.907 | 25.77 | GL + OE + K+ | 157.027 |
| 8 | 175.069 | 8.56 | 2GL + OE-H2O + H+ | 175.097 |
| 9 | 196.881 | 33.95 | GLYC + GL + CH3OH | 198.110 |
| 10 | 205.073 | 39.21 | 3GL − H2O + H+ | 205.108 |
| 11 | 219.069 | 11.52 | 2GL + 2OE − H2O + H+ | 219.123 |
| 12 | 249.074 | 96.62 | 3GL + OE − H2O + H+ | 249.134 |
| 13 | 263.063 | 6.15 | GLYC + 2GL + Na+ | 263.111 |
| 14 | 279.087 | 49.41 | GLYC + 2GL + K+ | 279.085 |
| 15 | 293.093 | 41.94 | GLYC + GL + 2OE + K+ | 293.100 |
| 16 | 323.097 | 52.76 | GLYC + 2GL + OE + K+ | 323.111 |
| 17 | 335.114 | 8.19 | GLYC + 2GL + 2O−H2O + Na+ | 333.153 |
| 18 | 353.106 | 20.29 | 5GL − H2O + H+ | 353.181 |
| 19 | 367.121 | 13.63 | 4GL + 2OE − H2O + H+ | 367.197 |
| 20 | 393.975 | 100.00 | GLYC + 2GL + 3OE + Na+ | 395.189 |
| 21 | 397.159 | 15.38 | GLYC + 3GL + OE + K+ | 397.148 |
| 22 | 411.143 | 8.06 | GLYC + 4GL + Na+ | 411.184 |
| 23 | 427.144 | 10.43 | 5GL + OE − H2O + CH3OH | 428.226 |
| 24 | 441.156 | 7.13 | GLYC + 3GL + 2OE + K+ | 441.174 |
| 25 | 471.167 | 8.45 | 6GL + OE−H2O + H+ | 471.244 |
| 26 | 515.197 | 7.26 | GLYC + 4GL + 2OE + K+ | 515.211 |
| 27 | 534.289 | 16.09 | 3GL + 7OE − H2O + Na+ | 535.273 |
| 28 | 575.851 | 9.48 | 7GL + OE − H2O + CH3OH | 576.299 |
| 29 | 590.888 | 58.08 | 7GL + 2OE − H2O + H+ | 589.307 |
| Polyol | Density (g/cm3) | Surface Tension (mN/m) | Hydroxyl Number (mg KOH/g) | |
|---|---|---|---|---|
| (CS + GL + H2O) + EC | 1.2789 | 6847 | 41.0 | 654 |
| (CS + GL + GLYC) + EC | 1.2702 | 1475 | 37.9 | 560 |
| (CS + GL) + EC | 1.3150 | 88,612 | 44.2 | 409 |
| (CS + GL + EC) | 1.3208 | 52,176 | 41.0 | 493 |
| Polyol | Composition (g/100 g of Polyol) | Isocyanate Index | Foaming Process | |||||
|---|---|---|---|---|---|---|---|---|
| pMDI | Water | Silicon L-6900 | TEA | Cream Time (s) | Rise Time (s) | Tack-Free Time (s) | ||
| (CS + H2O + GL) + EC | 180 | 2 | 3.0 | 2.0 | 1.1 | 28 | 22 | 5 |
| 190 | 3 | 3.9 | 1.7 | 1.2 | 33 | 46 | 4 | |
| (CS + GL + GLYC) + EC | 178 | 2 | 3.0 | 0.8 | 1.3 | 55 | 54 | 5 |
| 180 | 3 | 3.0 | 0.8 | 1.3 | 59 | 56 | 5 | |
| (CS + GL) + EC | 110 | 2 | 3.9 | 0.9 | 1.1 | 40 | 43 | 14 |
| 120 | 3 | 3.9 | 0.5 | 1.1 | 72 | 59 | 26 | |
| (CS + GL + EC) | 150 | 2 | 3.9 | 2.7 | 1.2 | 24 | 20 | 11 |
| 185 | 3 | 3.9 | 2.2 | 1.5 | 21 | 30 | 14 | |
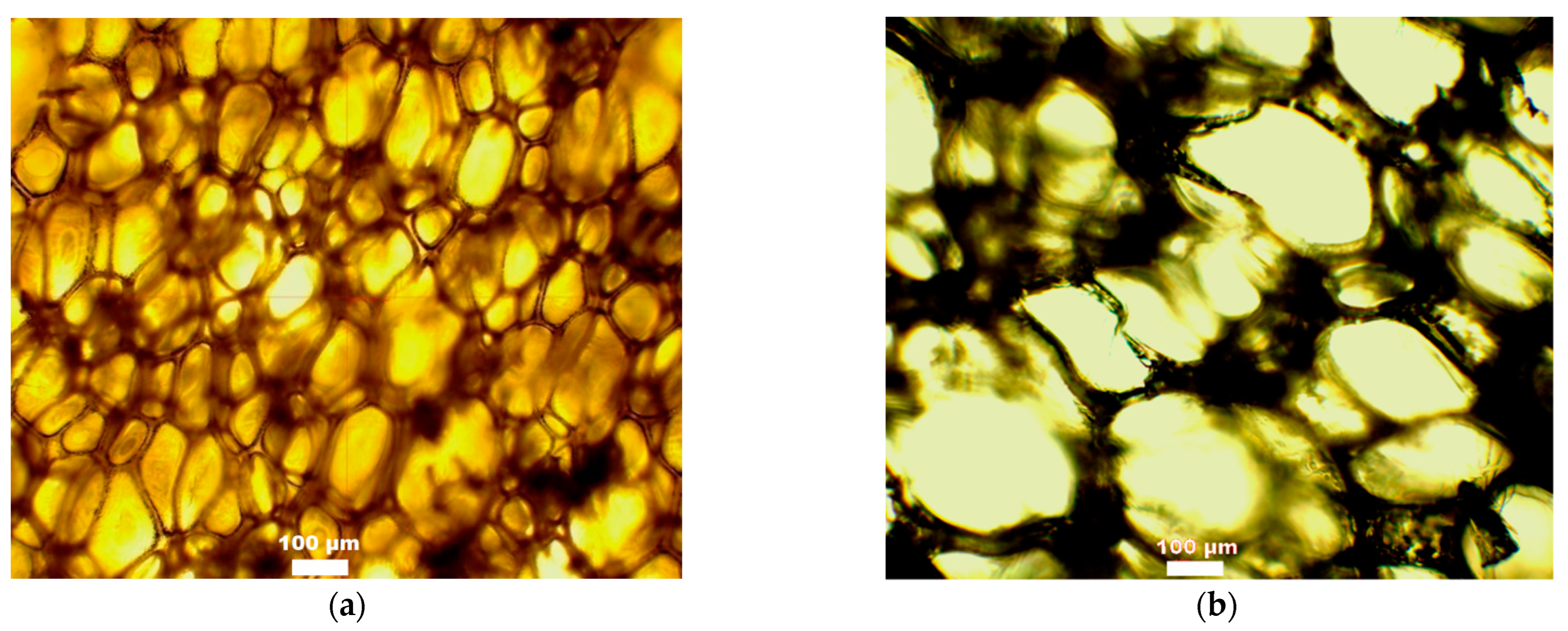
| Foam Obtained from Polyol | Amount of Water/100g of Polyol | Larger Diameter (µm) | Smaller Diameter (µm) | Thickness of Cell Wall (µm) |
|---|---|---|---|---|
| (CS + GL + H2O) + EC | 2 | 149 ± 16 | 129 ± 23 | 7 ± 1 |
| 3 | 237 ± 35 | 85 ± 35 | 15 ± 2 | |
| (CS + GL + GLYC) + EC | 2 | 169 ± 17 | 80 ± 12 | 11 ± 1 |
| 3 | 261 ± 36 | 149 ± 12 | 26 ± 7 | |
| (CS + GL) + EC | 2 | 209 ± 17 | 152 ± 23 | 20 ± 2 |
| 3 | 321 ± 55 | 96 ± 15 | 9 ± 2 | |
| (CS + GL + EC) | 2 | 305 ± 65 | 84 ± 18 | 12 ± 2 |
| 3 | 230 ± 24 | 146 ± 30 | 10 ± 1 |
| Foam Obtained From | Water (%) | Dimensional Stability (%) at 150 °C | |||||
|---|---|---|---|---|---|---|---|
| Length Change After | Width Change After | Height Change After | |||||
| 20 h | 40 h | 20 h | 40 h | 20 h | 40 h | ||
| (CS + GL + H2O) + EC | 2 | −2.27 | −2.82 | −1.14 | −0.97 | +2.95 | +1.22 |
| 3 | −0.80 | −0.67 | −0.88 | −1.38 | −4.25 | −2.30 | |
| (CS + GL + GLYC) + EC | 2 | −0.28 | −0.61 | −0.92 | −3.66 | −0.19 | −1.57 |
| 3 | −1.16 | −1.08 | +0.75 | +0.56 | −0.11 | −2.67 | |
| (CS + GL) + EC | 2 | +1.20 | +0.29 | +2.04 | +1.47 | +2.45 | +1.76 |
| 3 | −1.66 | −1.33 | +1.38 | −0.81 | −2.41 | −2.78 | |
| (CS + GL + EC) | 2 | −1.00 | −1.09 | −1.09 | −0.65 | −1.18 | −1.64 |
| 3 | −0.62 | −0.62 | −0.51 | −0.55 | −1.35 | −0.90 | |
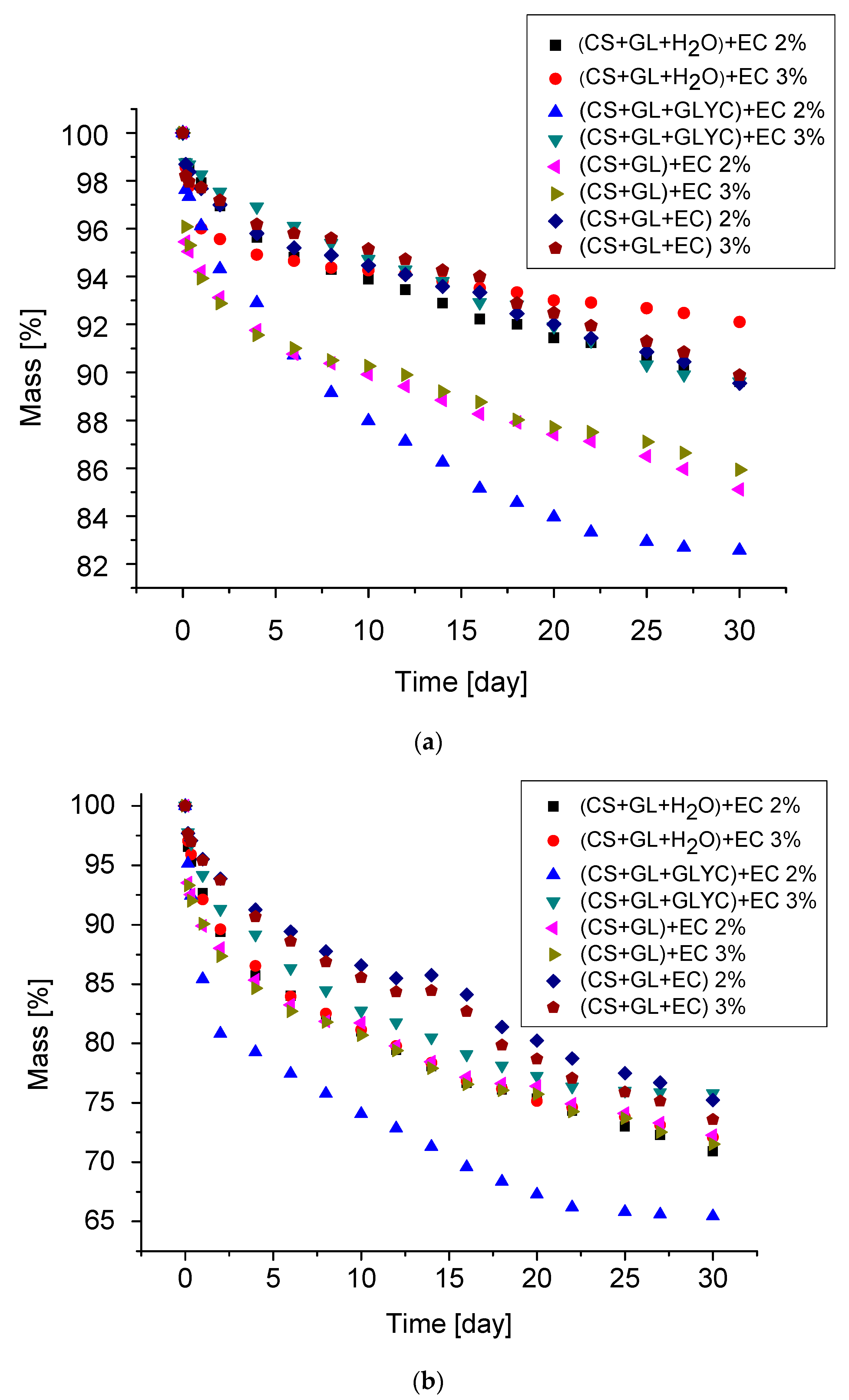
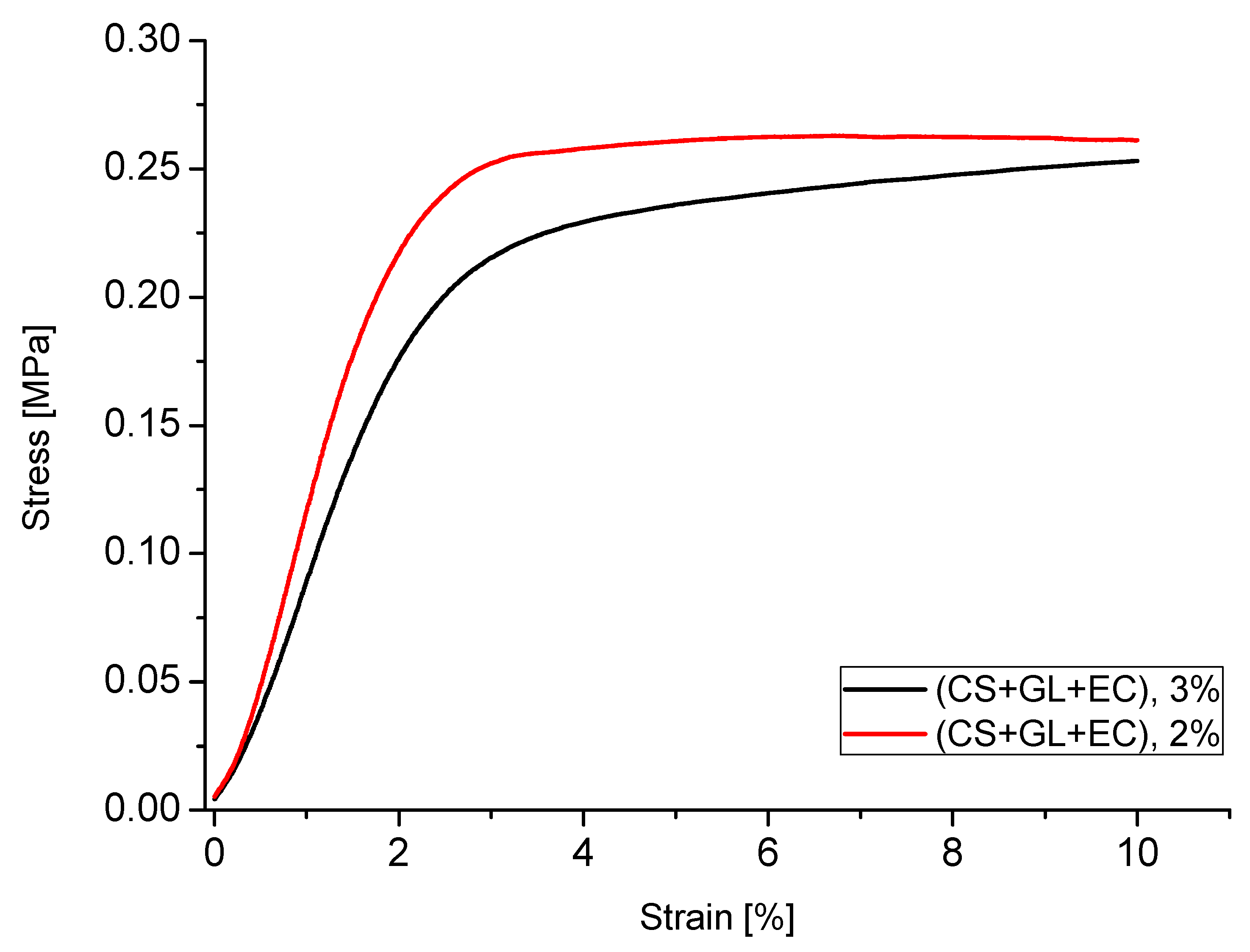
| Foam Obtained From | Water (%) | Mass Loss in %wt. after Exposure for 1 Month | Compressive Strength (MPa) | |||
|---|---|---|---|---|---|---|
| Before Exposure | After Exposure | |||||
| 150 °C | 175 °C | 150 °C | 175 °C | |||
| (CS + GL + H2O) + EC | 2 | 10.4 | 29.1 | 0.353 | 0.649 | 0.791 |
| 3 | 11.1 | 27.9 | 0.337 | 0.510 | 0.283 | |
| (CS + GL + GLYC) + EC | 2 | 17.4 | 34.6 | 0.513 | 0.694 | 0.791 |
| 3 | 10.4 | 24.2 | 0.337 | 0.510 | 0.238 | |
| (CS + GL) + EC | 2 | 14.9 | 27.7 | 0.119 | 0.271 | 0.373 |
| 3 | 14.1 | 28.5 | 0.203 | 0.235 | 0.219 | |
| (CS + GL + EC) | 2 | 10.4 | 24.8 | 0.238 | 0.336 | 0.328 |
| 3 | 10.1 | 26.4 | 0.227 | 0.249 | 0.248 | |
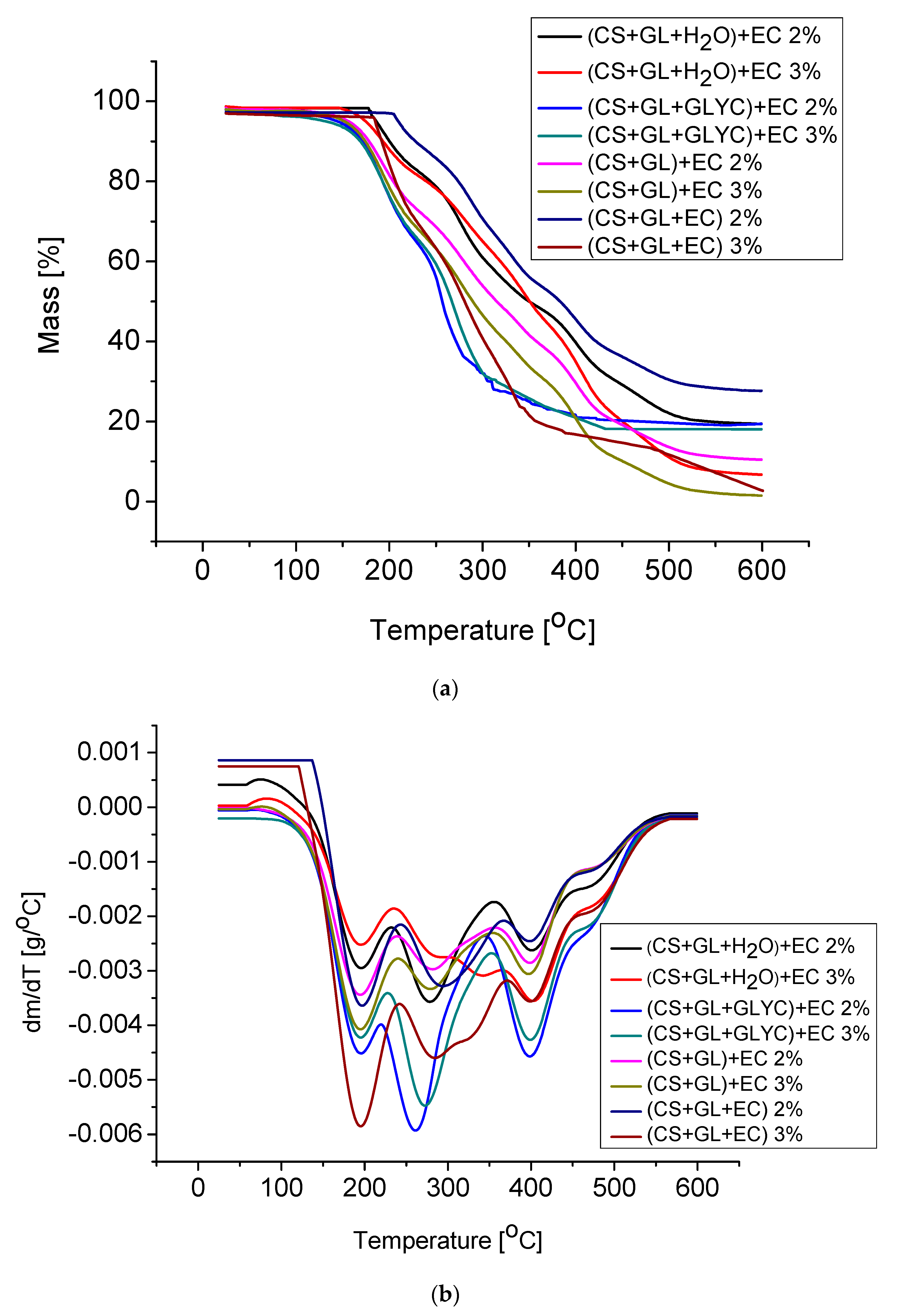
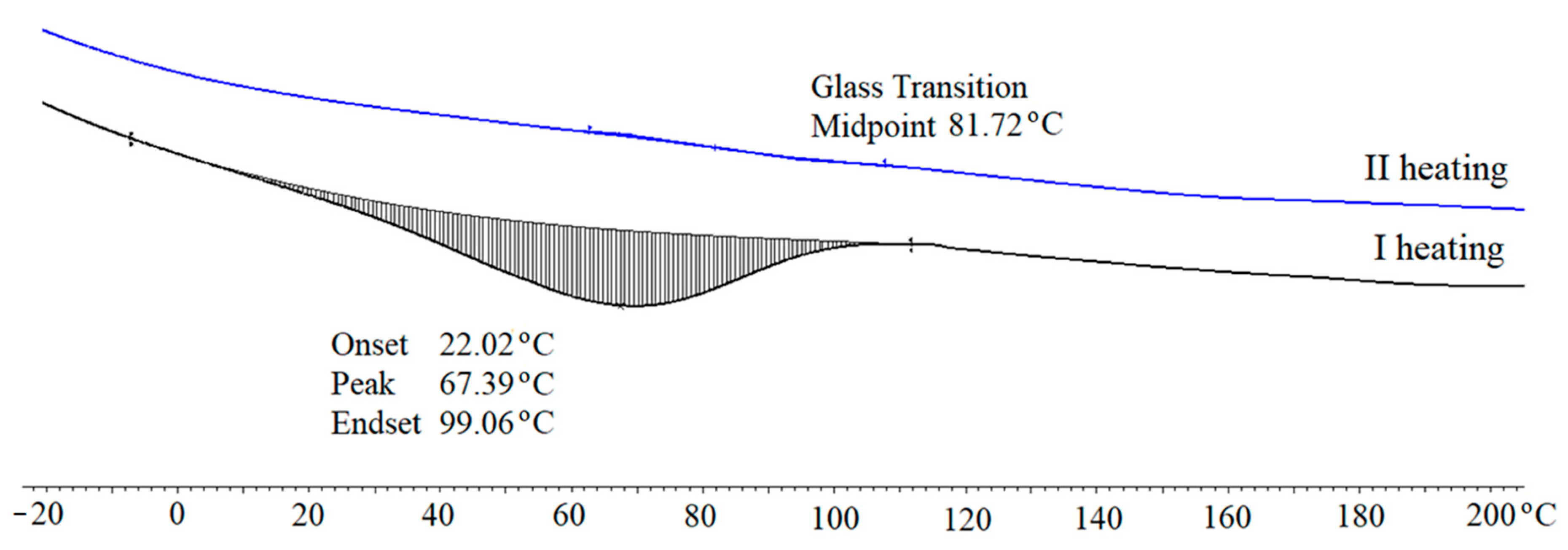
| Foam Obtained From | Water (%) | T5% (°C) | T10% (°C) | T25% (°C) | T50% (°C) | Tg (°C) |
|---|---|---|---|---|---|---|
| (CS + H2O + GL) + EC | 2 | 186 | 200 | 263 | 349 | 143 |
| 3 | 174 | 193 | 264 | 350 | 148 | |
| (CS + GLYC + GL) + EC | 2 | 144 | 171 | 202 | 256 | 111 |
| 3 | 131 | 168 | 203 | 266 | 111 | |
| (CS + GL) + EC | 2 | 156 | 178 | 220 | 315 | 91 |
| 3 | 152 | 175 | 208 | 289 | 82 | |
| (CS + GL + EC) | 2 | 210 | 229 | 288 | 382 | - |
| 3 | 184 | 192 | 216 | 280 | - |
| Sample | Element | |||
|---|---|---|---|---|
| C | H | O | N | |
| Polyol | 0.4693 | 0.0802 | 0.4505 | 0 |
| Polyurethane foam | 0.6102 | 0.0577 | 0.2636 | 0.0685 |
| Sample | BODx (mg/dm3) | BOD28 (mg/dm3) | Sample Mass (g) | TOD Counts | TOD (mg/dm3) | Dt (%) |
|---|---|---|---|---|---|---|
| Polyol | 116 | 107.5 | 0.19 | 8.4512 | 44.48 | 100 |
| Foam | 45.1 | 36.6 | 0.20 | 14.1264 | 70.63 | 51.82 |
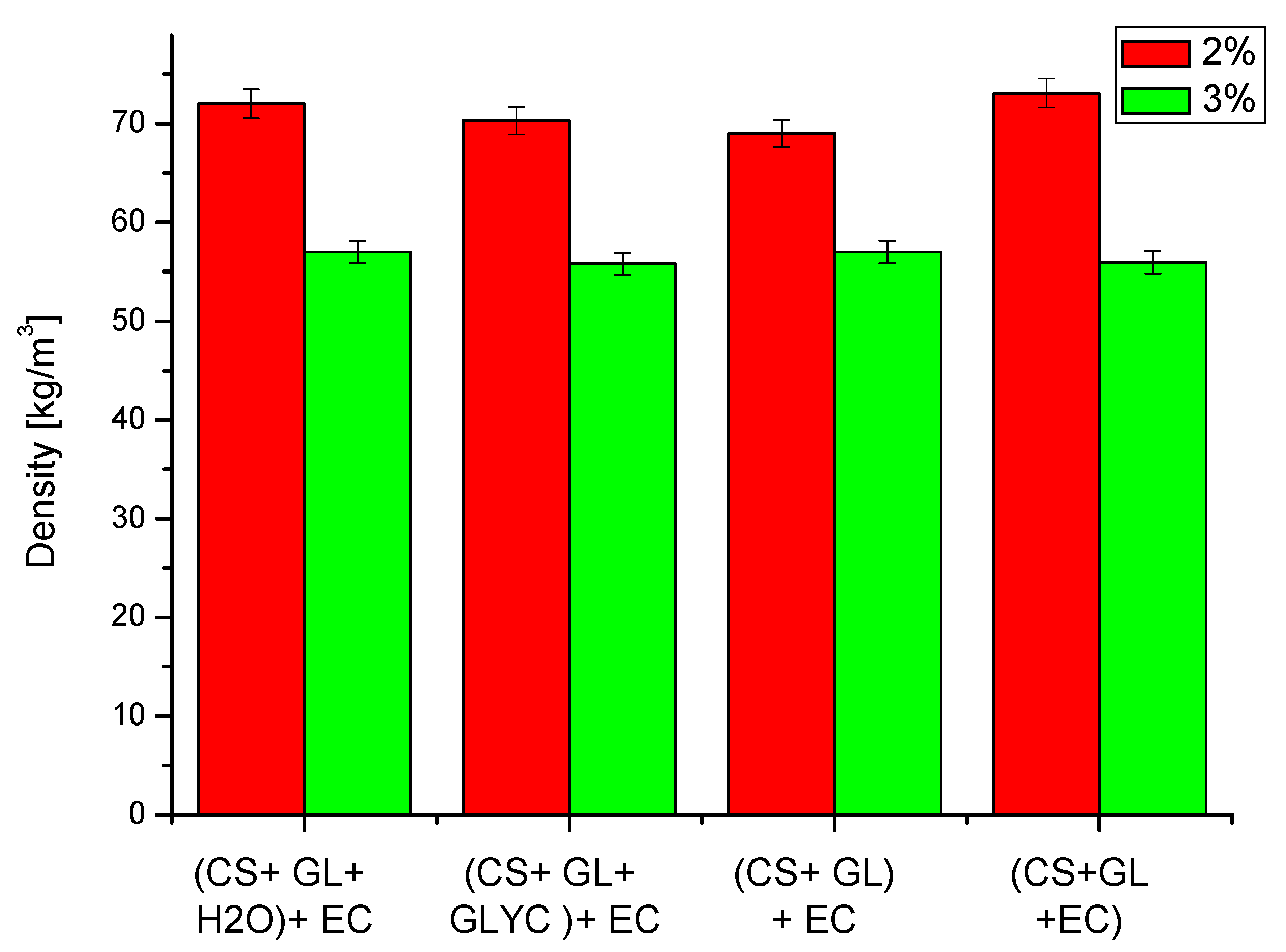
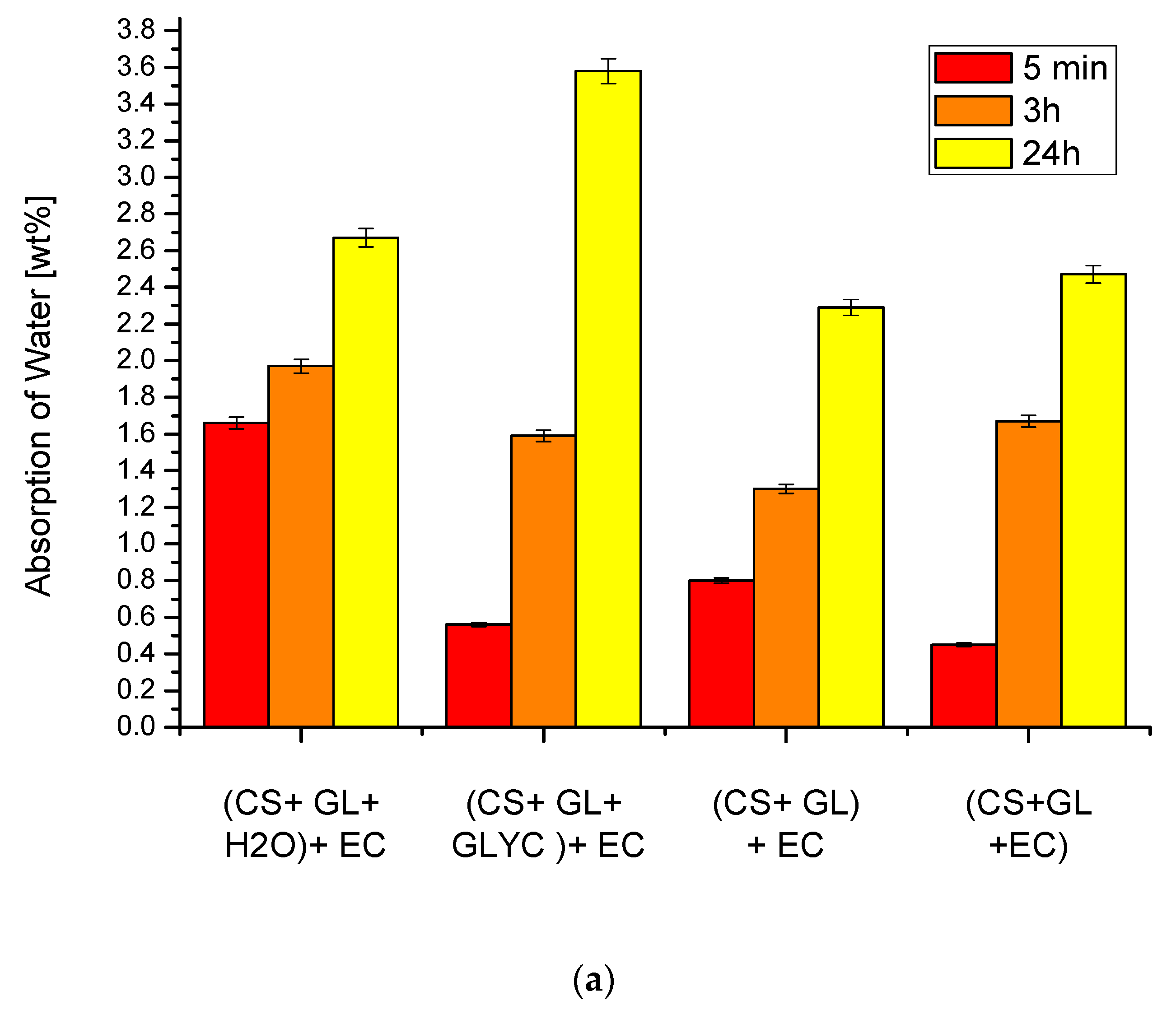
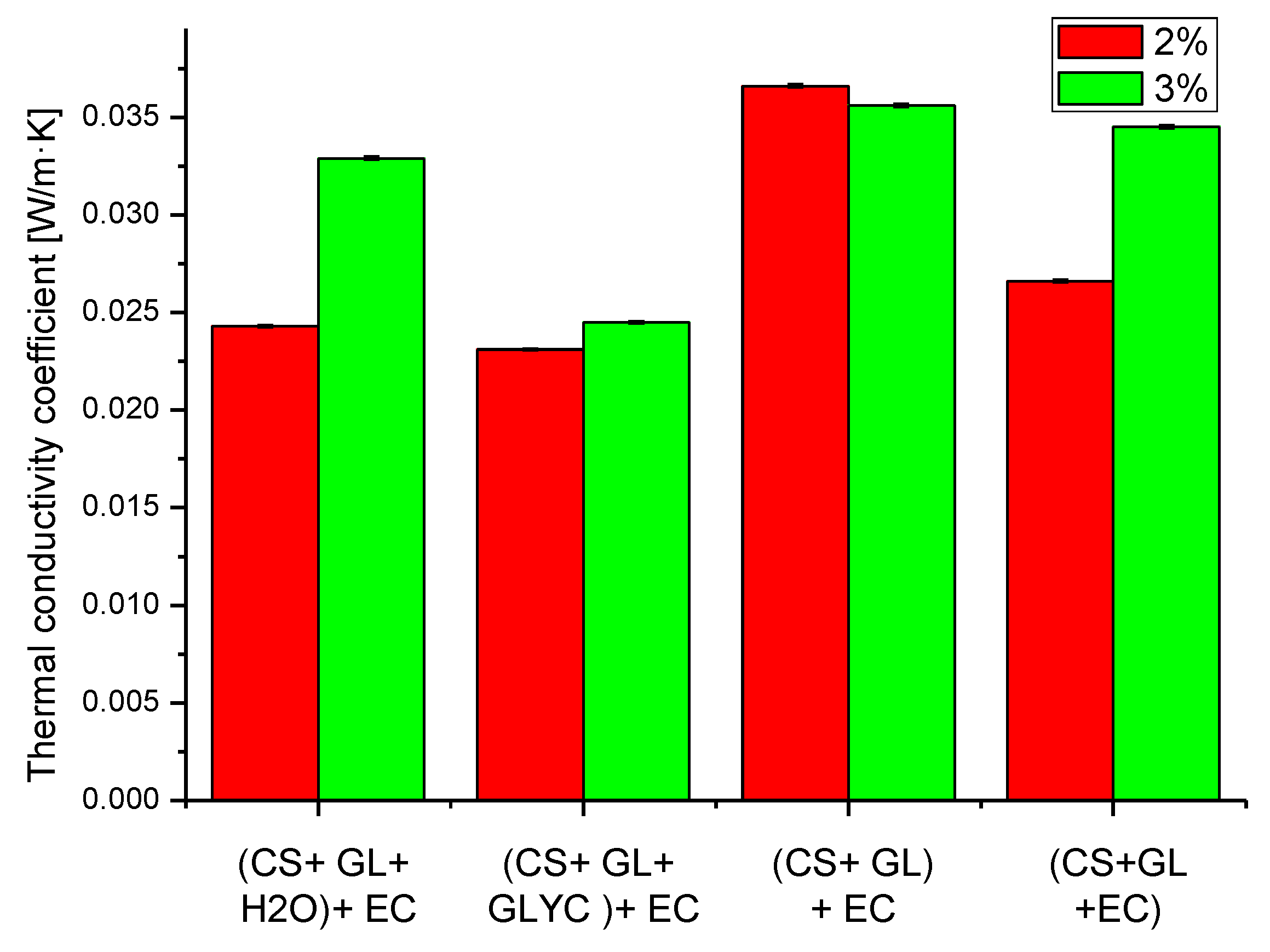
| Foam Obtained from Polyol | Water in Polyol (%) | Density (kg/m3) | Absorption of Water (wt.%) | Thermal Conductivity Coefficient (W/m·K) | Mass Loss after Exposure for 1 Month (wt%) | Compressive Strength (MPa) | |||
|---|---|---|---|---|---|---|---|---|---|
| Before Exposure | After Exposure | ||||||||
| 150 °C | 175 °C | 150 °C | 175 °C | ||||||
| (CS + GL + H2O) + EC | 2 | 72.0 | 2.67 | 0.0243 | 10.4 | 29.1 | 0.353 | 0.649 | 0.791 |
| 3 | 57.0 | 2.33 | 0.0329 | 11.0 | 27.1 | 0.337 | 0.510 | 0.283 | |
| (CS + GL + EC) | 2 | 73.0 | 2.47 | 0.0266 | 10.4 | 24.8 | 0.238 | 0.336 | 0.328 |
| 3 | 56.0 | 2.46 | 0.0345 | 10.1 | 26.4 | 0.227 | 0.249 | 0.248 | |
| S + H2O + EC [10] | 2 | 59.5 | 9.03 | 0.0367 | 15.2 | - | 0.223 | 0.924 | - |
| 3 | 61.9 | 7.24 | 0.0362 | 14.3 | - | 0.138 | 0.934 | - | |
| S + H2O + PC [10] | 2 | 43.2 | 7.07 | 0.0459 | 20.0 | 38.7 | 0.121 | 0.676 | 0.596 |
| 3 | 34.9 | 5.42 | 0.0561 | 16.4 | 34.6 | 0.120 | 0.247 | 0.375 | |
| S + GL + EC [32] | 4 | 37.3 | 4.75 | 0.0367 | 6.8 | 23.9 | 0.078 | 0.129 | 0.159 |
| S + GL + PC [32] | 4 | 26.0 | 5.00 | 0.0376 | 7.6 | 22.1 | 0.101 | 0.127 | 0.108 |
| (CEL + GL + H2O) + EC [11] | 2 | 60.5 | 6.56 | 0.0338 | 13.0 | 30.7 | 0.234 | 0.284 | 0.379 |
| CELhydrolysate + GL + EC [31] | 2 | 72.9 | 4.94 | 0.0364 | 7.6 | 21.0 | 0.112 | 0.325 | 0.410 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzałka, A.M.; Lubczak, J. Polyols and Polyurethane Foams Based on Water-Soluble Chitosan. Polymers 2023, 15, 1488. https://doi.org/10.3390/polym15061488
Strzałka AM, Lubczak J. Polyols and Polyurethane Foams Based on Water-Soluble Chitosan. Polymers. 2023; 15(6):1488. https://doi.org/10.3390/polym15061488
Chicago/Turabian StyleStrzałka, Anna Maria, and Jacek Lubczak. 2023. "Polyols and Polyurethane Foams Based on Water-Soluble Chitosan" Polymers 15, no. 6: 1488. https://doi.org/10.3390/polym15061488